Abstract
Changes in the blood expression levels of SAT1, PTEN, MAP3K3 and MARCKS genes have been reported as biomarkers of high versus low suicidality state (Le-Niculescu et al.). Here, we investigate these expression biomarkers in the Genome-Based Therapeutic Drugs for Depression (GENDEP) study, of patients with major depressive disorder on a 12-week antidepressant treatment. Blood gene expression levels were available at baseline and week 8 for patients who experienced suicidal ideation during the study (n=20) versus those who did not (n=37). The analysis is well powered to detect the effect sizes reported in the original paper. Within either group, there was no significant change in the expression of these four genes over the course of the study, despite increasing suicidal ideation or initiation of antidepressant treatment. Comparison of the groups showed that the gene expression did not differ between patients with or without treatment-related suicidality. This independent study does not support the validity of the proposed biomarkers.
Introduction
Suicide is a worldwide public health problem and is among the ten leading causes of death.1 Suicidal ideation is a risk factor for suicidal behavior, but its assessment has to rely on imprecise and subjective measures, hampered by patients' reluctance to report suicidal thoughts.2, 3 While many clinical variables are correlated with suicidality, they are insufficient to identify risk in individual patients.3, 4 Objectively measured biomarkers could contribute to better risk prediction and clinical care.
A recent study by Le-Niculescu et al.5 investigated biomarkers for suicidal ideation in a live discovery sample of patients with bipolar disorder (n=9). A Convergent Functional Genomics approach was used to prioritize genes which were differentially expressed between a high versus low suicidality state, on the basis of findings from postmortem brain gene expression studies of suicide victims, as well as genetic linkage or association studies on suicide. The top biomarkers were tested for differential expression in a validation sample of suicide victims (n=9) and for ability to predict past and future hospitalizations for suicidality in two follow-up cohorts with either bipolar disorder (n=42) or psychosis (n=46). It was reported that changes in the expression of four genes: spermidine/spermine N1-acetytransferase 1 (SAT1), phosphatase and tensin homolog (PTEN), mitogen-activated protein kinase kinase kinase 3 (MAP3K3) and myristoylated alanine-rich protein kinase C substrate (MARCKS) in the blood, could be used as biomarkers of a high versus low suicidal state and could predict hospitalizations for suicidality.5
Biomarker research, in general, is plagued with overestimation of results in discovery studies with subsequent lack of replication, and findings which usually have limited predictive ability.6, 7 Currently, in psychiatry there are no biomarkers of clinical utility.7 In suicidality biomarker research, the study of genetic, immunological and neuroendocrine biomarkers has generated inconsistent results, with little or no replication of initial findings.8 Replication in large independent samples by independent research groups is essential to validate the results of biomarker discovery studies. Here, we investigate the expression of the proposed biomarkers SAT1, PTEN, MAP3K3 and MARCKS, in patients with depression who experienced suicidal ideation during antidepressant treatment.
Materials and methods
Sample collection
The Genome-Based Therapeutic Drugs for Depression Study (GENDEP) is a prospective pharmacogenetic study of patients with major depressive disorder (n=868) receiving 12-week antidepressant treatment.9 Participants were recruited from nine European centers and diagnosed with major depressive disorder using the Schedules for Clinical Assessment in Neuropsychiatry Interview (SCAN), according to the International Classification of Diseases 10th edition (ICD-10) or Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV).10, 11, 12 SCAN interviews were conducted by psychologists or psychiatrists trained at World Health Organisation Training and Research Centres. Exclusion criteria were psychotic disorder with mood incongruent psychotic symptoms or bipolar disorder.9 Patients received a protocol-guided treatment with either escitalopram—a selective serotonin reuptake inhibiter or nortriptyline—a tricylic antidepressant.9 All participants in the GENDEP study were of Caucasian European parentage.9
Patients gave written informed consent and ethical approval was obtained from the local ethics committee at each center of recruitment. The GENDEP trial is registered at EudraCT (no. 2004-001723-38) and ISRCTN (no. 03693000).
Gene expression measurement
Blood was collected in PAXgene tubes (PreAnalytiX, Hombrechtikon, Switzerland) at both week 0 and week 8 for 136 participants and frozen at −80 °C.13 PAXgene tubes were allowed to thaw for 12 h at room temperature and mRNA was isolated from whole blood using the Qiagen PAXgene Blood miRNA Kit (PreAnalytiX) following the manufacturer's protocol.14 Genome-wide expression analysis was performed in four batches on Illumina Human HT-12 v4 BeadChip microarrays (Illumina, San Diego, CA, USA).
Quality control was performed using R 3.0.2. Gene expression values were log transformed. In the analysis of outliers of gene expression, 13 patients were excluded because the expression in one of their paired samples fell below 2 s.d. from the mean inter-array correlation. Additional filtering using sex-incongruent expression of probes within the XIST gene removed a further two samples. Detection score P-values were used for probe filtering (P<0.1 in at least one sample), and probes displaying little variation were also removed (where s.d. was in the lowest quartile <0.12030). After filtering, a total of 121 paired samples remained, with 29 765 probes. Data were normalized using quantile normalization, and ComBat was used to control for batch effects.15 Probes of interest were ILMN_1753342 (SAT1), ILMN_1701134 (PTEN), ILMN_1779010 and ILMN_2296697 (mean expression level was used for MAP3K3) and ILMN_1807042 (MARCKS).
Phenotype definition
Suicidal ideation was assessed weekly using items from the clinician-rated 17-item Hamilton Rating Scale for Depression, the Montgomery-Åsberg Depression Rating Scale and the self-report Beck Depression Inventory.16, 17, 18 Response options of these items are shown in Table 1.
Table 1. Range of response options for HRSD-17, MADRS and BDI suicide items.
| Scale | Score | Meaning |
|---|---|---|
| HRSD-17 | 0 | Absent |
| 1 | Feels life is not worth living | |
| 2 | Wishes he/she were dead, or any thought of possible death to self | |
| 3 | Suicide ideas or half-hearted attempt | |
| 4 | Attempts suicide | |
| MADRS | 0–1 | Enjoys life or take it as it comes |
| 2–3 | Weary of life. Only fleeting suicidal thoughts | |
| 4–5 | Probably better off dead. Suicidal thoughts are common, and suicide is considered as a possible solution, but without specific plans or intentions | |
| 6 | Explicit plans for suicide when there is an opportunity. Active preparation of suicide | |
| BDI | 0 | Absent |
| 1 | Thought of killing myself | |
| 2 | I would like to kill myself | |
| 3 | I would like to kill myself if I had a chance |
Abbreviations: BDI, Beck Depression Inventory; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale.
The three items were combined into a composite suicidal ideation score using item response theory.19 Significant suicidal ideation at baseline was defined as at least 1 s.d. above the minimum score on the composite scale.19 As previously described, treatment-worsening suicidal ideation was considered an increase of at least 0.5 s.d., above their original score in a patient with significant suicidal ideation at baseline. Treatment-emergent suicidal ideation was defined as surpassing the threshold for suicidal ideation and an increase of 0.5 s.d. above their original score, in patients without significant suicidal ideation at baseline.19 Individuals with either treatment-emergent suicidal ideation or treatment-worsening suicidal ideation at any point during the 12-week study were used as cases of treatment-related suicidal ideation (RxSI+ n=20). The worst week for suicidal ideation emerging or increasing was week 5 and none of the patients became suicidal or worsened after week 8.19 This definition corresponds to an increase of one unit on the Hamilton Rating Scale for Depression, as used by Le-Niculescu et al.5 Individuals with scores under the threshold for suicidal ideation at each week and who did not show an increase of >0.5 s.d. above their baseline score were used as controls (non-SI; n=37).19 The remaining individuals with paired transcriptomics data were excluded as they did not meet this case or control definition (n=64).
Statistical analysis
Two analyses were used to test for change in expression in RxSI+ patients and control (non-SI) patients. In a within-subjects design, a paired sample t-test was used to compare gene expression at week 0 and week 8 within the case and control groups, following the protocol used by Le-Niculescu et al.5 In a between-subjects design, the relationship between case (RxSI+) and control (non-SI) status and change in gene expression (week 8−week 0) was assessed using logistic regression, co-varying for age, sex, drug treatment, gene expression at week 0 and also center of recruitment, to capture any remaining variation in population structure.
Power calculation
This study had 98% power to detect a standardized difference in expression of 1.14 between cases with suicidal ideation versus non-suicidal controls, the largest reported difference in gene expression in the original paper.5 There was also good power to detect smaller changes in gene expression between cases and controls, with 80% power to detect an effect size of 0.79 and 60% power to detect an effect size of 0.62.
Results
Individuals with suicidal ideation were significantly older than controls (P=0.02), whereas there was no difference in sex or drug treatment between suicidal ideation cases and controls (Table 2).
Table 2. Characteristics of the GENDEP suicidal ideation sample.
| RxSI+ cases (n=20) (%) | Non-SI controls (n=37) (%) | P-value | |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 5 (25.0%) | 10 (27.0%) | |
| Female | 15 (75.0%) | 27 (72.9%) | |
| Mean age (years) (s.d.) | 48.7 (13.3) | 39.8 (12.8) | 0.020 |
| Drug | 0.779 | ||
| Escitalopram | 12 (60.0%) | 25 (67.6%) | |
| Nortriptyline | 8 (40.0%) | 12 (32.4%) |
Abbreviations: non-SI, controls without suicidal ideation; RxSI+, treatment-related suicidal ideation. P-value was determined using a chi-squared test, with the exception of age, where a nonparametric Mann–Whitney U-test was used.
Within-subjects comparison
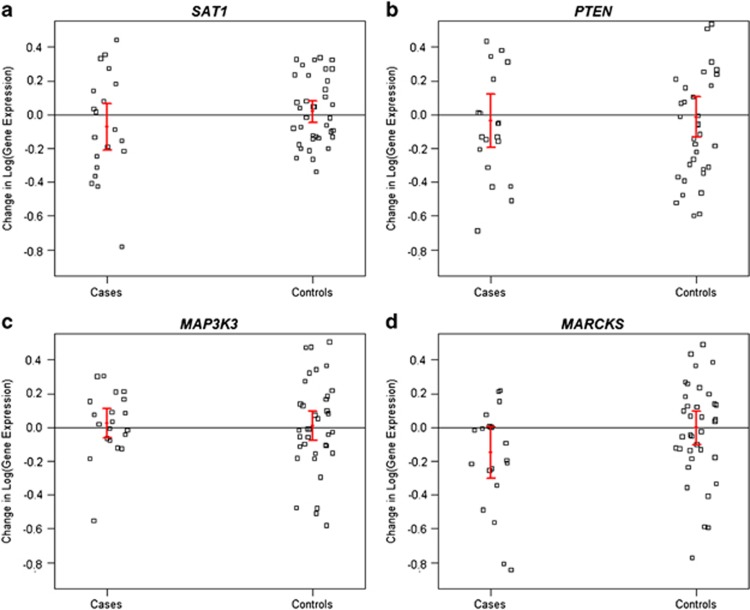
No significant difference in expression between week 0 and week 8 was detected for any gene, within either the RxSI+ cases or the non-SI controls (Table 3). Further, initiation of antidepressant treatment had no effect on the expression of these four genes in RxSI+ cases and non-SI controls (Figure 1).
Table 3. Difference in gene expression between week 0 and week 8.
| Gene |
RxSI+ cases |
Non-SI controls |
||
|---|---|---|---|---|
| Mean difference in expression (s.d.) | P-value | Mean difference in expression (s.d.) | P-value | |
| SAT1 | −0.072 (0.311) | 0.312 | 0.018 (0.196) | 0.570 |
| PTEN | −0.037 (0.361) | 0.648 | −0.014 (0.365) | 0.817 |
| MAP3K3 | 0.023 (0.194) | 0.591 | 0.009 (0.269) | 0.834 |
| MARCKS | −0.148 (0.353) | 0.075 | −0.003 (0.306) | 0.950 |
Abbreviations: non-SI, controls without suicidal ideation; RxSI+, treatment-related suicidal ideation. P-values were calculated using a paired t-test.
Figure 1.
(a–d) Change in gene expression (week 8−week 0) in RxSI+ case and non-SI control groups for (a) SAT1, (b) PTEN, (c) MAP3K3 and (d) MARCKS. Error bars represent 1 s.e.m. change in expression. RxSI+, treatment-related suicidal ideation; non-SI, controls without suicidal ideation.
Between-subjects comparison
The change in gene expression from week 0 to week 8 was compared between RxSI+ cases and non-SI controls using logistic regression, co-varying for age, sex, drug treatment, gene expression at week 0 and center of recruitment. There was no significant difference in the change in gene expression between cases versus controls for any gene tested (Table 4).
Table 4. Difference in change in gene expression between week 0 and week 8 in cases versus controls.
| Gene | Regression coefficient | Standard error | P-value |
|---|---|---|---|
| SAT1 | −2.067 | 1.707 | 0.226 |
| PTEN | −0.737 | 1.265 | 0.560 |
| MAP3K3 | −2.326 | 2.541 | 0.360 |
| MARCKS | −2.240 | 1.401 | 0.110 |
P-values were calculated using a logistic regression controlling for age, sex, drug, expression at week 0 and center of recruitment.
Discussion
Suicidal ideation is difficult to predict and assess, so the use of objectively measured biomarkers would be advantageous. Contrary to the findings of Le-Niculescu et al.,5 our analysis of the blood expression levels of SAT1, PTEN, MAP3K3 and MARCKS genes showed no difference between depressed patients with suicidal ideation versus those without.
Although the sample size in this study is small (n=20 cases and n=37 controls), it is larger than the primary analysis, which used a discovery cohort of nine patients and three small and heterogeneous replication samples.5 The original analysis was conducted in an all-male sample and so results may lack generalizability. The GENDEP sample is mixed and thus more representative of the natural epidemiology of suicidality in major depressive disorder.20, 21 The index of suicidal ideation used here incorporates three clinical scales (including that used in the original report) with a mixture of patient self-report and clinician ratings. The within-subjects analysis used to compare gene expression at week 0 and week 8 is a powerful design as it can remove the possible influence of genetics, as well as other patient-specific factors, on suicidal ideation during the study period.22, 23 Furthermore, this study has 98% power to detect the effect sizes previously reported.
Expression of SAT1 in our study was slightly lower in RxSI+ cases than in non-SI controls, though not significantly different. This is in the opposite direction to the findings reported by Le-Niculescu et al.,5 though in support of previous studies, which demonstrated decreased levels of SAT1 mRNA in several brain regions of suicide victims.24, 25, 26, 27, 28
Assessment of blood biomarkers may not be a reliable representation of brain function but it does provide easily obtainable measures which could be useful in patient monitoring. Suicidal ideation is a complex phenotype and its etiology is poorly understood. It is likely that larger sample sizes and a model including multiple clinical and biological risk factors, will be required to form a robust predictor with clinical utility.
Acknowledgments
The GENDEP study was funded by a European Commission Framework 6 grant, EC Contract Ref.: LSHB-CT-2003-503428. This work was funded in part by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London. This paper presents independent research in part funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. CML has received funding from the European Community's Seventh Framework Programme under the Marie Curie Industry-Academia Partnership and Pathways, grant agreement 286213. RU is supported by the Canada Research Chairs program (http://www.chairs-chaires.gc.ca/). KA holds an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta.
AF and PM have received consultancy fees and honoraria for participating in expert panels for pharmaceutical companies, including GlaxoSmithKline. CML has received consultancy honoraria from Eli Lilly. The remaining authors declare no conflict of interest.
References
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isometsa ET, Heikkinen ME, Marttunen MJ, Henriksson MM, Aro HM, Lonnqvist JK. The last appointment before suicide: is suicide intent communicated. Am J Psychiatry. 1995;152:919–922. doi: 10.1176/ajp.152.6.919. [DOI] [PubMed] [Google Scholar]
- Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983;40:249–257. doi: 10.1001/archpsyc.1983.01790030019002. [DOI] [PubMed] [Google Scholar]
- Blasco-Fontecilla H, Delgado-Gomez D, Ruiz-Hernandez D, Aguado D, Baca-Garcia E, Lopez-Castroman J. Combining scales to assess suicide risk. J Psychiatr Res. 2012;46:1272–1277. doi: 10.1016/j.jpsychires.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. 2013;18:1249–1264. doi: 10.1038/mp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA. 2011;305:2200–2210. doi: 10.1001/jama.2011.713. [DOI] [PubMed] [Google Scholar]
- Kobeissy F, Alawieh A, Mondello S, Boustany RM, Gold MS. Biomarkers in psychiatry: how close are we. Front Psychiatry. 2012;3:114. doi: 10.3389/fpsyt.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitzka U, Doucette S, Seemuller F, Grof P, Duffy AC. Biological indicators of suicide risk in youth with mood disorders: what do we know so far. Curr Psychiatry Rep. 2012;14:705–712. doi: 10.1007/s11920-012-0329-0. [DOI] [PubMed] [Google Scholar]
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . Diagnosis and Clinical Measurement in Psychiatry. A reference manual for SCAN. World Health Organisation: Geneva, Switzerland; 1998. [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th edn (DSM-IV) American Psychiatric Association: Washington DC, USA; 1994. [Google Scholar]
- Powell TR, Schalkwyk LC, Heffernan AL, Breen G, Lawrence T, Price T, et al. Tumor Necrosis Factor and its targets in the inflammatory cytokine pathway are identified as putative transcriptomic biomarkers for escitalopram response. Eur Neuropsychopharmacol. 2013;23:1105–1114. doi: 10.1016/j.euroneuro.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Qiagen PAXgene Blood miRNA Kit Handbook. . http://www.qiagen.com/products/catalog/sample-technologies/rna-sample-technologies/mirna/paxgene-blood-mirna-kit#resources , 2009
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Perroud N, Uher R, Marusic A, Rietschel M, Mors O, Henigsberg N, et al. Suicidal ideation during treatment of depression with escitalopram and nortriptyline in genome-based therapeutic drugs for depression (GENDEP): a clinical trial. BMC Med. 2009;7:60. doi: 10.1186/1741-7015-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Gender and mental health. . http://www.who.int/gender/documents/en/whopaper6.pdf , 2002
- Perroud N, Uher R, Ng MY, Guipponi M, Hauser J, Henigsberg N, et al. Genome-wide association study of increasing suicidal ideation during antidepressant treatment in the GENDEP project. Pharmacogenomics J. 2012;12:68–77. doi: 10.1038/tpj.2010.70. [DOI] [PubMed] [Google Scholar]
- Laje G, Allen AS, Akula N, Manji H, John Rush A, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. 2009;19:666–674. doi: 10.1097/FPC.0b013e32832e4bcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori LM, Bureau A, Labbe A, Croteau J, Noel S, Merette C, et al. Global gene expression profiling of the polyamine system in suicide completers. Int J Neuropsychopharmacol. 2011;14:595–605. doi: 10.1017/S1461145710001574. [DOI] [PubMed] [Google Scholar]
- Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, Vessaz M, et al. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L, et al. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, et al. Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr., et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]