Abstract
Narcissism is a complex phenomenon, involving a level of defensive self-enhancement. Narcissists have avoidant attachment styles, maintain distance in relationships and claim not to need others. However, they are especially sensitive to others’ evaluations, needing positive reflected appraisals to maintain their inflated self-views, and showing extreme responses (e.g. aggression) when rejected. The current study tested the hypothesis that narcissists also show hypersensitivity in brain systems associated with distress during exclusion. We measured individual differences in narcissism (Narcissistic Personality Inventory) and monitored neural responses to social exclusion (Cyberball). Narcissism was significantly associated with activity in an a priori anatomically defined social pain network (anterior insula, dorsal anterior cingulate cortex and subgenual anterior cingulate cortex) during social exclusion. Results suggest hypersensitivity to exclusion in narcissists may be a function of hypersensitivity in brain systems associated with distress, and suggests a potential pathway that connects narcissism to negative consequences for longer-term physical and mental health—findings not apparent with self-report alone.
Keywords: narcissism, social rejection, social exclusion, Cyberball, social pain network
INTRODUCTION
If no one turned around when we entered, answered when we spoke, or minded what we did, but if every person we met ‘cut us dead,’ and acted as if we were non-existing things, a kind of rage and impotent despair would ere long well up in us, from which the cruelest bodily torture would be a relief; for these would make us feel that, however bad might be our plight, we had not sunk to such a depth as to be unworthy of attention at all.
—William James, 1890, 293–4
Humans are fundamentally social beings, preferring to spend time with others for the majority of our waking hours (Kahneman et al., 2004). When we are not physically present with other people, they are present in our thoughts, dreams, fantasies, books, and televisions. Indeed, social connection has been found to be a robust predictor of psychological and physical health, including longevity (House et al., 1988; Holt-Lunstad et al. 2010; Konrath and Brown, 2012), and people who feel isolated or lonely are more likely to experience a variety of health problems (Cacioppo and Patrick, 2008). Interpersonal connections are so important that some scholars have argued that they fundamentally define the human experience (Baumeister and Leary, 1995; Cacioppo and Patrick, 2008).
Considering the primacy of social bonds to healthy functioning, it is important to understand what happens when those bonds are threatened by social exclusion. Exclusion experiences are painful to most people, and this is likely because at their core, they threaten our very sense of survival (Nezlek et al., 1997; Panksepp, 1998; Eisenberger and Lieberman, 2005; Macdonald and Leary, 2005). Without the protection offered by group members, the chances of survival in an evolutionary environment would dramatically diminish.
Thus, it is not surprising that rejection and social ostracism are associated with a number of negative immediate, shorter-term, and longer-term outcomes. In the immediate aftermath of ostracism, excluded people experience strong negative feelings such as anxiety or anger, high physiological arousal, and a sense of threat to their feelings of meaning and fulfillment (Leary, 1990; Shore et al., 1998; Snapp and Leary, 2001; Williams and Zadro, 2001; Zadro et al., 2004). Although internalizing responses to exclusion experiences are common (Parkhurst and Asher, 1992; Ladd, 2006), some individuals instead interpersonally externalize these experiences in the form of lower prosocial behaviour (Baumeister et al., 2002) and increased aggression (Twenge et al., 2001; Leary et al., 2003).
Individual differences in social exclusion
Negative responses to exclusion are nearly universally experienced (Williams and Sommer, 1997; Williams and Zadro, 2001; Zadro et al., 2004), even among infants (Tronick et al., 1978; Mesman et al., 2009). However, this does not rule out the possibility that some people are more susceptible to its negative effects. Some individual differences can have protective effects on social exclusion experiences such that even though these experiences remain unpleasant and painful, their longer-term negative consequences are attenuated. For example, individuals with more secure attachment styles and higher self-esteem are buffered from certain negative effects of social exclusion (Nezlek et al., 1997; Waldrip, 2007; DeWall et al., 2012). Several studies have also found that people who are highly self-conscious, those who have higher needs to belong and those with higher social anxiety have more detrimental reactions to social exclusion experiences (Fenigstein, 1979; Zadro et al., 2006; Waldrip, 2007).
Social exclusion in the brain
Previous research examining neural responses to social exclusion has found increased activation of the dorsal anterior cingulate cortex (dACC) and anterior insula (AI) during periods of exclusion vs inclusion in adults (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004; Eisenberger, 2012a), and activation in the subgenual anterior cingulate cortex (subACC) during social exclusion experiences in adolescents and young adults (Masten et al., 2009; Onoda et al., 2009). In addition, both empirical work and reviews have concluded that increased activation in the dACC correlates with self-reported increased feelings of social distress in response to exclusion (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004; Eisenberger, 2012a). Furthermore, in clinical studies the subACC has been implicated in depression and mood disorders (Greicius et al., 2007). Finally, the AI and dACC are activated during exposure to both physical and social pain, and may constitute an affective alarm system of pain and distress (Eisenberger, 2012a).
Individual and group differences have also been shown to alter exclusion sensitivity at the neural level. For example, differences in neural responses to social exclusion depend on individuals’ attachment style (DeWall et al., 2012). Attachment style is a marker of how individuals approach social relationships, and is one way that individuals satisfy their need to belong (Bowlby, 1977). Individuals with anxious attachment styles show increases in the dACC and AI during exclusion vs inclusion experiences, whereas those with avoidant attachment styles display significantly less activity in the dACC and AI (DeWall et al., 2012). As such, avoidant attachment styles may buffer individuals from negative social experiences by reducing their responsiveness to negative social cues (DeWall et al., 2012).
Self-esteem is also related to neural responses to social exclusion (Onoda et al., 2010). Those with higher trait self-esteem display less activation of the dACC (Onoda et al., 2010). These results converge with research finding that high trait self-esteem may serve as a buffer from negative social experiences (Nezlek et al., 1997). Finally, spending more time with friends during adolescence has also been found to decrease neural sensitivity to peer exclusion (Masten et al., 2012).
Narcissism and social exclusion
Taken together, there are a number of factors that are associated with buffered (e.g. secure attachment, avoidant attachment, self-esteem, positive social relationships) vs exaggerated (e.g. self-consciousness, need to belong, social anxiety, anxious attachment, adolescent age group) negative responses to social rejection, and many of these are also evident at the neural level. In the current study, we examine whether increases in the personality trait narcissism are associated with buffered vs exaggerated neural responses to rejection in adolescent males.
Narcissism is a personality trait that in the extreme can be a psychological disorder (APA, 1994). It involves an excessively positive focus on the self in combination with an extremely low regard for others (APA, 1994; Konrath et al., 2009), including difficulty with empathy-related tasks such as imaginatively engaging with others’ viewpoints or feeling concern and compassion for others’ suffering (Watson et al., 1984; Watson, 1994). This low regard for others is consequential: narcissists have difficulties in maintaining healthy relationships (Campbell et al., 2002) and they also tend to be hostile and aggressive (Baumeister et al., 1996; Bushman and Baumeister, 1998; Baumeister et al., 2000; Twenge and Campbell, 2003).
Despite a strong current understanding of behavioural characteristics associated with narcissism, little is known about the underlying neural mechanisms involved, especially with respect to responses to potential threats such as social exclusion (Konrath and Bonadonna, 2014). Therefore, an important question is how are individual differences in narcissism related to neural responses to social exclusion?
Two competing sets of hypotheses are plausible. It is possible that narcissism may buffer individuals’ responses to social exclusion. This is because at least on traditional self-report indices of mental health, narcissistic individuals look quite good. For example, they report being less depressed, anxious and lonely (Watson and Biderman, 1993; Sedikides et al., 2004) and also report higher self-esteem and more happiness compared with less narcissistic people (Watson and Biderman, 1993; Rose, 2002). As reviewed above, those with high self-esteem and avoidant attachment style (Watson and Biderman, 1993; Rose, 2002; Gjerde et al., 2004; Smolewska and Dion, 2005), personality characteristics associated with narcissism, show lower reactivity to social exclusion (Onoda et al., 2010; DeWall et al., 2012). Furthermore, narcissism has been shown to be associated with fewer negative internalized emotions in response to exclusion (Twenge and Campbell, 2003), which suggest that narcissists experience less internalized reactivity to exclusion. In that same study, however, narcissism was also associated with increased feelings of anger and increased aggression (Twenge and Campbell, 2003), both of which are self-protective responses that externalize blame to others (Tracy and Robins, 2003). Thus, it is difficult to determine how reactive narcissists are to social exclusion.
It is also possible that narcissism involves increased reactivity to social exclusion. Narcissism is a complex phenomenon, and involves a level of defensive self-enhancement. In fact, those scoring high in narcissism are especially sensitive to others’ evaluations. Narcissists need others’ admiration to maintain their positive self-views (Morf and Rhodewalt, 2001). Furthermore, even their supposed high self-esteem is in question; narcissists self-report having high self-esteem, but underlying feelings of low self-worth are evident when using implicit measures (Jordan et al., 2003; Zeigler-Hill, 2006). Moreover, narcissism and self-esteem are correlated, and both traits are associated with positive self-views on agentic traits like intelligence and competence. However, what differentiates them is that non-narcissistic people with high self-esteem also see themselves as high on communal traits such as warmth and caring, whereas narcissistic people do not (Campbell et al., 2002). Thus, their implicit low self-worth in combination with their troubled social interactions may suggest that narcissists’ high self-esteem is of a qualitatively different nature, and may not have the typical buffering effects.
Given the complexity of their self-views and social interactions, it might not be surprising that narcissistic people have excessive physiological responses (e.g. cortisol, cardiovascular reactivity) to socially threatening situations such as giving speeches (Kelsey et al., 2001, 2002; Sommer et al., 2009; Edelstein et al., 2010). Even in the absence of such stressors, in everyday low threat situations, their stress hormones are higher than people scoring low in narcissism (Reinhard et al., 2012). Thus, their underlying physiological responses betray their supposed psychological robustness and social nonchalance. In other words, narcissistic individuals might appear to be mentally healthy and socially nonchalant on the surface, but in reality, the trait is also associated with being socially needy and defensive. Taken together, there is evidence that narcissists are physiologically and behaviourally reactive to negative social situations and rejection; however, no research has explored the relationship between narcissism and neural responses to social exclusion.
The current study
The goal of this study was to investigate the relationship between neural responses to social exclusion and narcissism within a sample of adolescent boys. It is theoretically important to understand whether narcissistic individuals are hypersensitive to negative social experiences, despite possessing many potentially buffering characteristics (e.g. high self-reported self-esteem, low anxiety, avoidant attachment). In addition, although previous social cognitive and affective neuroscience investigations have demonstrated moderating relationships between individual differences such as attachment style (DeWall et al., 2012), social resources (Masten et al., 2012; Onoda et al., 2010) and neural responses to social exclusion, this literature has not been previously connected to the narcissism literature. Finally, it is important to examine these questions among adolescent males because adolescence is a critical developmental period when social acceptance becomes more important (Cotterell, 1996), and males tend to be more narcissistic than females (Foster et al., 2003).
Thus, the current study examines the relationship between male adolescents’ self-reported narcissistic personality characteristics and their responses to a social exclusion task. We examined their neural responses to exclusion using functional magnetic resonance imaging (fMRI) as well as their self-reported responses to exclusion. Findings from this study have important implications for individuals who score high in trait narcissism, which itself has been rising among American college students over time (Twenge et al., 2008). If they are indeed neurally hypersensitive to negative social experiences, this could have adverse consequences for the individuals themselves and also for their interpersonal functioning and ability to connect with and communicate effectively with others. Understanding the mechanisms that contribute to such hypersensitivity may offer scholars and practitioners clues to potentially effective intervention strategies, and also offers basic science insight into factors that impact neural responses to key social situations.
METHOD
Participants
Forty-three adolescent boys were recruited through the University of Michigan Transportation Research Institute as part of a larger study examining adolescent driving behaviour. Participants were between the ages of 16 to 17 (M = 16.8 years, s.d. = 0.47 years). All were right-handed, did not suffer from claustrophobia, were not currently taking any psychological medications, had normal (or corrected to normal) vision, did not have metal in their body that was contraindicated for fMRI, and did not typically experience motion sickness. One participant was excluded from the study due to a prior Autism diagnosis. Two other subjects were excluded from the analysis: one who did not complete the narcissism measure and another who fell asleep during the social exclusion task, resulting in a final sample size of 40 participants. Legal guardians provided written informed consent and teens provided written assent.
Materials and procedure
After the consent process, participants were introduced to two adolescent male confederates and were told that they would all be participating in a task together during their session. Participants were then put into separate rooms to complete a number of self-report survey measures, next they were scanned in an fMRI scanner, and finally they completed a number of post-scan survey measures. All participants were debriefed to alleviate negative responses to the social exclusion task.
Self-report measure of narcissism
Participants completed the Narcissism Personality Inventory (NPI; Raskin and Terry, 1988), which is a 40-item forced-choice measure in which participants must choose a more or less narcissistic response (e.g. ‘If I ruled the world it would be a better place’ or ‘The thought of ruling the world scares the hell out of me.’).
fMRI social exclusion task
To measure neural correlates of social exclusion, participants played the virtual ball tossing game Cyberball while undergoing an fMRI scan (Eisenberger et al., 2003). Participants were led to believe that they were playing with the two confederates from the beginning of the study, while in reality they were playing against two computer-generated participants. Participants were told that they could pass the ball to anyone they wanted, but that they should not hold the ball. In Round 1 (inclusion) of the game, the virtual participants played a game of catch in which everyone is included. In Round 2 (exclusion), the virtual participants began by including everyone, but soon excluded the participant from the game, only passing the ball between themselves.
fMRI data analysis
The data were pre-processed and analysed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). The fMRI data were pre-processed according to a standard pre-processing stream (including realignment to correct for head motion, coregistration, normalization and smoothing). Neural activity during exclusion (compared with inclusion) was modelled for each participant in Cyberball at the single subject level. Next, a random effects model was conducted, examining associations between scores on the NPI assessed prior to the scanning session and neural activity that was greater during exclusion compared with inclusion (see Supplementary Materials for complete details).
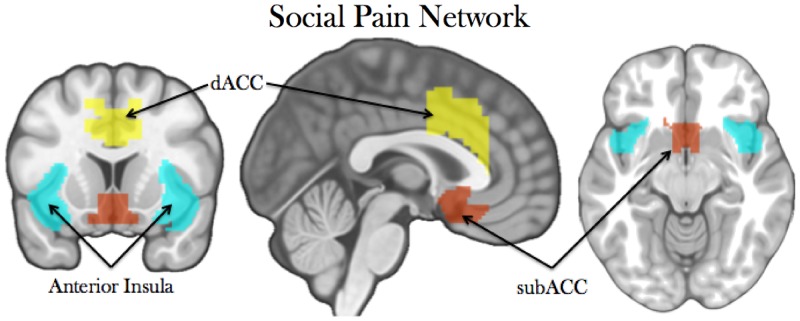
An a priori network of interest (NOI) analysis was conducted using MarsBaR (Brett et al., 2002). The regions of interest (ROIs) within the network were constructed based on prior work examining neural correlates of social exclusion, which included the AI, dACC and subACC [See Figure 1; (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004; Masten et al., 2009; Onoda et al., 2009; Eisenberger, 2012a)]. These regions were examined as a network and also analysed as individual ROIs (definitions in Supplementary Materials). Estimates of average percent signal change (exclusion > inclusion) within the entire NOI were extracted using MarsBaR, as well as within sub-regions of the network. We then examined the relationship between self-reported measures and NOI activation using multiple regression (with follow-up analyses conducted on sub-regions). Due to the sample size common of neuroimaging studies, bootstrap (10 000 samples) confidence intervals (CI) were also included.
Fig. 1.
The social pain network, constructed using the union of anatomically defined AI, subACC and dACC.
Self-report measures of threat following exclusion
Following the scanning session, participants completed a self-report assessment of feelings during the game (threat vs need satisfaction), using the Need Threat Scale (NTS; van Beest and Williams, 2006). Participants were instructed to rate their level of agreement with 20 statements on a seven-point scale ranging from ‘strongly disagree’ to ‘strongly agree’ (e.g. ‘I did not feel accepted by the other players’ and ‘I believed that my contribution to the game did not matter’). Results were coded such that higher scores indicated increased feelings of threat or distress.
RESULTS
Self-report measures
NPI scores ranged from 5 to 38 out of a total of 40 (M = 17.12, s.d. = 7.39, Cronbach’s α = 0.859), where higher scores corresponded to more narcissistic personality traits. Following the Cyberball exclusion experience, participants completed the NTS. On average participants scored near the midpoint of the seven-point scale (M = 3.11, s.d. = 0.97, Cronbach’s α = 0.908), where higher scores correspond to increased feelings of distress. These average self-reported distress scores following exclusion are consistent, within 1 s.d., with other neuroimaging studies examining Cyberball (3.25–3.99; Eisenberger et al., 2006; Sebastian et al., 2011; DeWall et al., 2012). We next examined whether self-reported distress following exclusion correlated with self-reported levels of narcissism. We found that, consistent with the demographic group being studied (teenage boys, who are often unwilling to explicitly express distress in social situations), feelings of distress following exclusion were uncorrelated with NPI scores (r = 0.12, P = 0.47; Figure 2).
Fig. 2.
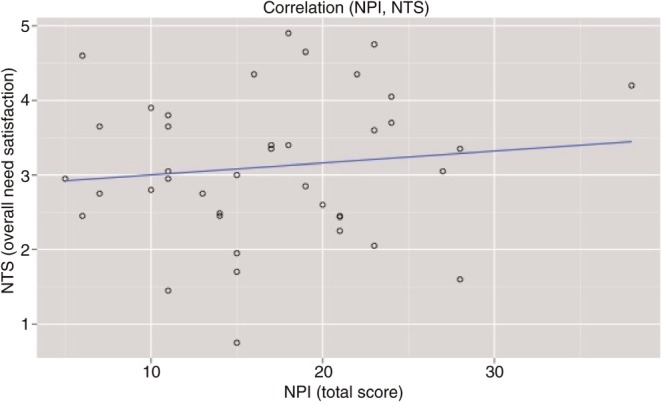
Correlation examining the relationship between self-reported NPI and NTS scores. Findings indicate that feelings of threat following exclusion (NTS) were uncorrelated with NPI scores (r = 0.12, P = 0.47).
Region of interest analyses
Narcissism and neural responses to exclusion
Activation of the social pain NOI (AI, dACC and subACC) was significantly correlated with NPI scores [r = 0.42, P = 0.009, CI = (0.12, 0.60); Figure 3]. Therefore, participants who reported higher levels of narcissism also had higher activation in the social pain network during social exclusion compared with baseline inclusion activations. In addition to the NOI analysis, we examined each individual region within the network separately. Independently, both the AI and dACC significantly correlated with NPI scores [r = 0.41, P = 0.01, CI = (0.15, 0.59) and r = 0.41, P = 0.01, CI = (0.10, 0.60), respectively]; however, the anatomically defined subACC ROI did not significantly correlate with NPI scores on its own [r = 0.17, P = 0.29, CI = (−0.17, 0.44)]. Finally, following all a priori specified NOI/ROI analyses, we conducted an exploratory whole brain analysis to determine which neural regions during exclusion > inclusion were most strongly associated with narcissism (see Supplementary Materials). These findings reinforce that hypersensitivity to exclusion in narcissists may be a function of hypersensitivity in brain systems associated with social pain, with clusters of activation observed in AI, dACC and subACC/medial prefrontal cortex, among other regions.
Fig. 3.

Correlation examining the relationship between the social pain NOI and NPI scores. Findings indicate that participants who reported higher levels of narcissism also had significantly higher activation in the social pain network during social exclusion compared with inclusion activations (r = 0.42, P = 0.009).
Self-reported threat and neural responses to exclusion
The social pain NOI was not significantly correlated with self-reported scores of threat following exclusion (r = −0.11, P = 0.51). In addition, self-reported threat following exclusion was uncorrelated with activity in the AI (r = −0.11, P = 0.50), dACC (r = −0.09, P = 0.58), and subACC (r = −0.09, P = 0.58). Results indicated that although participants showed reactivity within the social pain network in the brain, there may be variability in willingness to report, and awareness of the extent to which they are affected by social exclusion.
DISCUSSION
In this study, we expanded our current understanding of the potential intrapersonal costs of narcissism by uncovering narcissists’ neural responses to social exclusion. Adolescent males who scored higher in narcissism showed exaggerated neural responses in the putative social pain network during exclusion, compared with inclusion experiences. The current study also expands our understanding of individual differences in response to social exclusion, by exploring whether narcissistic individuals experience buffered vs exaggerated neural responses to social exclusion. Such exaggerated neural responses to social pain may indicate a neural mechanism that contributes to narcissists’ hypersensitivity to negative social experiences. These findings suggest that despite the potentially buffering correlates of narcissism (e.g. avoidant attachment style, high-trait self-esteem, low anxiety; Watson and Biderman, 1993; Rose, 2002; Gjerde et al., 2004; Smolewska and Dion, 2005), narcissistic individuals still have heightened neural responses to exclusion within the hypothesized social pain network. This may ultimately help to explain longer-term negative consequences of narcissism (Twenge et al., 2010). In fact, increased activity in regions associated with social threat or distress correlate with increased sympathetic nervous system and hypothalamic–pituitary–adrenal axis activity, which when chronically stressed have been associated with long-term disease; (for a review, see: Eisenberger and Cole, 2012). Furthermore, these results were not apparent when examining self-reported distress, thus demonstrating the utility of using neural imaging methodology to enhance our understanding of psychological phenomena.
Although these findings are consistent with current literature on narcissists’ behavioural hypersensitivity to social exclusion (e.g. aggression; Twenge and Campbell, 2003), they also point to the complex nature of narcissism. On the one hand, narcissists seem to benefit from an abundance of self-love and ego-protective traits (Watson and Biderman, 1993; Rose, 2002; Sedikides et al., 2004). However, on the other hand, they seem to be overly sensitive to social experiences that threaten their egos (Kelsey et al., 2001, 2002; Twenge and Campbell, 2003; Sommer et al., 2009; Edelstein et al., 2010; Reinhard et al., 2012). Some studies resolve this apparent contradiction by noting that narcissists’ positive self-views only seem to exist on explicit self-report measures, but negative self-views are evident in implicit measures, over which individuals have limited control (Jordan et al., 2003; Zeigler-Hill, 2006).
These results may shed light onto why narcissistic individuals are prone to certain behavioural reactions. Hypersensitivity to negative social experiences may be an underlying factor driving some of the prominent interpersonal behaviours associated with narcissism. In particular, narcissism is associated with interpersonal difficulties such as avoidant attachment styles, distance in social relationships and a potentially isolating sense of self-sufficiency and independence (Campbell, Foster, et al., 2002; Campbell and Foster, 2002; Campbell et al. 2004; Gjerde et al., 2004). However, this apparent lack of interest in social relationships may mask or be a coping mechanism for dealing with oversensitivity to exclusion. Hence, although narcissistic individuals tend to appear socially confident and relaxed on the surface, they may be focused on avoiding social exclusion because of exaggerated underlying physiological responses to negative social experiences (though the current data cannot directly address causality). Avoidant attachment styles may be one form of unconscious coping because avoidant attachment styles have been shown to buffer activation of the AI and dACC during social exclusion (DeWall et al., 2012). Such neural reflections of social pain may also help to explain narcissists’ aggressive responses after exclusion experiences (Twenge and Campbell, 2003). Furthermore, it is possible that to compensate, narcissistic people may experience a higher allostatic load—that is, narcissists may require extra physiological compensatory mechanisms to maintain external balance (Edelstein et al., 2010; Reinhard et al., 2012). This possibility suggests potential avenues to pursue linking narcissism with longer-term health outcomes (Konrath and Bonadonna, 2014).
There is a considerable body of research implicating our a priori identified neural ROIs in the distress of exclusion (so-called social pain). However, it should be noted that neural regions (dACC, AI and subACC) in the social pain network are also involved in other functions. Imaging studies have found the dACC to be associated with multiple functions including conflict monitoring, emotional awareness, cognitive dissonance, and reward-based decision making (Botvinick et al., 1999; Bush et al., 2002; Weissman et al., 2003; McRae et al., 2008; van Veen et al., 2009; Jarcho et al., 2011). Additionally, the AI has been implicated in various functions, including attention, decision making, intentions and awareness of sensations and movement; (for a review, see: Craig, 2009). Likewise, the subACC is implicated in depression and mood disorders (Greicius et al., 2007). Therefore, the idea of the social pain network is a possible neural mechanism that contributes to narcissists’ hypersensitivity to exclusion should be interpreted as one of several possible explanations. As with all studies of this type, the usual caution regarding reverse inference applies (Poldrack, 2006). Additionally, because the current work is correlational, there is the possibility that an unaccounted third variable may explain the current findings. Despite these limitations, the use of a priori ROIs and a theoretical framework strengthen our claim, and we suggest that this interpretation is parsimonious when considering other research on narcissism.
This study suggests the potential for several practical implications. For example, it can offer insight into potential testable interventions for narcissists. It is impossible to remove all sources of exclusion from a narcissist’s life; however, it may be possible to treat feelings of pain that result from them. As these feelings seem to be more strongly detected in physiological measures, perhaps effective treatments could also be physiological. For example, research has found acetaminophen, a commonly used over-the-counter pain reliever, is also effective at reducing social pain, whether that pain is measured using self-reports or fMRIs (Dewall et al., 2010). In one study, participants who took a 3-week regimen of acetaminophen reported having less hurt feelings, and participants in a separate experiment showed less neural activity in regions associated with social pain (dACC and AI), in response to social exclusion (Dewall et al., 2010). Indeed, narcissism is known to be associated with a variety of addictive and compulsive behaviours (Craig et al., 1985; Luhtanen and Crocker, 2005; Campbell et al., 2006; Rose, 2007; Carter et al., 2012), many which may serve self-medicating functions; as such, attention to multiple factors is warranted when considering the mental and physical health of narcissists. Furthermore, because neural mechanisms of social pain are closely tied with mechanisms of physical pain (Eisenberger, 2012b), coping strategies aimed at reducing physical pain may also be effective (Van Damme et al., 2008). For example, using relaxation techniques such as deep breathing may help to reduce tension in the body.
A more general question this study raises is under which contexts do individuals experience exaggerated vs buffered social pain? The current study capitalizes on the use of a homogeneous sample, consisting of males aged 16 to 17, in the context of social exclusion, outlining a clear context and demographic of individuals who show increased susceptibility to social exclusion. Prior work has found that narcissism is associated with high cortisol overall and in response to stressors, but only among males (Edelstein et al., 2010; Reinhard et al., 2012). Furthermore, adolescence is a key developmental period in which relations with peers are solidified and the brain undergoes rapid development in networks supporting social cognition and emotion regulation, yet cognitive control systems are slower to mature (Steinberg, 2007; Crone and Dahl, 2012). Future research should examine whether the current findings are consistent across different ages, among women, and in other populations. In addition, future work could further examine motivations, goals and the salience of negative social experiences in narcissistic individuals to better understand why they experience exaggerated social pain.
CONCLUSION
This study examined neural mechanisms associated with social exclusion and self-reported levels of narcissism. The current study enriches our understanding of individual differences that exaggerate effects of exclusion by (i) demonstrating that those who report higher levels of narcissism also experience exaggerated responses to exclusion in the brain, and (ii) suggesting that a social pain NOI, driven primarily by activity in the AI and dACC, may be one potential mechanism involved in this increased sensitivity to exclusion. Future investigations should examine the possibility that behavioural consequences of hypersensitivity to exclusion in narcissistic individuals may be a function of hypersensitivity in brain systems associated with distress more generally. Meanwhile, the current study suggests a pathway that may connect narcissism to negative consequences for longer-term physical and mental health—findings not apparent with self-report alone.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
This work was made possible by a grant from the University of Michigan Injury Center and NICHD (NIH-NICHD-DESPR-10-08; PI Bingham). In addition, S.K. was supported by Wake Forest University (The Character Project), the University of Notre Dame (Science of Generosity) and the John Templeton Foundation (Grant #47993). E.F. was supported by NIH 1DP2DA03515601 and NIH/NICHD 1R21HD073549-01A1 (PI Falk). Frank Tinney, Josh Carp, Matthew Brook O’Donnell, Jen LaRose, Johanna Dolle, Farideh Almani, Andrea Baretto, Alyssa Templar, Cary Welsh, Nicholas Wasylyshyn, Andrew Suzuki, Robin Liu, Ryan Bondy and Matthew Sweet assisted with data collection. Ray Bingham, Bruce Simons-Morton, Jean Shope, Anuj Pradhan, Marie-Claude Ouimet collaborated on the parent project. We thank the Pfeifer Lab for anatomical ROIs.
REFERENCES
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Baumeister RF, Bushman BJ, Campbell WK. Self-Esteem, narcissism, and aggression: does violence result from low self-esteem or from threatened egotism? Current Directions in Psychological Science. 2000;9(1):26. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497. [PubMed] [Google Scholar]
- Baumeister RF, Smart L, Boden JM. Relation of threatened egotism to violence and aggression: the dark side of high self-esteem. Psychological Review. 1996;103(1):5. doi: 10.1037/0033-295x.103.1.5. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Twenge JM, Ciarocco N. The inner world of rejection: Effects of social exclusion on cognition, emotion, and self-regulation. In: Forgas JP, Williams KD, editors. The Social Self: Cognitive, Interpersonal, and Intergroup Perspectives. New York: Psychology Press; 2002. pp. 161–74. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bowlby J. The making and breaking of affetional bonds. I. Aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. British Journal of Psychiatry. 1977;130:201–10. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. 2002. Region of interest analysis unsing an SPM toolbox. Presented at the The 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman BJ, Baumeister RF. Threatened egotism, narcissism, self-esteem, and direct and displaced aggression: does self-love or self-hate lead to violence? Journal of Personality and Social Psychology. 1998;75(1):219. doi: 10.1037//0022-3514.75.1.219. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Patrick W. Loneliness: Human Nature and the Need for Social Connection. New York: WW Norton & Company; 2008. [Google Scholar]
- Campbell WK, Bonacci AM, Shelton J, Exline JJ, Bushman BJ. Psychological entitlement: interpersonal consequences and validation of a self-report measure. Journal of Personality Assessment. 2004;83(1):29. doi: 10.1207/s15327752jpa8301_04. [DOI] [PubMed] [Google Scholar]
- Campbell WK, Foster CA. Narcissism and commitment in romantic relationships: an investment model analysis. Personality and Social Psychology Bulletin. 2002;28(4):484. [Google Scholar]
- Campbell WK, Foster CA, Finkel EJ. Does self-love lead to love for others? A story of narcissistic game playing. Journal of Personality and Social Psychology. 2002;83(2):340. doi: 10.1037/0022-3514.83.2.340. [DOI] [PubMed] [Google Scholar]
- Campbell WK, Rudich EA, Sedikides C. Narcissism, self-esteem, and the positivity of self-views: two portraits of self-love. Personality and Social Psychology Bulletin. 2002;28(3):358–68. [Google Scholar]
- Campbell WK, Shrira I, Foster JD. Theoretical models of narcissism, sexuality, and relationship commitment. Journal of Social and Personal Relationships. 2006;23(3):367. [Google Scholar]
- Carter RR, Johnson SM, Exline JJ, Post SG, Pagano ME. Addiction and “Generation Me”: narcissistic and prosocial behaviors of adolescents with substance dependency disorder in comparison to normative adolescents. Alcoholism Treatment Quarterly. 2012;30(2):163–78. doi: 10.1080/07347324.2012.663286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterell J. Social Networks and Social Influences in Adolescence. London: Taylor & Frances/Routledge; 1996. [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10(1):59. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig RJ, Verinis JS, Wexler S. Personality characteristics of drug addicts and alcoholics on the Millon Clinical Multiaxial Inventory. Journal of Personality Assessment. 1985;49(2):156–60. doi: 10.1207/s15327752jpa4902_10. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews. Neuroscience. 2012;13(9):636. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychological Science. 2010;21(7):931. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Masten CL, Powell C, et al. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7(2):184. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein RS, Yim IS, Quas JA. Narcissism predicts heightened cortisol reactivity to a psychosocial stressor in men. Journal of Research in Personality. 2010;44(5):565–72. doi: 10.1016/j.jrp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosomatic Medicine. 2012a;74(2):126. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews. Neuroscience. 2012b;13(6):421. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience. 2012;15(5):669. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126(1):132. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. The Social Outcast: Ostracisim, Social Exclusion, Rejection, and Bullying. New York: Cambridge University Press; 2005. Why it hurts to be left out: The neurocognitive overlap between physical and social pain; pp. 109–27. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fenigstein A. Self-consciousness, self-attention, and social interaction. Journal of Personality and Social Psychology. 1979;37(1):75. [Google Scholar]
- Foster JD, Campbell WK, Twenge JM. Individual differences in narcissism: inflated self-views across the lifespan and around the world. Journal of Research in Personality. 2003;37(6):469. [Google Scholar]
- Gjerde PF, Onishi M, Carlson KS. Personality characteristics associated with romantic attachment: a comparison of interview and self-report methodologies. Personality and Social Psychology Bulletin. 2004;30(11):1402–15. doi: 10.1177/0146167204264291. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Berkman ET, Lieberman MD. The neural basis of rationalization: cognitive dissonance reduction during decision-making. Social Cognitive and Affective Neuroscience. 2011;6(4):460–7. doi: 10.1093/scan/nsq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CH, Spencer SJ, Zanna MP, Hoshino-Browne E, Correll J. Secure and defensive high self-esteem. Journal of Personality and Social Psychology. 2003;85(5):969. doi: 10.1037/0022-3514.85.5.969. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306(5702):1776. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Ornduff SR, McCann CM, Reiff S. Psychophysiological characteristics of narcissism during active and passive coping. Psychophysiology. 2001;38(2):292. [PubMed] [Google Scholar]
- Kelsey RM, Ornduff SR, Reiff S, Arthur CM. Psychophysiological correlates of narcissistic traits in women during active coping. Psychophysiology. 2002;39(3):322. [PubMed] [Google Scholar]
- Konrath S, Bonadonna JP. Physiological and health-related correlates of the narcissistic personality. In: Besser A, editor. Psychology of Narcissism. Hauppauge, New York: Nova Science Publishers, Inc; 2014. (Vol. under review) [Google Scholar]
- Konrath S, Brown SL. The effects of giving on givers. In: Roberts N, Newman M, editors. Handbook of Health and Social Relationships. Washington, DC: American Psychological Association; 2012. [Google Scholar]
- Konrath S, Bushman BJ, Grove T. Seeing my world in a million little pieces: narcissism, self-construal, and cognitive-perceptual style. Journal of Personality. 2009;77(4):1197. doi: 10.1111/j.1467-6494.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- Ladd GW. Peer rejection, aggressive or withdrawn behavior, and psychological maladjustment from ages 5 to 12: an examination of four predictive models. Child Development. 2006;77(4):822. doi: 10.1111/j.1467-8624.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Leary MR. Responses to social exclusion: social anxiety, jealousy, loneliness, depression, and low self-esteem. Journal of Social and Clinical Psychology. 1990;9(2):221. [Google Scholar]
- Leary MR, Kowalski RM, Smith L, Phillips S. Teasing, rejection, and violence: case studies of the school shootings. Aggressive Behavior. 2003;29(3):202. [Google Scholar]
- Luhtanen RK, Crocker J. Alcohol use in college students. Psychology of Addictive Behaviors. 2005;19(1):99. doi: 10.1037/0893-164X.19.1.99. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131(2):202. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience. 2012;7(1):106. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41(2):648–55. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman J, van IJzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the still-face paradigm: a review and meta-analysis. Developmental Review. 2009;29(2):120. [Google Scholar]
- Morf CC, Rhodewalt F. Unraveling the paradoxes of narcissism: a dynamic self-regulatory processing model. Psychological Inquiry. 2001;12(4):177. [Google Scholar]
- Nezlek JB, Holgate S, Blevins T, Kowalski RM, Leary MR. Personality moderators of reactions to interpersonal rejection: depression and trait self-esteem. Personality and Social Psychology Bulletin. 1997;23(12):1235. [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4(5):443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience. 2010;5(4):385. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. The quest for long-term health and happiness: to play or not to play, that is the question. Psychological Inquiry. 1998;9(1):56–66. [Google Scholar]
- Parkhurst JT, Asher SR. Peer rejection in middle school: Subgroup differences in behavior, loneliness, and interpersonal concerns. Developmental Psychology. 1992;28(2):231. [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Raskin R, Terry H. A principal-components analysis of the Narcissistic Personality Inventory and further evidence of its construct validity. Journal of Personality and Social Psychology. 1988;54(5):890. doi: 10.1037//0022-3514.54.5.890. [DOI] [PubMed] [Google Scholar]
- Reinhard DA, Konrath S, Lopez WD, Cameron HG. Expensive egos: narcissistic males have higher cortisol. PloS One. 2012;7(1):e30858. doi: 10.1371/journal.pone.0030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P. The happy and unhappy faces of narcissism. Personality and Individual Differences. 2002;33(3):379. [Google Scholar]
- Rose P. Mediators of the association between narcissism and compulsive buying. Psychology of Addictive Behaviors. 2007;21(4):576. doi: 10.1037/0893-164X.21.4.576. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan G, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57(3):686. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sedikides C, Rudich EA, Gregg AP, Kumashiro M, Rusbult C. Are normal narcissists psychologically healthy?: self-esteem matters. Journal of Personality and Social Psychology. 2004;87(3):400–16. doi: 10.1037/0022-3514.87.3.400. [DOI] [PubMed] [Google Scholar]
- Shore WJ, Williams KD, Grahe JE. The silent treatment: perceptions of its behaviors and associated feelings. Group Processes and Intergroup Relations. 1998;1(2):117. [Google Scholar]
- Smolewska K, Dion K. Narcissism and adult attachment: a multivariate approach. Self and Identity. 2005;4(1):59. [Google Scholar]
- Snapp CM, Leary MR. Hurt feelings among new acquaintances: moderating effects of interpersonal familiarity. Journal of Social and Personal Relationships. 2001;18(3):315. [Google Scholar]
- Sommer KL, Kirkland KL, Newman SR, Estrella P, Andreassi JL. Narcissism and cardiovascular reactivity to rejection imagery. Journal of Applied Social Psychology. 2009;39(5):1083. [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55. [Google Scholar]
- Tracy JL, Robins RW. “Death of a (narcissistic) salesman:” An integrative model of fragile self-esteem. Psychological Inquiry. 2003;14:57–62. [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17(1):1. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can’t join them, beat them: effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81(6):1058. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Campbell WK. “Isn’t it fun to get the respect that we’re going to deserve?” Narcissism, social rejection, and aggression. Personality and Social Psychology Bulletin. 2003;29(2):261. doi: 10.1177/0146167202239051. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Gentile B, DeWall CN, Ma D, Lacefield K, Schurtz DR. Birth cohort increases in psychopathology among young Americans, 1938–2007: a cross-temporal meta-analysis of the MMPI. Clinical Psychology Review. 2010;30(2):145. doi: 10.1016/j.cpr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Konrath S, Foster JD, Campbell WK, Bushman BJ. Egos inflating over time: a cross-temporal meta-analysis of the narcissistic personality inventory. Journal of Personality. 2008;76(4):875. doi: 10.1111/j.1467-6494.2008.00507.x. [DOI] [PubMed] [Google Scholar]
- Van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91(5):918. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C. Coping with pain: a motivational perspective. Pain. 2008;139(1):1. doi: 10.1016/j.pain.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS, Krug MK, Schooler JW. Neural activity predicts attitude change in cognitive dissonance. Nature Neuroscience. 2009;12(11):1469. doi: 10.1038/nn.2413. [DOI] [PubMed] [Google Scholar]
- Waldrip AM. The power of ostracism: Can personality influence reactions to social exclusion? 2007. University of Texas. Requirements for the Degree of doctor of philosophy. [Google Scholar]
- Watson PJ. Empathy, sex role orientation, and narcissism. Sex Roles. 1994;30(9–10):701. [Google Scholar]
- Watson PJ, Biderman MD. Narcissistic personality inventory factors, splitting, and self-consciousness. Journal of Personality Assessment. 1993;61(1):41–57. doi: 10.1207/s15327752jpa6101_4. [DOI] [PubMed] [Google Scholar]
- Watson PJ, Grisham SO, Trotter MV, Biderman MD. Narcissism and empathy: validity evidence for the narcissistic personality inventory. Journal of Personality Assessment. 1984;48(3):301–5. doi: 10.1207/s15327752jpa4803_12. [DOI] [PubMed] [Google Scholar]
- Weissman D, Giesbrecht B, Song A, Mangun G, Woldorff M. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage. 2003;19(4):1361–8. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Williams KD, Sommer KL. Social ostracism by coworkers: does rejection lead to loafing or compensation? Personality and Social Psychology Bulletin. 1997;23(7):693. [Google Scholar]
- Williams KD, Zadro L. Interpersonal Rejection. New York: Oxford University Press; 2001. Ostracism: on being ignored, excluded and rejected; pp. 21–53. [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42(5):692. [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-steem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40(4):560. [Google Scholar]
- Zeigler-Hill V. Discrepancies between implicit and explicit self-esteem: implications for narcissism and self-esteem Instability. Journal of Personality. 2006;74(1):119. doi: 10.1111/j.1467-6494.2005.00371.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.