Abstract
Maternal anxiety during pregnancy has been consistently shown to negatively affect offspring neurodevelopmental outcomes. However, little is known about the impact of positive maternal traits/states during pregnancy on the offspring. The present study was aimed at investigating the effects of the mother’s mindfulness and anxiety during pregnancy on the infant’s neurocognitive functioning at 9 months of age. Mothers reported mindfulness using the Freiburg Mindfulness Inventory and anxiety using the Symptom Checklist (SCL-90) at ±20.7 weeks of gestation. Event-related brain potentials (ERPs) were measured from 79 infants in an auditory oddball paradigm designed to measure auditory attention—a key aspect of early neurocognitive functioning. For the ERP responses elicited by standard sounds, higher maternal mindfulness was associated with lower N250 amplitudes (P < 0.01, η2 = 0.097), whereas higher maternal anxiety was associated with higher N250 amplitudes (P < 0.05, η2 = 0.057). Maternal mindfulness was also positively associated with the P150 amplitudes (P < 0.01, η2 = 0.130). These results suggest that infants prenatally exposed to higher levels of maternal mindfulness devote fewer attentional resources to frequently occurring irrelevant sounds. The results show that positive traits and experiences of the mother during pregnancy may also affect the unborn child. Emphasizing the beneficial effects of a positive psychological state during pregnancy may promote healthy behavior in pregnant women.
Keywords: event-related potential, auditory attention, cognitive development, mindfulness, anxiety, infant
INTRODUCTION
Research under the ‘prenatal programming hypothesis’ examines the short- and long-term consequences of the conditions of the prenatal environment for the offspring’s health and disease risk (Barker, 2004). To date, accumulating evidence has demonstrated that prenatal exposure to maternal psychological distress affects neurocognitive functioning in the child. This effect probably results from induced alterations to fetal brain development and physiology (Van den Bergh et al., 2005; Räikkönen et al., 2011; Van den Bergh, 2011). Although the majority of the research in this field has focused almost exclusively on effects of exposure to early adverse conditions, prenatal exposure to maternal well-being might also ‘program’ infant development and health. For example, Sriboonpimsuay and colleagues (2011) showed that the incidence of preterm birth was reduced in mothers who received meditation intervention during pregnancy as compared with the control group (i.e. those who underwent routine prenatal care). These results highlight the relevance of positive maternal experiences during pregnancy and their possible beneficial effects for both the mother and the child. Therefore, to cover the full scope of prenatal programming, one should also study the effects of positive experiences and traits during pregnancy on the offspring.
A good candidate for such a positive trait might be mindfulness, as many studies to date have reported associations between self-reported mindfulness (as a trait) and psychological health (for a review, see Keng et al., 2011). Being mindful refers to a state of mind consisting of two key elements: (i) An alert mode of perceiving all mental contents (i.e. perceptions, sensations, cognitions and emotions) and (ii) a friendly, accepting and non-judgmental attitude toward those mental contents (Kohls et al., 2009). Being more mindful has been associated with better work–family balance among working parents (Allen and Kiburz, 2012) and better emotion regulation (Goodall et al., 2012). These two factors could contribute to a more healthy pregnancy, which, in turn, may provide a better environment for the fetus. Mindfulness interventions have been developed recently for pregnant women, and their effects on psychological stress experience during pregnancy have been evaluated. The initial trials suggested beneficial effects of mindfulness interventions for normal populations of pregnant women, as they reduced anxiety and depressive symptoms in mothers both pre- and postnatally (Vieten and Astin, 2008; Dunn et al., 2012). A recent randomized controlled trial also found reduced maternal anxiety after mindfulness intervention in pregnant women with elevated anxiety levels (Guardino et al., 2014). To our knowledge, only the study conducted by Sriboonpimsuay and colleagues (2011) reported effects of mindfulness intervention during pregnancy on birth outcomes (i.e. lower preterm birth weight in the intervention group) and no studies have been published on child developmental and health outcomes. Hence, the current study investigated the effects of dispositional maternal mindfulness during pregnancy and its effects on infant neurocognitive functioning.
A key aspect of early neurocognitive functioning is auditory attention. Auditory attention is an essential building block for developmental milestones, such as speech and language acquisition (Molfese, 2000; Benasich et al., 2002, 2006; Kushnerenko et al., 2013). It is important for infants to learn to organize the auditory input by rapidly extracting higher-level relationships and regularities from the sensory environment while becoming less responsive to variance in primary sensory features (Kushnerenko et al., 2007). Auditory event-related potentials (ERPs) have long been used to study these processes in infants and young children. The ERP method is suitable for testing this target group, as ERPs can be recorded without the need for a behavioral response and in the absence of focused attention. Further, neurophysiological measures, such as ERPs, can be linked to the neurobiological processes involved in information processing. Despite these advantages, few human studies examining prenatal programming have incorporated neurophysiological measures (Mennes et al., 2009; Buss et al., 2010; Hunter et al., 2012). In the current study, we measured ERPs from 9 month old infants in a passive auditory oddball paradigm designed to measure the processing of frequent and rare sound events. Infants were presented with a repetitious train of ‘standard’ sounds, which was occasionally interspersed with acoustically (i.e. white noise sounds and novel sounds) or temporally deviant sounds.
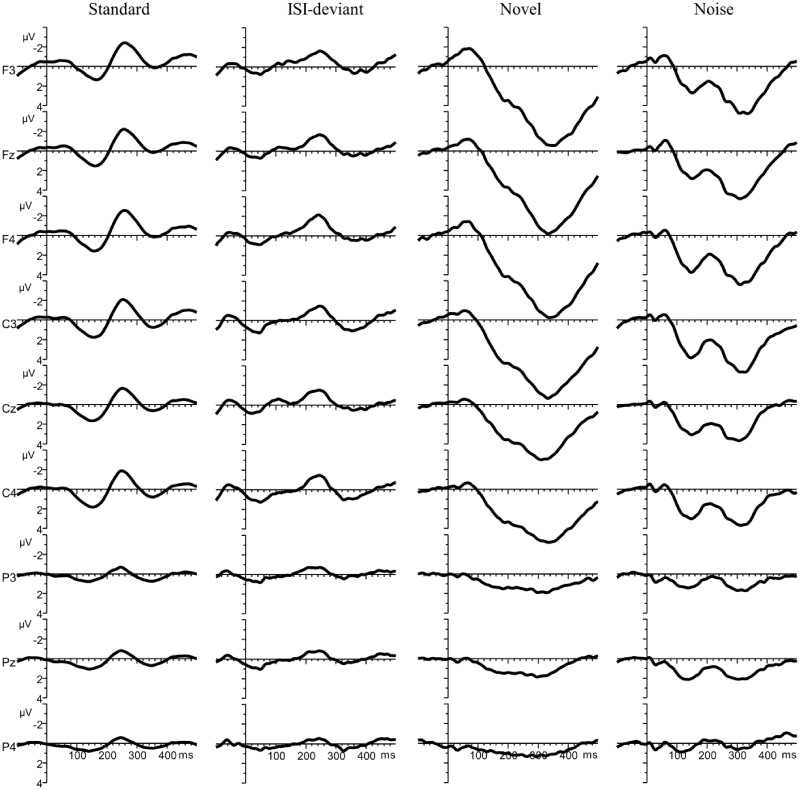
Although the auditory ERP components observed in infants are often not easily comparable with those obtained in adults (de Haan, 2006), the results of a longitudinal study by Kushnerenko et al. (2002) have shown similarities between some of the infantile ERP components and those observed in children and adults. The authors have suggested that the P150 component found in infants may be the precursor of the P100 response observed in children, which then develops into the adult P1 response. Functionally, this component is typically interpreted as an indicator of preferential attention to the auditory input and suppression of unattended information (Key et al., 2005). Kushnerenko et al. (2002) also argued that the infant N250 recorded at 12 months of age could be considered similar to the N250 in children and the adult N2. The adult N2 has been associated with the orienting response and target selection (Key et al., 2005). Finally, development of the infantile large positive component (PC) leads to the emergence of the P3a response in children and adults (Kushnerenko et al., 2002). The P3a has been proposed to reflect stimulus-driven attention switching (Knight, 1996; Escera et al., 2000; Polich, 2007). In 9 month old infants, the ERP response elicited by standard and temporal deviant sounds typically carry both the P150 and the N250; the responses elicited by rare environmental (novel) sounds usually only show a PC response; white noise sounds typically elicit an ERP-response that comprises all three of the above components (Kushnerenko et al., 2013).
ERP-studies in adults have associated individual differences in dispositional mindfulness with differences in information processing (e.g. Brown et al., 2013). Here we examine the possible effects of dispositional mindfulness during pregnancy on neurocognitive functioning in the offspring. Support for the notion that maternal traits/states during pregnancy may affect neurocognitive functioning of the offspring comes from ERP studies examining the effects of prenatal and perinatal exposure to other maternal traits/states, such as prenatal maternal anxiety. These studies consistently associated prenatal (Mennes et al., 2009; Hunter et al., 2012; Van den Bergh et al., 2012) and perinatal exposure (Harvison et al., 2009) to elevated maternal anxiety with altered neurocognitive functioning in the offspring. For example, Mennes and colleagues (2009) found altered ERP patterns in response to an endogenous cognitive control task in 17 year old boys exposed to high maternal anxiety during pregnancy. Because mindfulness and anxiety are negatively correlated (e.g. Brown and Ryan, 2003; Walsh et al., 2009), we hypothesized that prenatal exposure to maternal mindfulness and anxiety would affect the offspring in opposite ways.
METHODS
Participants
The current study was part of the Prenatal Early Life Stress project, an ongoing prospective cohort study, following pregnant women and their offspring from the first trimester of pregnancy onward. All participating parents provided written informed consent. The medical ethical committee of a local hospital approved the study, which was conducted in full compliance with the Helsinki declaration.
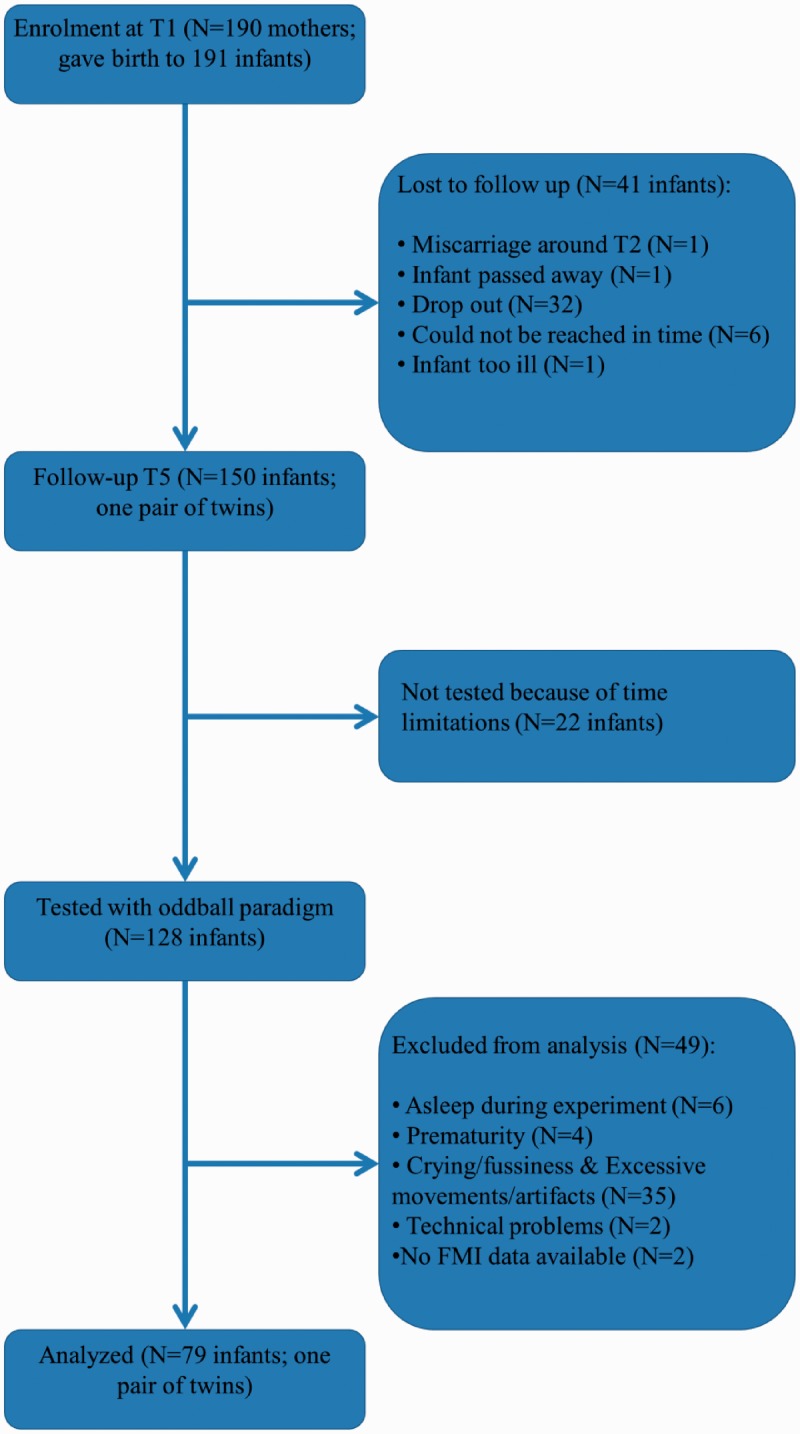
A total of 178 women had been recruited in the 15th week and 12 women between the 16th and 22nd week of pregnancy from a general hospital and four midwife practices. Tests were administered to them three times during pregnancy (T1, T2, T3; once in each trimester) and they were invited with their infant for postnatal observations either at 2? or 4? (T4) and once again at 9 months after birth (T5). Here, we analyzed the data of those mother–infant dyads of which both maternal mindfulness and anxiety data at T2 and ERP data at 9 months were available. From the 128 infants that were brought to the ERP measurement, 2 were excluded due to missing mindfulness and anxiety data, 2 due to technical problems, 35 due to excessive movements/artifacts and or excessive crying/fussiness and 4 infants because of premature birth (i.e. before week 36 of gestation and/or a birth weight <2500 grams). We also excluded six infants who fell asleep during the experiment because previous studies suggested that the state of alertness affects the auditory ERPs (Friederici et al., 2002; Otte et al., 2013). For a full overview of the inclusion and exclusion of mother–infant dyads, see the flowchart in Figure 1.
Fig. 1.
Flowchart of inclusion and exclusion of the participating mothers–infant dyads.
The final sample consisted of 78 mothers and their 79 infants of 9 months (42 girls, one pair of twins). The infants had a mean age of 43.90 weeks (s.d. = 1.84) and a mean gestational age at birth of 39.98 weeks (s.d. = 1.26). The mothers had a mean age of 32.09 years (s.d. = 5.55) at the time of the ERP measurement. All infants were healthy and had passed a screening test for hearing impairment performed by a nurse from the infant health-care clinic between the fourth and seventh day after birth. The screening test was a simple, non-invasive test using otoacoustic emissions for detecting hearing deficits.
Measurements
Table 1 describes the sample of the mothers and their infants including demographic characteristics and scores on the mindfulness and anxiety questionnaires.
Table 1.
Characteristics of the participating mother–infant dyad sample
| Infants (N = 79) | N | % | M (s.d.) |
|---|---|---|---|
| Age at EEG-measurement (weeks) | 79 | 43.9 (1.84) | |
| Sex | |||
| Girl | 42 | 53.2 | |
| Boy | 37 | 46.8 | |
| Birth weight (grams) | 79 | 3495.25 (487.26) | |
| Gestational age at birth (weeks) | 77 | 39.98 (1.26) | |
| Mothers (N = 78) | |||
| Age (years) | 78 | 32.09 (5.55) | |
| FMIs-14 sum at T2 | 78 | 40.66 (6.30) | |
| SCL-90 sum at T2 | 78 | 13.13 (3.39) | |
| SCL-90 sum at T5 | 57a | 13.26 (5.02) | |
| Education | |||
| Primary or secondary | 6 | 7.7 | |
| General vocational training | 22 | 28.2 | |
| Higher vocational training | 34 | 43.6 | |
| University degree or higher | 16 | 20.5 | |
| Primigravida | 29 | 37.2 |
Note: FMIs-14 = Freiburg Mindfulness Inventory short form; SCL-90 = anxiety subscale of the Symptom Checklist; T2 = during second trimester; T5 = ca. 10 months after birth.
aN = 21 mothers included in the study did not complete the postnatal questionnaire.
Mindfulness
Maternal mindfulness was measured using the Dutch short version of the Freiburg Mindfulness Inventory (FMIs-14; Walach et al., 2006). The original FMI is a self-report questionnaire developed in line with the concept of Buddhist psychology, but requires no knowledge of Buddhism or meditation to complete (Walach et al., 2006). The shorter FMIs-14 consists of 14 items with four-point Likert scales ranging from 1 (rarely) to 4 (almost always). Higher aggregate scores indicate higher mindfulness. The FMIs-14 was shown to measure a single dimension and shows good internal consistency (α = 0.86; Walach et al., 2006).
Anxiety
Maternal anxiety was measured using the Dutch version of the anxiety subscale of the Symptom Checklist (SCL-90; Arrindel and Ettema, 1981). This SCL-90 anxiety subscale is a self-report measure of anxiety symptoms, consisting of 10 items with five-point Likert scales ranging from 0 (not at all) to 4 (extremely). Higher aggregate scores indicate higher anxiety. The scale has good convergent and divergent validity and has good internal consistency (α = 0.88 for the anxiety subscale; Arrindell and Ettema, 2003).
ERP paradigm
ERPs were measured in a passive auditory oddball paradigm. The stimulus sequences consisted of four different types of sound events: a frequent standard sound (probability of 0.7) and three types of infrequent deviant events (each with a probability of 0.1). The standard was a complex tone with 500 Hz base frequency presented following an interstimulus interval (ISI; offset-to-onset) of 300 ms. Standard sounds were constructed from the three lowest partials, with the intensity of the second and third partials set 6 and 12 dB lower, respectively, than that of the base harmonic. The deviant sounds were (i) the same tone as the standard but following a shorter ISI of 100 ms (‘ISI-deviant’); (ii) a white noise segment (‘white noise’, 300 ms ISI); and (iii) 150 unique environmental sounds such as a slamming door, a barking dog, etc. (‘novel sound’, 300 ms ISI). All stimuli had durations of 200 ms and were presented at an intensity of 75 dB SPL. In total, 1500 stimuli were delivered. They were divided into five stimulus blocks, each containing 300 stimuli. The stimuli were presented in a semi-random order with the restriction that novel/white noise sounds were always preceded by at least two standard sounds and consecutive ISI-deviants were always separated by at least two sounds with a regular ISI (standard, novel or white noise).
Procedure
Mindfulness and anxiety questionnaires were administered to mothers at the beginning of the second trimester (T2: mean weeks of pregnancy = 20.72 weeks, s.d. = 2.06) in their home. To control for postnatal anxiety, the same anxiety questionnaire was also administered to most mothers (N = 57) ca. 10 months after birth (T5: mean = 44.09 weeks, s.d. = 1.84). The ERP-measurement took place ca. 9 months after birth in a dimly lit and sound-attenuated room at the Developmental Psychology Laboratory of the university. The complete procedure took ∼60 min including electrode placement and removal. During the EEG recording, infants sat on their parents’ lap with two loudspeakers placed at a distance of 80 cm from the infant’s head, one on each side. The whole experimental procedure was recorded with two cameras of which the first one was placed behind and the other facing the infant and the parent. The camera recordings were used to detect episodes when the baby was crying or moving; these episodes were then excluded from the analyses.
ERP measurement and data processing
EEG was recorded with BioSemi ActiveTwo amplifiers (www.biosemi.com) with a sampling rate of 512 Hz, using caps with 64-electrode locations placed according to the extended International 10-20 system. We analyzed data from the following nine electrode sites: F3, Fz, F4, C3, Cz, C4, P3, Pz and P4. The standard BioSemi reference (CMS-DRL) was used (see www.biosemi.com/faq/cms&drl.htm for details) and two additional electrodes were placed on the left and right mastoids, respectively, and mathematically combined off-line to produce an average mastoids reference derivation. All electrophysiological analyses were conducted using the BrainVision Analyzer 2 software package (Brain Products, Munich, Germany). Off-line filter settings consisted of a 50 Hz notch filter and a zero-phase Butterworth bandpass 1.0–30 Hz (slope 24 dB) filter. Subsequently, the data were segmented into epochs of 600 ms duration including a 100 ms pre-stimulus period. Epochs with a voltage change exceeding 150 µV within a sliding window of 200 ms duration or with changes exceeding the speed of 80 μV/ms at any of the nine electrodes were rejected from further analysis. Trials that preceded the ISI deviant were removed from the analysis because late responses to these sounds overlapped the early responses elicited by the ISI deviant. The average numbers of remaining trials included in the analyses of the four stimulus types were as follows: standard, 730; ISI-deviant, 118; white noise, 102; novel, 105. ERPs were averaged separately for the four different stimulus types (standard, ISI-deviant, white noise, novel sound) and baseline-corrected to the average voltage in the 100 ms pre-stimulus period.
Time windows for measuring the various ERP components were selected on the basis of the grand average ERPs measured from the nine electrode locations, separately for the standard and the three oddball stimuli (Figure 2). Mean amplitudes were measured from the following time windows/stimuli: a window from 100 to 200 ms for the standard, the ISI-deviant and the white noise sound to capture the P150-waveform; for the N250, the window was set from 200 to 300 ms for the standard sound and the ISI-deviant and from 150 to 250 ms for the white noise sound in the response to which this component peaked earlier; the window for the PC component was set between 250 and 400 ms for the white noise and novel sounds (the only ones eliciting this component).
Fig. 2.
Group-average (N = 79) ERP responses to standard tones, ISI-deviants, novel sounds and white noise segments (columns) at electrodes F3, Fz, F4, C3, Cz, C4, P3, Pz, P4 (rows).
Statistical analysis
Firstly, using Pearson’s correlation, we checked whether the correlation between mindfulness and anxiety measured at T2 was negative, as was expected on the basis of previous results (e.g. Brown and Ryan, 2003; Walsh et al., 2009). Three series of repeated-measures ANCOVAs were then conducted to test the effects of maternal mindfulness and anxiety on the infant’s ERP amplitudes: one with ‘Mindfulness’, one with prenatal (T2) ‘Anxiety’ and one with postnatal (T5) ‘Anxiety’ as the continuous predictor. The latter was introduced for comparing the effects of pre- and postnatal anxiety on the ERP responses. Instead of dichotomizing the variables, continuous predictors were used because dichotomizing might lead to loss of information, effect size, power, risks missing non-linear effects and may cause problems in comparing and aggregating findings across studies (e.g. MacCallum et al., 2002). In each ANCOVA, two within-subject factors ‘Frontal–Central–Parietal’ × ‘Left–Middle–Right’ were also included for assessing effects of the scalp topography of these components. Separate ANCOVAs were performed per stimulus type (standard, ISI-deviant, white noise and novel sounds) and peak (P150, N250 and PC, where applicable). For significant interactions between the target variables (mindfulness and anxiety) and the ‘Frontal–Central–Parietal’ factor, post hoc tests were conducted by separate ANCOVAs for the frontal (F3, Fz, F4), central (C3, Cz, C4) and parietal arrays of electrodes (P3, Pz, P4). Except for the ANCOVAs with T5 anxiety, gestational age and birth weight of the infants were selected as covariates because previous studies showed effects of gestational age and birth weight on cognitive functioning (e.g. Fellman et al., 2004; Shenkin et al., 2004) and brain development (e.g. Poston, 2012). Postnatal anxiety at T5 was also selected as a covariate to control for possible postnatal effects of anxiety. The covariates were first correlated with the AERP measures using Pearson’s correlation and only added to the ANCOVAs if significant correlations were found. All statistical analyses were performed using IBM SPSS 19.0 for Windows. All significant results are reported together with the partial η2 effect size values; α = 0.05.
RESULTS
Maternal mindfulness and anxiety during pregnancy (T2) were negatively correlated (r = −0.270; P < 0.05). Maternal anxiety measured during pregnancy (T2) and maternal anxiety measured ca. 10 months after birth (T5) were positively correlated (r = 0.308; P < 0.05).
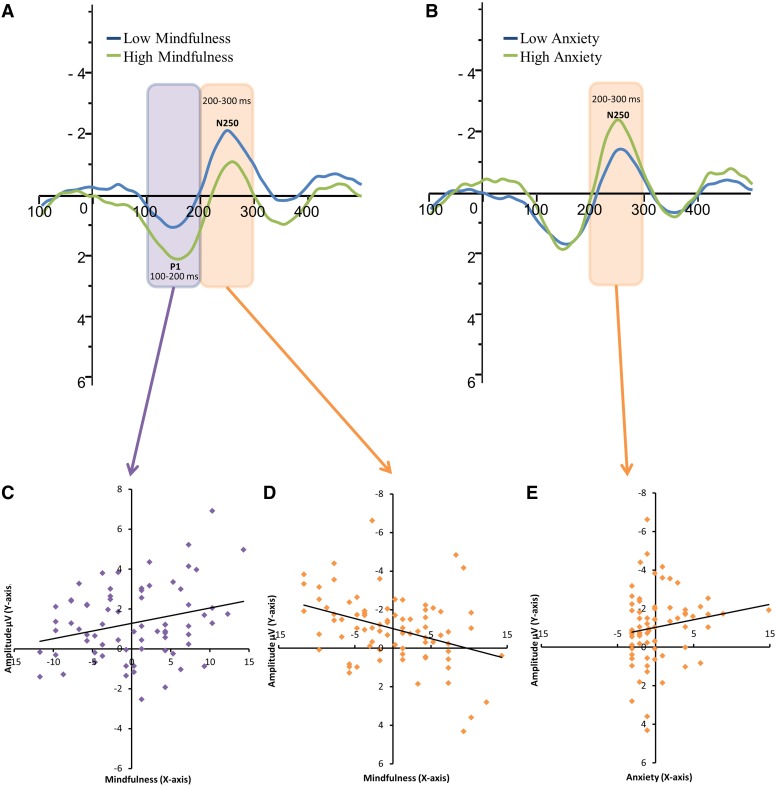
Figure 2 shows that all major components are clearly fronto-centrally distributed. The ERP effects of maternal mindfulness and anxiety at T2 on the infants’ ERP response to the standard sound is illustrated in Figure 3A and B. For illustration purposes only, the infants were divided into low and high maternal mindfulness/anxiety groups and the responses separately averaged for these groups. A mean cutoff was used for the mindfulness measure; the cutoff score (15) for the SCL-90 anxiety subscale was taken from the average for the normal population (Derogatis et al., 1974). Note that in the statistical analyses both of these measures were included as continuous predictors. Because we found no significant association (P > 0.05) between the P150 and N250 amplitudes and the covariates (i.e. gestational age, birth weight and maternal anxiety at T5), no covariates were entered for the analysis of the results reported below.
Fig. 3.
Group-average (N = 79) central (Cz) ERP response to the standard sound of infants of mothers with low (blue line) and high (green line) mindfulness (A) and anxiety (B). The scatterplots shows the correlation between maternal mindfulness and the amplitude of (C) the first positive-going wave ‘P150’ (measured from the 100–200 ms post-stimulus interval) and (D) the first negative-going wave ‘N250’ (200–320 ms). Panel (E) shows the scatterplot for the correlation between maternal anxiety and the amplitude of the ‘N250’ component. Notes: The statistical analyses were performed with mindfulness and anxiety as continuous predictors (C, D and E). Panels A and B are for illustration purposes only.
For the ERPs elicited by standard sounds a significant positive association was obtained between maternal mindfulness and the P150 amplitude [F(1,77) = 10.476, P = 0.002, η2 = 0.120; Figure 3C] and a significant negative association between maternal mindfulness and the N250 amplitude [F(1,77) = 8.504, P = 0.005, η2 = 0.099; Figure 3D]. For the N250 amplitude, also a significant interaction between mindfulness and the ‘Frontal–Central–Parietal’ factor was found [F(2,77) = 14.743, P = 0.009, η2 = 0.066]. Follow-up tests showed that the effect of mindfulness was significant at frontal and central scalp locations [F(1,77) = 8.515, P = 0.005, η2 = 0.100, and F(1,77) = 8.841, P = 0.004, η2 = 0.103, respectively], but not at parietal sites. For the rare deviant stimuli (i.e. ISI-deviant, white noise and novel sound), no significant associations were found between maternal mindfulness and any of the ERP amplitudes.
Higher prenatal (T2) maternal anxiety was significantly associated with larger N250 amplitudes for the standard sound [F(1,77) = 8.177, P = 0.005, η2 = 0.096; Figure 3E]1. Scalp distribution factors did not yield significant main effects or interactions with other factors. For the rare deviant stimuli (i.e. ISI-deviant, white noise and novel), no significant associations were found between maternal anxiety and any of the ERP amplitudes. Finally, postnatal (T5) maternal anxiety was not significantly associated with the measured ERP amplitudes.
DISCUSSION
The aim of the present study was to examine the effects of the mother’s mindfulness and anxiety during pregnancy on neurocognitive functioning in the offspring. We found significant opposite effects of maternal mindfulness and anxiety during pregnancy on how infants processed repetitive sounds. In contrast, none of the ERPs elicited by rare auditory events were significantly affected by the independent variables. Although effects of maternal anxiety during pregnancy on offspring neurocognitive functioning have already been previously reported (Mennes et al., 2009; Buss et al., 2010; Van den Bergh et al., 2012), our results show that maternal mindfulness may also affect neurocognitive functioning in the offspring.
Associations between maternal mindfulness and anxiety during pregnancy and their effects on the infants’ auditory ERP responses
As was expected from results of previous studies (Brown and Ryan, 2003; Walsh et al., 2009), a negative correlation was observed between maternal mindfulness and anxiety in the current group of pregnant women and, in line with our hypothesis, we found opposite effects of maternal mindfulness and anxiety on the ERPs elicited by the frequent standard sounds: higher maternal mindfulness was associated with lower N250 amplitudes, whereas higher maternal anxiety was associated with higher N250 amplitudes. Higher maternal mindfulness was also associated with higher P150 amplitudes. P1, the putative adult analogue of P150 is regarded as reflecting early pre-attentive processes extracting sound features (e.g. Näätänen and Winkler, 1999; Picton, 2010). Thus, higher P150 amplitudes may reflect more thorough/elaborate feature extraction in babies of mothers with higher mindfulness scores. In adults, the various subcomponents of N2 (N2a, b and c—Pritchard et al., 1991), the putative analogue of the infantile N250, have been associated with various cognitive processes (e.g. selective attention, stimulus identification) evaluating the incoming sound. The lower N250 amplitudes found for infants exposed to higher levels of maternal mindfulness during pregnancy might be a consequence of these infants having formed more accurate pre-attentive representations (i.e. higher P1) and, therefore, not needing to process the repetitious standard sound more elaborately. In sum, infants prenatally exposed to higher levels of maternal mindfulness may devote less processing to the uninformative repetitious sounds, whereas infants prenatally exposed to higher levels of maternal anxiety may process such sounds more extensively. These results suggest that prenatal exposure to maternal mindfulness and anxiety affects neurocognitive functioning in the offspring in at least partly opposite ways.
Significant effects were only observed for the frequent standard sound but not for the rare deviant sounds. This suggests that the infants’ deviance detection mechanism was not affected by exposure to maternal mindfulness (or anxiety) during pregnancy. The fact that both mindfulness and anxiety only exerted influence on the responses elicited by the standard sound could mean that these maternal states/traits affect processes involving habituation. Neural habituation has been defined as a process by which the neural response decreases over time during repeated stimulation (Thompson and Spencer, 1966). This is more likely to take place for the high-probability standard sound than for the low-probability deviant sounds. Because extensive and continuous processing of the oft-repeated standard is largely unnecessary, habituation to this stimulus could be considered as adaptive. Building on this line of reasoning, the lower amplitude of the N250 for infants prenatally exposed to higher mindfulness could indicate stronger habituation processes in these infants, which might be a sign of more adaptive brain functioning. In contrast, the fact that higher N250 amplitude was associated with higher maternal anxiety could be interpreted as reflecting weaker habituation processes in these infants, possibly indicating less adaptive brain functioning. The latter suggestions is compatible with the results of Turner et al. (2005), who reported that children of anxious parents were less likely to habituate to fear-relevant auditory and visual stimuli. More research is necessary, however, to test whether infants prenatally exposed to higher levels of maternal mindfulness possess stronger habituation processes.
The non-significant correlations for the ERP responses elicited by the deviant sounds may be possibly attributed to relatively higher noise levels in the deviant-sound responses due to the lower average number of trials for the deviant sounds compared with that for the standard sounds. However, at least the novel sounds and the white noise segment used in our study have been shown to elicit ERP responses of higher amplitudes than the standard sound. Therefore, the signal-to-noise ratio for equal numbers of trials is better for these sounds than for the tone stimuli, such as the standard (Kushnerenko et al., 2007), and thus, fewer number of trials are needed to achieve the same S/N ratio. Further, the average trial numbers for all three deviants was relatively high and the study includes a large group of infants compared with most studies recording auditory ERPs in infants. Taken these together, the lack of significant correlations between maternal mindfulness/anxiety during pregnancy and the ERP responses elicited by the deviant sounds is likely a robust finding of this study. Future research could focus on exploring the mechanisms selectively affecting the processing of repetitive sounds but leaving the deviance detection mechanism intact.
Possible mechanisms
The fact that mindfulness and anxiety exert influence on the same type of information processing might point to shared underlying mechanisms. However, the mechanisms by which the psychological traits/states of the mother ‘program’ the infants’ brain during pregnancy are not yet known. One often proposed mechanism is the exposure to excessive levels of cortisol, commonly known as the ‘stress hormone’. The human fetus is relatively protected against direct exposure to high cortisol concentrations, as in the placenta 50–90% of maternal cortisol is converted to biologically inactive cortisone by the enzyme 11β-hydroxysteroid-dehydrogenase (11β-HSD-2) (Mulder et al., 2002). However, high maternal anxiety in pregnancy may lead to downregulation of placental 11β-HSD-2 (O'Donnell et al., 2012). Although cortisol is crucial for fetal tissue proliferation and differentiation, exposure to excessive levels of this hormone due to maternal stress can have negative consequences for birth outcome and development, such as e.g. increased risk for premature birth (Sandman et al., 2006) and impaired cognitive performance (Davis and Sandman, 2010). Because dispositional mindfulness has been linked to reduced cortisol levels (Brown et al., 2012) and anxiety is associated with enhanced cortisol levels (Pluess et al., 2010), the suggestion of cortisol secretion as one of the underlying mechanisms appears to be reasonable. Being mindful during pregnancy could make the mother less vulnerable to stressful events. Indeed mindfulness has been linked to psychological health (Keng et al., 2011), better work–family balance (Allen and Kiburz, 2012) and better emotion regulation (Goodall et al., 2012). In sum, mindfulness could prevent the negative consequences of prenatal exposure to stress on the fetus, whereas anxiety during pregnancy might enhance vulnerability to stress in the fetus.
Although ERPs were measured early in development, postnatal influences could have also affected the neurocognitive functioning of the infants. Maternal anxiety and mindfulness during pregnancy may be related to attachment, parenting styles and ways of taking care of the infant. These factors may, therefore, be partly responsible for the observed association between maternal prenatal anxiety/mindfulness and some of the ERP responses. On the other hand, we have found no significant association between the mothers’ postnatal anxiety at 9 months after birth and the P150 and N250 amplitudes. Thus, the effects found cannot be attributed to the influences of anxiety on the mother–child interaction between birth and 9 months of age. Unfortunately the mothers’ postnatal mindfulness was not measured. More mindful mothers could have, for instance, (also) affected their infants’ brain through more mindful parenting or less postnatal stress. Possible genetic factors cannot be disregarded either; mindful mothers might give birth to babies more disposed toward mindfulness. Nevertheless, human studies evaluating the consequences of stressful life events on development, such as natural disasters (Laplante et al., 2004), suggest that the effects of prenatal stress cannot be explained by genetic predispositions alone. To isolate the effects of prenatal maternal mindfulness from possible postnatal effects and to eliminate genetic influences, future randomized controlled trials are needed with mindfulness interventions for pregnant women.
Clinical applications
The current results could help to suggest directions to nurses, midwives, general practitioners and gynecologists in providing pregnant women with information about potentially positive prenatal programming effects and in this way contribute to healthier pregnancy. Highly anxious mothers may feel guilty in response to the message that being anxious during pregnancy negatively affects their baby and might in turn experience increased anxiety levels instead of decreased levels. By stressing the potential of mindfulness instead, a positive message can be provided to them. Further, the current results emphasize the possible strengths of a mindfulness intervention for pregnant women. Such an intervention for pregnant women suffering from anxiety may be a desirable alternative to pharmacological interventions, as several studies have described the detrimental effects of psychopharmacological treatment during pregnancy on the fetus (e.g. Mulder et al., 2011). However, more research into the effects of maternal mindfulness during pregnancy is necessary before firm conclusions about the potential benefits for the infants’ neurocognitive functioning can be drawn.
CONCLUSION
The results of the current study indicate that (i) infants’ processing of the standard but not the deviant sounds (ISI-deviant, white noise, novel) is affected by prenatal exposure to maternal mindfulness and anxiety and (ii) mindfulness and anxiety exert, at least partly, opposite effects on the infant’s ERP responses to repetitive sounds. We suggest that infants prenatally exposed to higher levels of maternal mindfulness devote less in-depth processing to frequently occurring irrelevant stimuli and/or they habituate faster to these stimuli. In contrast, infants prenatally exposed to higher levels of maternal anxiety process such uninformative sounds more extensively and/or they habituate slower to these stimuli. This difference might stem from infants prenatally exposed to higher maternal mindfulness pre-attentively forming more accurate perceptual representations. The current study contributes to the field of prenatal programming by showing that negative traits and experiences of the mother during pregnancy are not the only ones to have an effect on the child, as is often emphasized in the literature. Positive traits of the mother during pregnancy may also ‘program’ the infant.
Conflict of Interest
None declared.
Acknowledgments
The authors are grateful to the parents and infants for their participation in our study and to the students who helped with the data collection. The study described is part of the Prenatal Early Life Stress (PELS) project, one of four collaborative project of ‘EuroSTRESS’, a EUROCORES programme focused on stress and mental health and launched by European Science Foundation (ESF) in 2008. B.V.d.B. is project leader of PELS, and principal investigator (PI) for the Netherlands. We thank Vivette Glover (Imperial College London; PI for UK), Stephan Claes (KU Leuven; PI for Belgium) and Alina Rodriguez, associated partner (Uppsala University Sweden) for the collaboration in the PELS project. Research funding was generated from the Brain and Cognition Programme of the Dutch Organization for Scientific Research (NWO), through European Science Foundation. B.V.d.B. is supported by European Commission Seventh Framework Programme (FP7–HEALTH.2011.2.2.2-2 BRAINAGE, Grant agreement no: 279281). I.W. is supported by the Hungarian Scientific Research Fund (OTKA K101060) grant.
Footnotes
1 The association remained significant [F(1,76) = 6.319, P = 0.014, η2 = 0.077] even after removing the extreme case (SCL-score = 28; see Figure 3E) from the analysis.
References
- Allen TD, Kiburz KM. Trait mindfulness and work–family balance among working parents: the mediating effects of vitality and sleep quality. Journal of Vocational Behavior. 2012;80(2):372–79. [Google Scholar]
- Arrindel WA, Ettema JHM. Dimensionele Structuur, Betrouwbaarheid en Validiteit van de Nederlandse bewerking van de Symptom Checklist (SCL-90) [Dimensional Structure, Reliability and Validity of the Dutch Version of the Symptom Checklist (SCL-90)] Lisse: Swets & Zeitlinger; 1981. [Google Scholar]
- Arrindell WA, Ettema JHM. SCL-90. Symptom Checklist. Handleiding bij een Multidimensionele Psychopathologie-Indicator [Symptom Checklist. Manual of a Multidimensional Psychopathology-Indicator] Lisse: Swets Test Publishers; 2003. [Google Scholar]
- Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23(6 Suppl):588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppänen PH. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Developmental Psychobiology. 2002;40(3):278–92. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Goodman RJ, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Social Cognitive and Affective Neuroscience. 2013;8(1):93–9. doi: 10.1093/scan/nss004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84(4):822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown KW, Weinstein N, Creswell JD. Trait mindfulness modulates neuroendocrine and affective responses to social evaluative threat. Psychoneuroendocrinology. 2012;37(12):2037–41. doi: 10.1016/j.psyneuen.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35(1):141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81(1):131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M. Infant EEG and Event-Related Potentials. Hove, England: Psychology Press; 2006. [Google Scholar]
- Dunn C, Hanieh E, Roberts R, Powrie R. Mindful pregnancy and childbirth: effects of a mindfulness-based intervention on women's psychological distress and well-being in the perinatal period. Archives of Women's Mental Health. 2012;15(2):139–43. doi: 10.1007/s00737-012-0264-4. [DOI] [PubMed] [Google Scholar]
- Escera C, Alho K, Schroger E, Winkler I. Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiology and Neuro-otology. 2000;5(3-4):151–66. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Näätänen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatric Research. 2004;56(2):291–7. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Friedrich M, Weber C. Neural manifestation of cognitive and precognitive mismatch detection in early infancy. NeuroReport. 2002;13(10):1251–4. doi: 10.1097/00001756-200207190-00006. [DOI] [PubMed] [Google Scholar]
- Goodall K, Trejnowska A, Darling S. The relationship between dispositional mindfulness, attachment security and emotion regulation. Personality and Individual Differences. 2012;52(5):622–6. [Google Scholar]
- Guardino CM, Dunkel Schetter C, Bower JE, Lu MC, Smalley SL. Randomized controlled pilot trial of mindfulness training for stress reduction during pregnancy. Psychology and Health. 2014;29(3):334–49. doi: 10.1080/08870446.2013.852670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvison KW, Molfese DL, Woodruff-Borden J, Weigel RA. Neonatal auditory evoked responses are related to perinatal maternal anxiety. Brain and Cognition. 2009;71(3):369–74. doi: 10.1016/j.bandc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Mendoza JH, D'Anna K, Zerbe GO, McCarthy L, Hoffman C, Freedman R, Ross RG. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. The American Journal of Psychiatry. 2012;169(6):616–24. doi: 10.1176/appi.ajp.2012.11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review. 2011;31(6):1041–56. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Developmental Neuropsychology. 2005;27(2):183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383(6597):256–9. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kohls N, Sauer S, Walach H. Facets of mindfulness – Results of an online study investigating the Freiburg mindfulness inventory. Personality and Individual Differences. 2009;46(2):224–30. [Google Scholar]
- Kushnerenko E, Čeponienė R, Balan P, Fellman V, Huotilaine M, Näätäne R. Maturation of the auditory event-related potentials during the first year of life. NeuroReport. 2002;13(1):47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Winkler I, Horváth J, Näätänen R, Pavlov I, Fellman V, Huotilainen M. Processing acoustic change and novelty in newborn infants. European Journal of Neuroscience. 2007;26(1):265–74. doi: 10.1111/j.1460-9568.2007.05628.x. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Van den Bergh BRH, Winkler I. Separating acoustic deviance from novelty during the first year of life: a review of event related potential evidence. Frontiers in Psychology. 2013;4:595. doi: 10.3389/fpsyg.2013.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56(3):400–10. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Mennes M, Van den Bergh B, Lagae L, Stiers P. Developmental brain alterations in 17 year old boys are related to antenatal maternal anxiety. Clin Neurophysiol. 2009;120(6):1116–22. doi: 10.1016/j.clinph.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72(3):238–45. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Ververs FF, de Heus R, Visser GH. Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology. 2011;36(10):1961–71. doi: 10.1038/npp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJH, Robles de Medina PG, Huizink AC, Van den Bergh BRH, Buitelaar JK, Visser GHA. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Human Development. 2002;70(1–2):3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychological Bulletin. 1999;125(6):826–59. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37(6):818–26. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Otte RA, Winkler I, Braeken MA, Stekelenburg JJ, van der Stelt O, Van den Bergh BRH. Detecting violations of temporal regularities in waking and sleeping two-month-old infants. Biological Psychology. 2013;92(2):315–22. doi: 10.1016/j.biopsycho.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Picton TW. Human Auditory Evoked Potentials. San Diego: Plural Publishing; 2010. [Google Scholar]
- Pluess M, Bolten M, Pirke KM, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biological Psychology. 2010;83(3):169–75. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Practice and Research Clinical Endocrinology and Metabolism. 2012;26(5):627–39. doi: 10.1016/j.beem.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Shappell SA, Brandt ME. Psychophysiology of N200/N400: a review and classification scheme. In: Jennings JR, Ackles PK, editors. Advances in Psychophysiology: A Research Annual. London: Jessica Kingsley; 1991. [Google Scholar]
- Räikkönen K, Seckl JR, Pesonen AK, Simons A, Van den Bergh BRH. Stress, glucocorticoids and liquorice in human pregnancy: programmers of the offspring brain. Stress. 2011;14(6):590–603. doi: 10.3109/10253890.2011.602147. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychological Bulletin. 2004;130(6):989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- Sriboonpimsuay W, Promthet S, Thinkhamrop J, Krisanaprakornkit T. Meditation for preterm birth prevention: a randomized controlled trial in Udonthani, Thailand. International Journal of Public Health Research. 2011;1(1):31–9. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Roberson-Nay R. Offspring of anxious parents: reactivity, habituation, and anxiety-proneness. Behaviour Research and Therapy. 2005;43(10):1263–79. doi: 10.1016/j.brat.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Developmental Medicine and Child Neurology. 2011;4:19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews. 2005;29(2):237–58. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Otte RA, Braeken MAKA, van den Heuvel MI, Winkler I. Prenatal exposure to maternal anxiety is associated with sensory-cognitive development in 2-month-olds (abstract) Developmental Medicine and Child Neurology. 2012;54(Suppl 2):22. [Google Scholar]
- Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Archives of Women’s Mental Health. 2008;11(1):67–74. doi: 10.1007/s00737-008-0214-3. [DOI] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmuller V, Kleinknecht N, Schmidt S. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI) Personality and Individual Differences. 2006;40(8):1543–55. [Google Scholar]
- Walsh JJ, Balint MG, Smolira Sj DR, Fredericksen LK, Madsen S. Predicting individual differences in mindfulness: the role of trait anxiety, attachment anxiety and attentional control. Personality and Individual Differences. 2009;46(2):94–9. [Google Scholar]