Abstract
The hippocampus—crucial for memory formation, recall and mood regulation—is involved in the pathophysiology of dementia and depressive disorders. Recent genome-wide association studies (GWAS) have identified five genetic loci associated with hippocampal volume (HV). Previous studies have described psychosocial and clinical factors (for example, smoking, type 2 diabetes and hypertension) to have an impact on HV. However, the interplay between genetic, psychosocial and clinical factors on the HV remains unclear. Still, it is likely that genetic variants and clinical or psychosocial factors jointly act in modifying HV; it might be possible they even interact. Knowledge of these factors might help to quantify ones individual risk of or rather resilience against HV loss. We investigated subjects (N=2463; 55.7% women; mean age 53 years) from the Study of Health in Pomerania (SHIP-2; SHIP-TREND-0) who underwent whole-body magnetic resonance imaging (MRI) and genotyping. HVs were estimated with FreeSurfer. For optimal nonlinear model fitting, we used regression analyses with restricted cubic splines. Genetic variants and associated psychosocial or clinical factors were jointly assessed for potential two-way interactions. We observed associations between HV and gender (P<0.0001), age (P<0.0001), body height (P<0.0001), education (P=0.0053), smoking (P=0.0058), diastolic blood pressure (P=0.0211), rs7294919 (P=0.0065), rs17178006 (P=0.0002), rs6581612 (P=0.0036), rs6741949 (P=0.0112) and rs7852872 (P=0.0451). In addition, we found three significant interactions: between rs7294919 and smoking (P=0.0473), rs7294919 and diastolic blood pressure (P=0.0447) and between rs7852872 and rs6581612 (P=0.0114). We suggest that these factors might have a role in the individual susceptibility to hippocampus-associated disorders.
Introduction
The hippocampus—a core region in the limbic system—represents an important brain structure for memory1 and stress regulation.2 Decreased hippocampal volume (HV) is associated with executive dysfunction.3,4 In addition, HV and hippocampus-dependent memory measures (such as short-delay retention, long-delay retention and discriminability) are positively correlated.5 Patients with hippocampal degradation seem to have negative clinical outcome in depression.3,6 Cumulative factors lead to differences in the HV with increasing age. Therefore, it seems to be important to investigate factors influencing HV changes.
A broad range of clinical and psychosocial determinants influencing the HV and its function have been identified. Depression,7,8 alcohol consumption9, 10, 11 and smoking have been associated with decreased HVs.12 In addition, cardiometabolic factors have been associated with decreased HV: abdominal obesity,13,14 hypertension,15,16 type 2 diabetes17, 18, 19 and elevated total cholesterol levels.12 In contrast, exercise intervention increased the HV and subsequently improved memory function.20,21 High education buffered the age-related decrease in the HV.22
In twin studies, the heritability of HV has been estimated at 40–64%.23,24 Two genome-wide association studies (GWAS) have been performed to search for genetic markers related to HV.25,26 We have contributed with our samples (SHIP-2, SHIP-TREND-0) to these analyses, leading to the identification of the intergenic variant rs7294919,26 replicated by Bis et al.25 Rs7294919 is located on 12q24.22, a chromosomal region comprising the genes TESC, HRK and FBXW8. The biological mechanism how rs7294919 influences on HV is currently unknown. However, all three neighboring genes could be involved. TESC has an important role in cell proliferation and differentiation for HV and brain development.26,27 Harakiri (HRK) is a regulator of apoptosis, which exhibits death-inducing activity in cells28,29 FBXW8 encodes a member of the F-box protein family, which modifies neuronal dendrite growth and elaboration.30 Furthermore, Bis et al.25 identified promising loci at 12q14 (WIF1 and MSRB3), 2q24 (within DPP4, dipeptidylpeptidase) and 9p33 (within ASTN2).
Those markers may be of relevance to the physiology of the hippocampus: Wingless-related integration site (WNT) inhibitory factor 1 is a protein that inhibits WNT proteins, which have an important role in the embryonic development, in the regulation of morphogenesis in the central nervous system and which can affect neuronal functions in adulthood.31,32 MSRB3 encodes proteins that catalyze the reduction of methionine sulfoxide to methionine, thus preserving the biological activity of proteins after oxidative damage.33,34 DPP4 is a protein encoded by the DPP4 gene. DPP4 inhibitors, as a new antidiabetic treatment approach, inhibit the degradation of Glucagon-like Peptide 1. The DPP-4 inhibitor moderated the impaired neuronal insulin receptor function and brain mitochondrial function caused by high-fat diet and showed improved cognitive behavior in an animal model.35 Astrotactin2 (ASTN2) is expressed in the brain among others in the dendate gyrus of the hippocampus and, together with ASTN1, involved in neuronal migration.36
However, the interplay between genetic, psychosocial and clinical factors on the HV remains poorly understood. These factors might jointly act or interact in modifying HV. Knowledge of these factors and their interactions might help to quantify ones individual risk of or rather resilience against hippocampus-related diseases (for example, dementia) with HV loss and hippocampal loss function. It might improve our understanding of structural changes from this vulnerable brain tissue. We included the above mentioned, well-described and highly epidemiological relevant clinical and psychosocial factors to affect HV in our study.
The aim of our study was to explore direct associations of genetic candidate markers and potential psychosocial and clinical factors with HV in the general adult population. Moreover, we investigated putative gene–gene and gene–environment interactions in relation to HVs.
Our aims were threefold:
to explore clinical and psychosocial determinants of HV in large adult population-based samples (SHIP-2 and SHIP-TREND-0);
to test associations between single-nucleotide polymorphisms (SNPs; known from GWAS 25,26 ) and HV;
to examine two-way interactions between those SNPs and psychosocial and clinical factors and interactions between these SNPs.
Materials and methods
Participants
Data from the Study of Health in Pomerania (SHIP-2 and SHIP-TREND-0) were used.37, 38, 39 SHIP-0 comprised adult German residents in northeastern Germany living in three cities and 29 communities, with a total population of 212 157. A two-stage stratified cluster sample (aged 20–79 years at baseline) was randomly drawn from local registries. In all, 4308 Caucasian subjects participated at baseline SHIP-0 (1997–2001). The first follow-up was conducted 5 years later (SHIP-1; N=3300 subjects). The second follow-up examination (SHIP-2; N=2333) was carried out about 10 years after baseline. In 2007, the LEGEND study was started (evaluation of lifetime diagnosis of depression in the SHIP-2 sample).37 Concurrent with SHIP-2, a new independent sample in the same area was drawn in 2008 and similar examinations were undertaken (SHIP-TREND-0; N=4420).
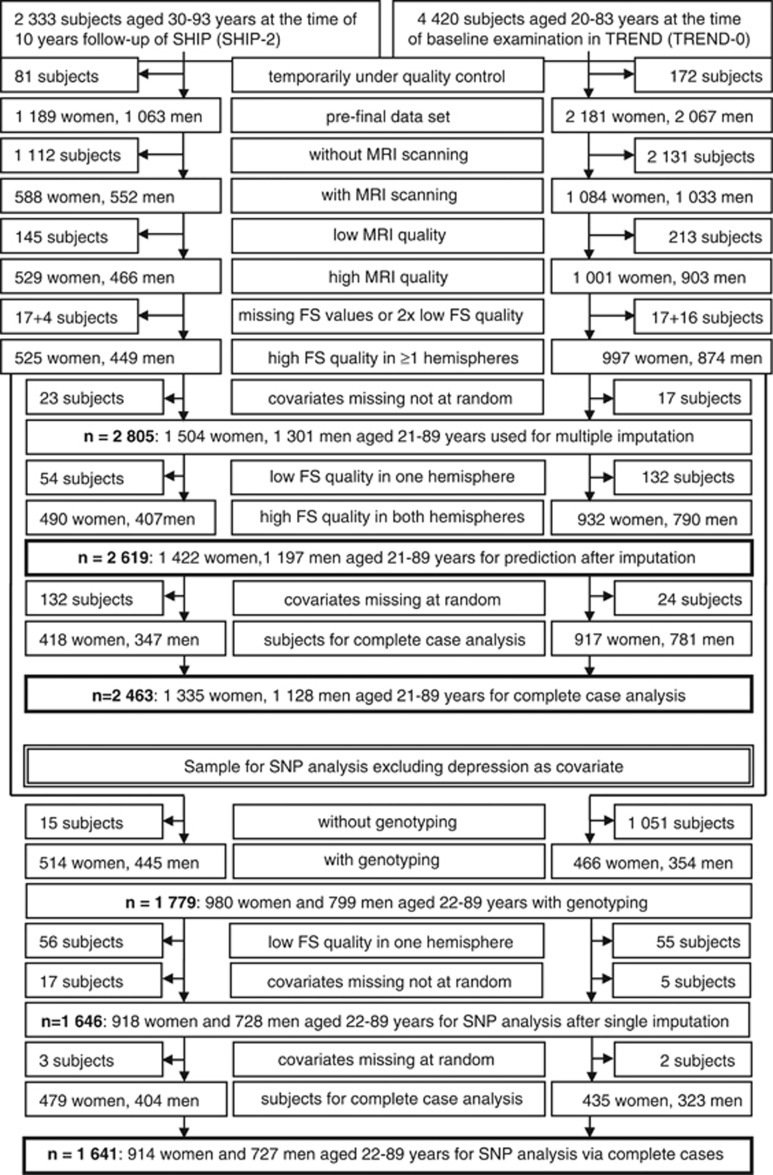
For inclusion and exclusion criteria see flow chart (Figure 1).
Figure 1.
Flow chart: inclusion and exclusion criteria for participants from the Study of Health in Pomerania (SHIP-2 and SHIP-TREND-0).
Magnetic resonance imaging (MRI) scanning and segmentation of the hippocampus
All images were obtained from the same scanner (1.5 Tesla Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). We used the multiplanar reconstruction T1-weighted axial MRI sequence with the following parameters: 1900 ms repetition time, 3.4 ms echo time, flip angle=15° and a voxel size of 1.0 × 1.0 × 1.0 mm. After file format conversion from DICOM to NIfTI, the whole preprocessing of the T1-weighted images and segmentation of the hippocampus was carried out with FreeSurfer v5.1.0 (Cambridge, MA, USA).
Interview (psychosocial factors) and clinical examination
Sociodemographic factors and medical history were assessed by a computer-assisted face-to-face- interview. Smoking was defined as current smoking (occasional; 1–14 cigarette (s) per day; ⩾15 cigarettes per day), former smoking (occasional; 1–14 cigarette (s) per day; ⩾15 cigarettes per day) and never smoking. The highest level of education was assessed by interview and was divided into three groups of school education: 8 years, 10 years and 12 years. Leisure time physical activity (weekly sportive activity or exercise, for example, jogging, biking and swimming) was assessed by interview in three categories: no activity (neither in summer nor in winter), high activity (>2 h per week as well in summer and winter) and moderate activity (residual category). Alcohol consumption was calculated from the interview in g per day as a continuous variable. Having completed the interview, participants underwent medical examinations: including the measurement of height and weight to calculate the body mass index (continuous variable). Waist circumference was measured in cm (continuous variable). After a 5-min resting period, blood pressure was measured three times on the right arm of seated subjects using a digital blood pressure monitor (HEM-705CP, Omron, Tokyo, Japan), with each reading being followed by a further resting period of 3 min. Cuffs were applied according to the circumference of the participant's arm. The mean of the second and third measurements (mm Hg) was used for the analyses (continuous variables). All subjects were informed to bring in their packing containers of all medication they had taken during the last 7 days, as well as their drug prescription sheets. Every compound was recorded and categorized according to the Anatomical Therapeutic Chemical classification.40 We focused on antidepressants (ATC-code N06A*), antihypertensives (C02*, C03*, C07*, C08* and C09*), antidiabetic drugs (A10*) and lipid-lowering drugs (C10*).
Assessment of depression
The lifetime diagnosis of depressive disorders was assessed with the Munich-Composite International Diagnostic Interview (M-CIDI41) in SHIP-LEGEND37 and in SHIP-TREND-0. The M-CIDI is a standardized, fully structured instrument for assessing psychiatric disorders over the lifespan according to DSM-IV criteria.
Genotyping
Serum, EDTA, citrate plasma and DNA are stored at −80 °C in a biobank. In SHIP-0, 4081 individuals were successfully genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix, Santa Clara, CA, USA). The overall genotyping efficiency of the GWA was 98.6%. Imputation of genotypes in the SHIP cohort was performed with the software IMPUTE v0.5.0 (Oxford, UK) against the HapMap II (CEU v22, Build 36; International HapMap Consortium) reference panel using 869 224 genotyped SNPs. The total number of SNPs after imputation and quality control was 2 748 910.
Genotyping of the SHIP-TREND-0 subjects (N=986) was performed using the Illumina HumanOmni2.5-Quad (San Diego, CA, USA). DNA from whole blood was prepared using the Gentra Puregene Blood Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Subsequent sample processing and array hybridization were performed as described by the manufacturer (Illumina) at the Helmholtz Zentrum München. The final sample call rate was 99.51%. Imputation of genotypes in the SHIP-TREND-0 cohort was performed with the software IMPUTE v2.1.2.3 against the HapMap II (CEU v22, Build 36) reference panel. The total number of SNPs after imputation and quality control was 3 437 411. Allele frequencies were all in Hardy–Weinberg equilibrium P>0.2 and imputation quality was P>0.90, except for rs17178006 (Supplementary eTable 1).
Purity and concentration of DNA was determined using a NanoDrop ND-1000 UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA, USA). The integrity of all DNA preparations was validated with electrophoresis using 0.8% agarose-1x TBE gels stained with ethidium bromide. Genotypes in SHIP-0 were determined using the Birdseed2 clustering algorithm. Genotypes in SHIP-TREND-0 were called with the GenCall algorithm of GenomeStudio Genotyping Module v1.0 (Illumina). Arrays with a call rate below 94%, duplicate samples as identified by estimated IBD as well as individuals with reported and genotyped gender mismatch were excluded. The genetic data analysis workflow was created using the Software InforSense (London, UK). Genetic data were stored using the database Caché (InterSystems, Cambridge, MA, USA). Hardy–Weinberg equilibrium P-values are: rs7294919: P=0.913; rs17178006: P=0.844; rs6581612: P=0.658; rs6741949: P=0.215 and rs7852872: P=0.4233.
Laboratory work
Glycated hemoglobin (HbA1c, measured in %) concentrations were determined with high-performance liquid chromatography (Bio-Rad Diamat, Munich, Germany). Skilled technical personnel performed all assays according to the manufacturer's recommendations. In addition, the laboratory participates in official quarterly German external proficiency testing programs. HbA1c, glucose, triglycerides, total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were meassured as dimensional scores. Triglyceride and HDL cholesterol concentrations were measured photometrically (Hitachi 704, Roche, Mannheim, Germany), whereas follow-up HDL concentrations were quantified with lipid electrophoresis (HELENA SAS-3 system, Helena 7 BioSciences Europe, Tyne & Wear, UK). Triglyceride and glucose concentrations were determined enzymatically using reagents from Roche Diagnostics (Hitachi 717, Roche Diagnostics).
To ensure comparability in the longitudinal HDL analyses, we used baseline HDL concentrations as the reference and calculated corrected follow-up HDL concentrations based on a previously published conversion formula (HDL_fu_corr=−80+(1.158*HDL_fu).42 Doing so, we found that the average HDL concentrations produced by the two methods were virtually identical, suggesting that the differences in HDL will be small within the range of practical relevance. Serum LDL was measured by applying a precipitation procedure using dextran sulphate (Immuno, Heidelberg, Germany) on an Epos 5060 (Eppendorf, Hamburg, Germany).
Statistical methods
As recommended,43 we applied advanced statistical methods implemented in the 'rms: Regression Modeling Strategies' R package44 (including redundancy analysis with the 'redun' procedure, cluster analysis and Spearman correlations). Intracranial volume (ICV) was not considered to be a confounding factor because two of the three criteria for a confounding factor do not meet.45 First, the ICV includes the HV and is a consequence rather than a source of HV. Second, if an exposure affects the ICV, then the ICV is an intermediate step in the causal path between the exposure and the HV and, therefore, to be excluded from analysis.
Because of redundancy, systolic blood pressure, total cholesterol, triglycerides, glucose and body mass index were removed from further analysis. To avoid power loss and unjustified linearity assumptions, continuous variables were modeled as restricted cubic splines.43
To test interactions between genetic and psychosocial or clinical factors, we chose an approach in two steps. In the first step we identified statistically significant psychosocial or clinical factors in the sample with 2463 subjects. A statistically significant psychosocial or clinical factor had to meet two conditions: (1) significance (P⩽0.05) and (2) for factors that had to be controlled for medication, the related group of clinical predictors including medication had to be significant in the corresponding joint test; a categorical variable was tested by joint test across categories (P⩽0.05). (Specification of the related groups for joint test: included major depression and antidepressants; HbA1c and antidiabetics; HDL, LDL and lipid-lowering drugs; and diastolic blood pressure and antihypertensive medication.)
In the second step, interactions between genetic variants and clinical or psychosocial factors (significant in the previous step) were modeled and analyzed in 1641 subjects with complete genetic and clinical data (data not shown).
We restricted interactions to be linear in continuous predictors. All two-way interactions that met the conditions were modeled in a single model. All factors from the first step were left in the interaction model.43 Only depression was excluded from this step because the proportion of missing values was higher than 5% (N=116), and results in the first step were not significant.
Analyses and graphics were performed using the R software46 and the STATA/MP software, release 12.1 (Stata, College Station, TX, USA).
Sensitivity analysis
Effects of current and ex-smoking showed no statistical significance (contrast between current and ex-smoking in occasional smokers: 32.4±43.5 mm3, P=0.4575; contrast between current and ex-smoking across three categories each 7.5±21.4, P=0.7254). As a result, these six categories were collapsed for further analyses.
Imputation
In addition to the complete case analysis (N=2463), we conducted an extra analysis for the clinical prediction model with imputed data (N=2619) to validate the consistency of the associations with larger sample sizes (Table 1).
Table 1. Clinical prediction model.
| Factor |
Complete case analysis (n=2463) |
Imputation (n=2619) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Effects estimated |
Conventional estimation |
Penalized estimation |
Conventional estimation |
|||||||||
| Low (median =50th percentile) | High (90th percentile) | Difference or reference | Effect | Robust s.e. | P (factor) | Effect | Robust s.e. | P (factor) | Effect | Robust s.e. | P (factor) | |
| Women | Men | −199.7 | 25.3 | <0.0001 | −170.0 | 20.2 | <0.0001 | −205.9 | 24.3 | <0.0001 | ||
| Age, years | 52.1 | 69.9 | 17.8 | −290.7 | 25.0 | <0.0001 | −291.4 | 18.1 | <0.0001 | −288.2 | 24.4 | <0.0001 |
| Body height, cm | 170 | 182 | 12 | 129.8 | 25.6 | <0.0001 | 140.0 | 17.0 | <0.0001 | 126.0 | 24.9 | <0.0001 |
| Education | 0.0053 | 0.0120 | 0.0148 | |||||||||
| Education, 10 years | 8 Years | 21.3 | 23.7 | 0.3684 | 14.7 | 20.3 | 0.4705 | 18.6 | 22.8 | 0.4144 | ||
| Education, 12 years | 8 Years | 65.6 | 24.5 | 0.0076 | 53.2 | 21.3 | 0.0125 | 57.3 | 23.8 | 0.0160 | ||
| Cigarette smoking | 0.0058 | 0.0329 | 0.0007 | |||||||||
| Ex-smoker, occasional | Never | −49.3 | 20.3 | 0.0151 | −41.7 | 18.5 | 0.0241 | −52.3 | 19.7 | 0.0079 | ||
| Ex-smoker, 1–14 cigarette per day | Never | −67.4 | 24.6 | 0.0063 | −53.3 | 22.0 | 0.0160 | −67.4 | 23.4 | 0.0040 | ||
| Ex-smoker, ⩾15 cigarette per day | Never | −41.8 | 23.4 | 0.0748 | −34.2 | 20.1 | 0.0896 | −51.0 | 22.8 | 0.0256 | ||
| Current smoker, occasional | Never | −16.9 | 41.7 | 0.6853 | −24.6 | 27.5 | 0.3711 | −19.4 | 41.4 | 0.6390 | ||
| Current smoker, 1–14 cigarette per day | Never | −81.5 | 23.9 | 0.0007 | −65.4 | 21.1 | 0.0019 | −92.8 | 23.2 | <0.0001 | ||
| Current smoker, ⩾15 cigarette per day | Never | −37.5 | 28.1 | 0.1825 | −31.9 | 22.0 | 0.1481 | −43.8 | 27.2 | 0.1072 | ||
| Alcohol consumption, g per day | 4.0 | 22.3 | 18.3 | −9.7 | 24.1 | 0.6885 | −5.3 | 12.9 | 0.9456 | −16.6 | 23.3 | 0.5852 |
| Major depression, lifetime | No | 3.1 | 19.0 | 0.8692 | −0.5 | 17.0 | 0.9765 | 5.2 | 18.7 | 0.7821 | ||
| Physical activity | 0.1468 | 0.2256 | 0.1299 | |||||||||
| >0–2 h Per week | 0 h Per week | 36.3 | 19.2 | 0.0588 | 29.7 | 17.4 | 0.0872 | 36.4 | 18.4 | 0.0484 | ||
| >2 h Per week | 0 h Per week | 19.7 | 22.6 | 0.3843 | 18.4 | 19.9 | 0.3557 | 21.2 | 21.8 | 0.3305 | ||
| Waist circumference, cm | 88.6 | 106.0 | 17.4 | −5.6 | 17.4 | 0.2654 | −2.6 | 16.4 | 0.3717 | −10.1 | 17.0 | 0.1601 |
| HbA1c, % | 5.2 | 6.0 | 0.8 | −24.7 | 14.6 | 0.1374 | −13.7 | 8.6 | 0.2695 | −34.3 | 14.5 | 0.0375 |
| HDL cholesterol, mmol l−1 | 1.42 | 1.98 | 0.56 | 30.0 | 14.9 | 0.1166 | 20.3 | 14.9 | 0.3957 | 35.9 | 14.0 | 0.0368 |
| LDL cholesterol, mmol l−1 | 3.37 | 4.61 | 1.24 | 4.5 | 15.3 | 0.5089 | 5.4 | 13.4 | 0.5249 | 3.9 | 14.7 | 0.3934 |
| Diastolic blood pressure, mm Hg | 77.5 | 91.0 | 13.5 | −38.2 | 13.7 | 0.0211 | −31.4 | 13.6 | 0.0654 | −35.5 | 13.0 | 0.0219 |
| Antidepressants | No | −60.5 | 30.9 | 0.0500 | −42.8 | 25.4 | 0.0926 | −52.5 | 29.8 | 0.0787 | ||
| Antidiabetics | No | −37.7 | 36.2 | 0.2971 | −22.8 | 30.4 | 0.4522 | −29.7 | 35.6 | 0.4053 | ||
| Lipid-lowering drugs | No | −16.3 | 27.1 | 0.5473 | −16.3 | 22.3 | 0.4647 | −6.2 | 26.1 | 0.8129 | ||
| Antihypertensive medication | No | −26.2 | 17.4 | 0.1336 | −27.8 | 16.3 | 0.0886 | −35.7 | 17.0 | 0.0353 | ||
| SHIP-2 | TREND-0 | 1.1 | 15.7 | 0.9449 | −1.0 | 17.0 | 0.9480 | −1.5 | 14.9 | 0.9221 | ||
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein. P for joint test including major depression, antidepressants: robust/penalized/imputed: 0.1404/0.2308/0.2109. P for joint test including HbA1c, antidiabetics: robust/penalized/imputed: 0.0408/0.1859/0.0116. P for joint test including HDL, LDL, lipid-lowering drugs: robust/penalized/imputed: 0.3037/0.5607/0.1501 P for joint test including diastolic blood pressure, antihypertensive medication: robust/penalized/imputed: 0.0232/0.0429/0.0091.
Imputation of missing data is recommended in general;47 therefore, two imputations were planned, one for the model without genetic data and one for the gene interaction model.
Additional 156 subjects revealed data 'missing at random' (for example, blood pressure, blood lipids; for details see Table 2). The overall proportion (SHIP-2/TREND combined) of missing data was 6% (SHIP-2 sample separate: 15%), which is acceptable for single imputation.43 SHIP-2 and TREND-0 data were separately imputed using chained equations. One hundred sixteen subjects did not participate in the psychiatric diagnostic interview (highest number of missing data in the diagnosis of lifetime depression); however, most of them took part in the CID screener from SHIP-0 and SHIP-1. Two items of the CID screener are also part of the depression interview and were used for recursive partitioning and imputation of depression. Observations with missing data of HV in one hemisphere were multiply-imputed (all variables were used); however, observations with missing outcome data dropped.47 Data not missing at random (for example, questions of alcohol consumption) were not imputed.
Table 2. Variables in clinical and genotype models.
| Characteristic |
Clinical prediction model |
Analysis of gene interactiona |
|
|---|---|---|---|
| Included (n=263) | Excluded (n=156) | (n=1641) | |
| Mean hippocampal volume, mean (s.d.), mm3 | 3947.8 (423.7) | 3892.5 (415.9) | 3935.0 (430.4) |
| Women, N (%) | 1335 (54.2) | 87 (55.8) | 914 (55.7) |
| Age, mean (s.d.), years | 52.1 (13.6) | 55.8 (12.7)b | 53.0 (13.1) |
| Body height, mean (s.d.), cm | 170 (9.3) | 169 (9.3) | 170 (9.2) |
| Education, mean (s.d.) | |||
| <10 Years | 372 (15.1) | 37 (23.9)c | 253 (15.4) |
| 10 Years | 1382 (56.1) | 83 (53.6) | 924 (56.3) |
| >10 Years | 709 (28.8) | 35 (22.6) | 464 (28.3) |
| Cigarette smoking, N (%) | |||
| Non-smoker | 991 (40.2) | 56 (38.6) | 677 (41.3) |
| Former smoker, occasional | 389 (15.8) | 13 (9.0) | Former: |
| Former smoker, 1–14 cigarettes per day | 206 (8.4) | 18 (12.4) | 624 (38.0) |
| Former smoker, ⩾15 cigarettes per day | 310 (12.6) | 24 (16.6) | |
| Current smoker, occasional | 79 (3.2) | 2 (1.4) | Current: |
| Current smoker, 1–14 cigarettes per day | 253 (10.3) | 17 (11.7) | 340 (20.7) |
| Current smoker, ⩾15 cigarettes per day | 235 (9.5) | 15 (10.3) | |
| Alcohol consumption, N (%) | |||
| 0 g Per day | 314 (12.8) | 18 (11.5) | 171 (10.4) |
| >0–5 g Per day | 1035 (42.0) | 57 (36.5) | 693 (42.2) |
| >5–10 g Per day | 427 (17.3) | 37 (23.7) | 303 (18.5) |
| >10–20 g Per day | 393 (16.0) | 18 (11.5) | 257 (15.7) |
| >20–30 g Per day | 137 (5.6) | 14 (9.0) | 97 (5.9) |
| >30 g Per day | 157 (6.4) | 12 (7.7) | 120 (7.3) |
| Major depression, lifetimed, N (%) | 446 (18.1) | 3 (15.0)e | 254 (16.8) |
| Physical activity, N (%) | |||
| No | 429 (17.4) | 37 (23.9) | 269 (16.4) |
| >0–2 h Per week | 1459 (59.2) | 87 (56.1) | 971 (59.2) |
| >2 h Per week | 575 (23.4) | 31 (20.0) | 401 (24.4) |
| Waist circumference (cm), N (%) | 88.9 (12.8) | 90.5 (13.1)f | 88.5 (12.6) |
| Body mass index (kg m2−1), N (%) | 27.4 (4.4) | 27.7 (4.3) | 27.3 (4.3) |
| HbA1c (%) mean (s.d.) | 5.3 (0.7) | 5.4 (0.8) | 5.3 (0.7) |
| Glucose (mmol l−1) | 5.6 (1.3) | 5.6 (1.5) | 5.5 (1.1) |
| Triglycerides (mmol l−1), mean (s.d.) | 1.6 (1.1) | 1.8 (1.4)c,g | 1.6 (1.2) |
| Total cholesterol (mmol l−1), mean (s.d.) | 5.5 (1.1) | 5.5 (1.1)g | 5.5 (1.1) |
| HDL-cholesterol (mmol l−1), mean (s.d.) | 1.5 (0.4) | 1.5 (0.4)g | 1.5 (0.4) |
| LDL cholesterol (mmol l−1), mean (s.d.) | 3.4 (0.9) | 3.3 (1.0)g | 3.4 (0.9) |
| Diastolic blood pressure (mm Hg), mean (s.d.) | 78.0 (9.9) | 80.2 (10.4)c,h | 78.6 (10.0) |
| Systolic blood pressure (mm Hg), mean (s.d.) | 127.2 (17.3) | 131.8 (19.9)b,h | 127.7 (17.7) |
| Medication | |||
| Antidepressants, N (%) | 138 (5.6) | 11 (7.0) | 90 (5.5) |
| Antidiabetics, N (%) | 95 (3.9) | 13 (8.3)c | 43 (2.6) |
| Lipid-lowering drugs, N (%) | 257 (10.4) | 18 (11.5) | 181 (11.0) |
| Antihypertensive medication, N (%) | 772 (31.3) | 51 (32.7) | 514 (31.3) |
| rs7294919 (TESC, HRK, FBXW8), N (%) | |||
| CC | 18 (1.1) | ||
| CT | 326 (19.9) | ||
| TT | 1297 (79.0) | ||
| rs17178006 (MSRB3), N (%) | |||
| GG | 21 (1.3) | ||
| GT | 284 (17.3) | ||
| TT | 1336 (81.4) | ||
| rs6581612 (WIF1), N (%) | |||
| AA | 908 (55.3) | ||
| AC | 613 (37.4) | ||
| CC | 120 (7.3) | ||
| rs6741949 (DPP4), N (%) | |||
| CC | 265 (16.2) | ||
| CG | 773 (47.1) | ||
| GG | 603 (36.8) | ||
| rs7852872 (ASTN2), N (%) | |||
| CC | 636 (38.8) | ||
| CG | 763 (46.5) | ||
| GG | 242 (14.8) | ||
Excluded major depression, lifetime diagnosis.
P<0.01.
P<0.05.
N=1 510.
N=20.
N=156.
N=153.
N=152.
Given the insignificant P-value for depression in the second step of analysis, we decided to remove depression from the model, thereby avoiding a need for imputation in gene interaction analysis. Using imputed values sample data (N=2619), however, HbA1c and HDL cholesterol additionally achieved significant associations with HV (Table 1).
Penalized regression
Results of penalized regression based on the 'pentrace' procedure were presented because 'A shrinkage approach will often result in regression coefficient estimates that while biased are lower in the mean squared error and hence are more likely to be close to the true unknown parameter values'.43
Mediator and pathway analyses
For two genes—rs17178006 and rs6581612—a mediator analysis (data not shown) was conducted. For the gene–gene interaction—rs7852872 and rs6581612—a pathway analyses was conducte48 (see supplement and discussion).
Results
Baseline characteristics
The description of the sample is given in Table 2.
Associations of psychosocial and clinical factors with HV(N=2463)
Besides gender, age and body height, education, smoking and diastolic blood pressure were associated with HV (Table 1). Participants with high education had larger HV than participants with low education (P=0.0076; Table 1). Current and former smokers revealed smaller HV than non-smokers (P=0.0058; Table 1). Diastolic blood pressure was negatively associated with HV in participants having more than 80 mm Hg and slightly positively associated in participants having less than 75 mm Hg (P=0.0211; Table 1). Although antidepressant medication was associated with smaller HV, the corresponding joint test (including depression) did not indicate an association.
We did not find significant associations for the following psychosocial and clinical factors: waist circumference, alcohol consumption, physical activity, lifetime major depression diagnosis, HDL cholesterol, LDL cholesterol and HbA1c (Table 1).
Associations of genetic variants with HV (N=1641)
We confirmed significant associations for genetic variants with HV (Table 3).
Table 3. Genetic markers associated with hippocampal volume (comparison with Bis et al. 22 ).
| SNP |
SHIP/TREND N=1641 |
Bis et al.22 | SHIP/TREND (N=1641) versus Bis et al.22 | |||
|---|---|---|---|---|---|---|
| Allele coded | Effect adjusted for sex and age | Robust s.e. | P- value | Effect tested | P-value | |
| rs7294919 (TESC, HRK, FBXW8) | T | −52.7 | 19.4 | 0.0065 | −107.8 | 0.0045 |
| rs17178006 (MSRB3) | G | −72.9 | 19.7 | 0.0002 | −123.8 | 0.0100 |
| rs6581612 (WIF1) | C | −41.2 | 14.1 | 0.0036 | −63.3 | 0.1194 |
| rs6741949 (DPP4) | G | −31.7 | 12.5 | 0.0112 | −52.8 | 0.0916 |
| rs7852872 (ASTN2) | C | −26.6 | 13.3 | 0.0451 | −47.7 | 0.1126 |
Interactions between clinical or psychosocial factors and genetic markers (N=1641)
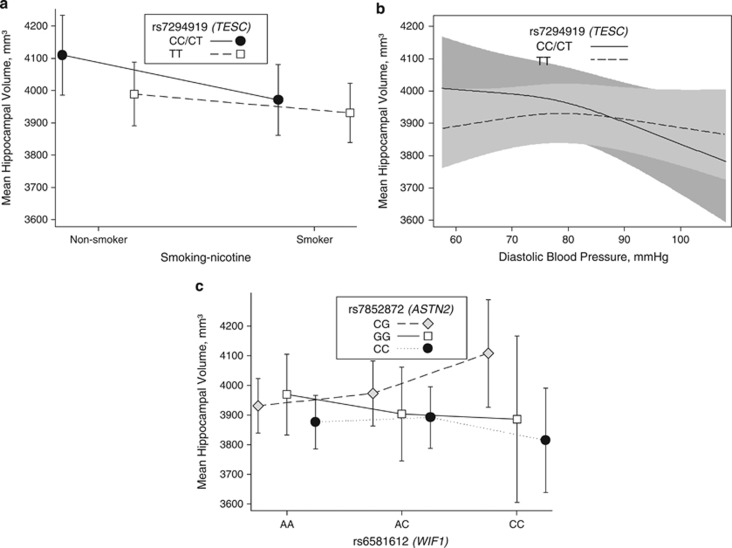
We identified two interactions between genetic markers und clinical factors. Rs7294919 modified both: the effects of smoking and diastolic blood pressure. In non-smokers, carriers of the C allele had larger HVs than non-smokers with TT genotype. In smokers, C allele carriers and non-carriers did not differ (Figure 2a; P for interaction=0.0473). Moreover, participants with low diastolic blood pressure carrying the C allele had larger HVs than TT carriers (Figure 2b; P indication of interaction=0.0447).
Figure 2.
(a) The interaction between rs7294919 and smoking status for the mean hippocampal volume (mm3) is depicted. In this plot, an interaction becomes evident between carriers of the CC/CT genotype of rs7294919 and non-smoker for a larger hippocampal volume (P=0.0473). Number of subjects for each combination of genotype and smoking status are given below: rs7294919CT/CC and non-smoker N=140; rs7294919TT and non-smoker N=537; rs7294919CT/CC and smoker N=204; rs7294919TT and smoker N=760. (b) The interaction between rs7294919 and diastolic blood pressure for the mean hippocampal volume (mm3) is depicted. In this plot, an interaction becomes evident between carriers of the CC/CT genotype of rs7294919 and lower diastolic blood pressure for a larger hippocampal volume (P=0.0447). (c) Gene–gene interaction between rs7852872 (ASTN2) and rs6581612 (WIF1) is depicted for the mean hippocampal volume in mm3. This plot shows a considerable interaction for a larger hippocampal volume in CG-genotype carriers of rs7852872 and the CC-genotype carriers of rs6581612 (P=0.0114). Number of subjects for each combination of genotype are given below: rs6581612AA and rs7852872CC: N=350; rs6581612AA and rs7852872GG: N=141; rs6581612AA and rs7852872CG: N=417; rs6581612AC and rs7852872CC: N=242; rs6581612AC and rs7852872GG: N=290; rs6581612AC and rs7852872CG: N=81; rs6581612CC and rs7852872CC: N=44; rs6581612CC and rs7852872GG:N=20; rs6581612CC and rs7852872CG; N=56.
Interactions between genetic markers (N=1641)
We found one gene–gene interaction: CG carriers of rs7852872 combined with CC genotype of rs6581612 had larger HVs than CG carriers combined with the A allele of rs6581612 (Figure 2c; P for interaction=0.0114).
Discussion
In this adult population-based study, we confirmed genetic, psychosocial and clinical factors that are associated with HV: gender, age and body height, as well as education, smoking and diastolic blood pressure. As genetic factors, we found rs7294919 to modify effects of smoking and diastolic blood pressure. Moreover, we identified an interaction between genetic markers known as candidates of neural function.
The novelty of this study consists of the joint analysis of potential predictors within one model, allowing for nonlinear relationship between continuous predictors and HV in a large population-based sample.
Associations of psychosocial and clinical factors with HV
The findings for education are in line with a study in healthy elders49 and support the idea of an education-related biological and cognitive reserve (for review see Stern50); higher reserve could counterbalance aging or pathological effects. The result for smoking is consistent with observations from Durazzo et al.51,52 who found smaller HVs in smokers compared with non-smokers. Although several studies indicate that nicotine induces neuronal cell loss, the exact pathomechanism of how nicotine affects brain tissue in general and the hippocampus in particular remains unclear. There is evidence that smoking affects the hippocampus by decreasing the regional cerebral blood flow.53 Smoking was associated with atrophy compared with non-smoking in several brain regions in early stages of Alzheimer's.51,54 In animal studies, hippocampal neuronal damage after nicotine exposure has been demonstrated.55,56 Nicotine induced cell death in hippocampal progenitors.57 Diastolic blood pressure was associated with HV, which is in line with previous studies.58, 59, 60 Hypertension is known as a risk factor for Alzheimer's, although causality might be difficult to determine because of high prevalence of hypertension and brain atrophy while aging.61 Hypertension increases the risk of atherosclerosis that might lead to decreased blood flow in the hippocampus.62 In animal models, blood pressure-related cerebral changes promoted neuronal death and brain atrophy.63,64 This is consistent with Beauchet et al.60 who concluded in their systematic review and meta-analysis that brain volume reduction in hypertension cannot solely be explained by age and use of hypertensive drugs. Several psychosocial and clinical factors were not confirmed to influence the HV in our study: waist circumference did not raise an association with HV. This result differs from other studies that could find associations between overweight/obese participants and smaller HV. In contrast to our study, their samples were not population-based or subjects were elderly.13,14 Effects of obesity on the HV may increase with age and in combination with other clinical determinants. Alcohol consumption was not associated with HV in contrast to the study from Agartz et al.9 who investigated 52 hospitalized alcohol-addicted patients.
The effect of physical activity in our study was not convincing. The rather crude assessment with low threshold (>2 h per week) of physical activity in our study may have missed protective effects in subjects with higher physical activity. Furthermore, socially desired answers have possibly led to an overestimation of sportive activities.
Previous studies about HVs and major depression are contradictory: although there is some evidence for decreased HVs or decreased subregions,65, 66, 67 other studies did not find associations between depression and decreased brain volumes.68,69 Although we had data from clinical interviews (DSM-IV), we could not support any association for HV with depression. We neither analyzed severity or recurrence of depression nor measured hippocampal subregions. We controlled results for medication as effects from diseases on brain structures might be concealed or caused by the medication. Antidepressants have been demonstrated to induce hippocampal neuroneogenesis.70, 71, 72 Antidepressants—independent from the diagnosis of depression—did have negative impact on HV in our study. This result appears counterintuitive. Although the intake of antidepressants may largely be associated with depressive disorders, they are also prescribed for patients suffering from pain, anxiety or sleep disturbances. Among those participants suffering from depression, the use of antidepressants may indicate as well chronicity, recurrence or severity of depression, which lead to decrease of HV. In addition, loss of HV or reduction of hippocampal cell proliferation might not be a direct effect of antidepressants. Antidepressants could also be understood as a proxy for distress by pain, depression or anxiety, which lead to hippocampal volume loss. Effects of the chronic diseases might not be completely removed from the neural plastic and proliferative effects of antidepressants.
We could not confirm that diabetes or elevated cholesterol levels were associated with smaller HV. This is contradictory to previous findings of association of diabetes and total cholesterol levels and HV in a sample of older male subjects.12
Associations of genetic markers with HV
We confirmed not only rs7294919 but also the four loci discovered by Bis et al. (rs17178006, rs6741949, rs6581612 and rs7852872) as predictors for HV. These findings are consistent with our hypotheses to confirm all genetic loci named above.
Rs17178006 (MSRB3, methionine sulfoxide reductase B3) is a gene that encodes a protein involved in the reduction of methionine sulfoxide to methionine, preserving the biological activity of proteins after oxidative damage.33,34 Although pathophysiology is unclear, MSRB3 seems to be cell-protective in the hippocampus and may prevent apoptosis leading to HV loss.73,74 As rs17178006 (MSRB3) and rs6581612 (WIF1) were in low linkage disequilibrium (r2=0.2), we performed conditional analyses on their association on HV: both effects were attenuated but only the association with rs17178006 remained significant. Although both genes are excellent candidates of neural function and protection, this finding implies a single locus or complex mediation effects (for example, through gene transcription modulation or protein networks). A pathway analysis revealed a putative connection between MSRB3 and WIF1 via leptin and APOB (via text mining; Supplementary Figure 1).
Rs6741949 (DPP) was not involved in interactions or pathways but in a genetic association. In animal models, DPP-4- inhibitors moderated brain mitochondrial function showed improved cognitive behavior35 and reduced deposits of amyloid in Alzheimer models.75,76
Interactions between clinical or psychosocial factors and genetic markers
Three interactions were associated with HV: the two interactions with rs7294919 are in line with both GWAS.25,26 C-allele carriers had larger HVs than non-carriers, but only in non-smokers or participants with low diastolic blood pressure. The intergenic variant rs7294919 comprises the genes TESC, HRK and FBXW8. TESC encodes a protein signaling complex77 and has an important role in cell proliferation and brain development.26,27 HRK is a regulator of apoptosis,29,78 which exhibits death-inducing activity in cells.28,29 FBXW8 encodes a member of the F-box protein family, which modifies neuronal dendrite growth and configuration.30
Interactions between genetic markers
Carriers of CC genotype of rs6581612 (WIF1) and the CG genotype of rs7852872 (ASTN2) were associated with larger HVs. ASTN2 is expressed in the hippocampus and is involved in neuronal migration.36 WIF1 also known as WNT inhibitory factor 1 is a protein that is encoded by the WIF1 gene79,80 that inhibits WNT proteins from triggering signals and affects neuronal functions.31,32 The WNT signaling pathway is part of the cell–cell communication between neurons and astrocytes, which may be disturbed in neurogenenerative disorders.81 At the physiological level, an interaction between mechanisms of neuronal migration and cell–cell communication seems conceivable.
Landmarks of this study provide: the joint analysis of potential factors affecting HV in a large general population sample, applying the same sampling methods and investigated in the same scanner with identical protocols. Modeling with restriced cubic splines made it possible to detect nonlinear associations with HV. The statistical model for interaction analysis comprised all main effects and interaction terms that lead to robust adjustment for all confounding effects and circumvent the problem of multiple testing.
Limitations of our study are: first, our results may have a limited generalizability as distributions of risk/protective factors in different populations vary and lead to different associations. Second, selection bias because of MRI participation cannot be excluded. Third, unmeasured confounding and information bias could have influenced our results. Fourth, we did not measure hippocampal subregions. Some studies demonstrated that volume loss may rather be a process affecting hippocampal subregions without showing significant change in the whole hippocampus. Fifth, the cross-sectional nature of our study limits causal inference. Last, additional cognitive tests may be helpful to underlie the functional differences in HV.
This study confirmed the impact of education, diastolic blood pressure, smoking and genetic variants on HV. Complex interactions between these factors seem to have an important role in HV changes. We suggest composite scores for the individual estimate of HV and the biological reserve capacity.
Acknowledgments
The Study of Health in Pomerania (SHIP) is supported by the German Federal Ministry of Education and Research (grants 01ZZ9603, 01ZZ0103 and 01ZZ0403) and the German Research Foundation (DFG; GR 1912/5-1). Genome-wide data and MRI scans were supported by the Federal Ministry of Education and Research (grant 03ZIK012) and a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg–West Pomerania. The University of Greifswald is a member of the Center of Knowledge Interchange program of the Siemens AG. SHIP-TREND-0: The authors from SHIP are grateful to M. Stanke for the opportunity to use his Server Cluster for SNP Imputation. This cohort is a part of the Community Medicine Research net (CMR) of the University of Greifswald, which is funded by the German Federal Ministry of Education and Research and the German Ministry of Cultural Affairs, as well as by the Social Ministry of the Federal State of Mecklenburg–West Pomerania. CMR encompasses several research projects that share data from the population-based SHIP (see URLs). The work is also supported by the Greifswald Approach to Individualized Medicine (GANI_MED) network funded by the Federal Ministry of Education and Research (grant 03IS2061A). MRI scans were supported by the Federal Ministry of Education and Research (grant 03ZIK012) and a joint grant from Siemens Healthcare and the Federal State of Mecklenburg–West Pomerania. We thank all staff members and participants of the SHIP studies, as well as all of the genotyping staff for generating the SHIP SNP data set. We are especially grateful to Alexander Teumer PhD (Interfaculty Institute for Genetics and Functional Genomics; University Medicine Greifswald, Germany) for his critical comments and help to improve the paper. D. J. is supported by a scholarship from the Gerhard-Domagk programme of the University Medicine Greifswald. SD is a recipient of a Chaire d'Excellence Grant from the Agence Nationale de la Recherche, in collaboration with INSERM Unit U708 and the University of Versailles Saint-Quentin en Yvelines. This study was supported by HELIOS- Kliniken GmbH grand-ID: 001283.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schaub A, Banac S, Charypar M, Jager M, Kummler P, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–323. [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Meyer P, Cacciaglia R, Liebscher C, Ridder S, Flor H. Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct Funct. 2014;219:255–267. doi: 10.1007/s00429-012-0497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, et al. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Sigurdsson T, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med. 2012;43:317–328. doi: 10.1017/S0033291712001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Qiu C, Zhang Y, Bronge L, Herlitz A, Aspelin P, Backman L, et al. Medial temporal lobe is vulnerable to vascular risk factors in men: a population-based study. Eur J Neurol. 2012;19:876–883. doi: 10.1111/j.1468-1331.2011.03645.x. [DOI] [PubMed] [Google Scholar]
- Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Map. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes care. 2006;29:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Shah K, Villareal DT, Head D. Cognitive and neural correlates of aerobic fitness in obese older adults. Exp Aging Res. 2012;38:131–145. doi: 10.1080/0361073X.2012.659995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, et al. Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. NeuroImage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Bis JC, DeCarli C, Smith AV, van der Lijn F, Crivello F, Fornage M, et al. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44:545–551. doi: 10.1038/ng.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Hudson QJ, Perera EM, Akan L, Tobet SA, Smith CA, et al. Expression and evolutionary conservation of the tescalcin gene during development. Gene Expr Patterns. 2009;9:273–281. doi: 10.1016/j.gep.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ding L, Chen S, Nunez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L) EMBO J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Barrera-Vilarmau S, Obregon P, de Alba E. Characterization of a novel interaction between Bcl-2 members Diva and Harakiri. PLoS ONE. 2010;5:e15575. doi: 10.1371/journal.pone.0015575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterman N, Ikeuchi Y, Gallardo G, O'Connell BC, Sowa ME, Gygi SP, et al. An OBSL1-Cul7Fbxw8 ubiquitin ligase signaling mechanism regulates Golgi morphology and dendrite patterning. PLoS Biol. 2011;9:e1001060. doi: 10.1371/journal.pbio.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development (Cambridge, England) 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H, Etienne F, Hoshi T, Heinemann SH, Lowther WT, Matthews B, et al. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch Biochem Biophys. 2002;397:172–178. doi: 10.1006/abbi.2001.2664. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–849. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- Wilson PM, Fryer RH, Fang Y, Hatten ME. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. J Neurosci. 2010;30:8529–8540. doi: 10.1523/JNEUROSCI.0032-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40:294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, et al. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001;46:186–194. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- ATC-Index. Anatomisch- therapeutisch- chemische Klassifikation mit Tagesdosen Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland, 2007
- H.-U.Wittchen HPa. DIA-X-Interviews: Manual für Screening Verfahren und Interview; Interviewheft Längsschnittuntersuchung (DIA-X-Lifetime); Ergänzungsheft (DIA-X-Lifetime); Interviewheft Querschnittsuntersuchung (DIA-X-12 Monate); Ergänzungsheft (DIA-X-12 Monate). Swets & Zeitlinger Frankfurt, 1997
- Nauck M, Winkler K, Marz W, Wieland H. Quantitative determination of high-, low-, and very-low-density lipoproteins and lipoprotein(a) by agarose gel electrophoresis and enzymatic cholesterol staining. Clin Chem. 1995;41:1761–1767. [PubMed] [Google Scholar]
- Harrell FE., Jr . Logistic Regression, and Survival Analysis. Springer: New York; 2001. Regression modeling strategies: with applications to linear models. [Google Scholar]
- Harrell FE., Jrrms: Regression Modeling Strategies. R package, version 3.5-0. http://CRAN.R-project.org/package=rms ; 2012
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Wolters Kluwer|Lippincott Williams & Wilkins: Philadelphia; 2008. [Google Scholar]
- R Core Team R: A language and environment for statistical computing, version 2.15.3, ISBN 3-900051-07-0, URL http://www.R-project.org/ . 2.15.3edn, R Foundation for Statistical Computing: Vienna, Austria; 2013 [Google Scholar]
- von Hippel PT. Regression with missing Ys: an improved strategy for analyzing multiply imputed data. Sociol Methodol. 2007;37:83–117. [Google Scholar]
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, et al. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mezenge F, Perrotin A, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage. 2013;83C:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Insel PS, Weiner MW. Greater regional brain atrophy rate in healthy elderly subjects with a history of cigarette smoking. Alzheim Dement. 2012;8:513–519. doi: 10.1016/j.jalz.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Dependence. 2013;133:704–711. doi: 10.1016/j.drugalcdep.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, et al. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens. 2013;31:1502–1516. doi: 10.1097/HJH.0b013e32836184b5. [DOI] [PubMed] [Google Scholar]
- Appelman AP, Exalto LG, van der Graaf Y, Biessels GJ, Mali WP, Geerlings MI. White matter lesions and brain atrophy: more than shared risk factors? A systematic review. Cerebrovas Dis (Basel, Switzerland. 2009;28:227–242. doi: 10.1159/000226774. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sarraf H, Philip L. Increased brain uptake and CSF clearance of 14C-glutamate in spontaneously hypertensive rats. Brain Res. 2003;994:181–187. doi: 10.1016/j.brainres.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Al-Sarraf H, Philip L. Effect of hypertension on the integrity of blood brain and blood CSF barriers, cerebral blood flow and CSF secretion in the rat. Brain Res. 2003;975:179–188. doi: 10.1016/s0006-8993(03)02632-5. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Map. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Tiemeier H, Luijendijk HJ, van der Lijn F, Koudstaal PJ, Hofman A, et al. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biol Psychiatry. 2011;70:191–197. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, NY) 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000;18:259–270. doi: 10.1016/s0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Walss-Bass C, Soto-Bernardini MC, Johnson-Pais T, Leach RJ, Ontiveros A, Nicolini H, et al. Methionine sulfoxide reductase: a novel schizophrenia candidate gene. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:219–225. doi: 10.1002/ajmg.b.30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault VA, Holscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol. 2008;587:112–117. doi: 10.1016/j.ejphar.2008.03.025. [DOI] [PubMed] [Google Scholar]
- D'Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Exp Gerontol. 2010;45:202–207. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Baumgartner M, Patel H, Barber DL. Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- Coultas L, Terzano S, Thomas T, Voss A, Reid K, Stanley EG, et al. Hrk/DP5 contributes to the apoptosis of select neuronal populations but is dispensable for haematopoietic cell apoptosis. J Cell Sci. 2007;120:2044–2052. doi: 10.1242/jcs.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat Struct Mol Biol. 2011;18:886–893. doi: 10.1038/nsmb.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caruso A, Caraci F, Iacovelli L, Sortino MA, et al. The Wnt pathway, cell-cycle activation and beta-amyloid: novel therapeutic strategies in Alzheimer's disease. Trennd Pharmacol Sci. 2003;24:233–238. doi: 10.1016/s0165-6147(03)00100-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.