Abstract
The dysregulation of inflammation has been associated with depression and, more recently, with suicidal behaviors. The reports regarding the relationship between interleukin-6 (IL-6) and suicide attempts are inconsistent. Personality traits such as impulsivity and aggression are considered endophenotypes and important factors that underlie suicidal behaviors. The aim of the current study was to assess whether plasma and cerebrospinal fluid (CSF) levels of IL-6 are associated with personality traits among suicide attempters. We assessed the relationships among personality traits, IL-6 and violent suicide attempts. The plasma and CSF levels of IL-6 were measured in suicide attempters (plasma=58, CSF=39) using antibody-based immunoassay systems. Personality domains were assessed using the Karolinska Scale of Personality (KSP). IL-6 levels in plasma and CSF were used to predict personality domains via regression models. Plasma IL-6 was significantly and positively correlated with extraversion as well as the KSP subscales impulsivity and monotony avoidance. CSF IL-6 was positively correlated with monotony avoidance. Violent suicide attempts tended to be associated with high plasma IL-6 levels. Plasma and CSF levels of IL-6 were not significantly associated with each other. These results indicate that impulsivity and the choice of a violent suicide attempt method might be related to higher levels of IL-6 in individuals who attempt suicide. The neuroinflammation hypothesis of suicidal behavior on the basis of elevated IL-6 levels might be partly explained by the positive association between IL-6 and impulsivity, which is a key element of the suicidal phenotype.
Introduction
Suicidal behaviors constitute a major global health burden and lead to the annual death of one million people worldwide.1 Suicidal behavior is independently inherited from other concomitant psychiatric morbidities.2,3 An endophenotypic approach is a rational way of linking biological factors with established traits or probable risk factors to dissect the etiology of a complex and multifactorial behavior such as suicide.4,5
Certain personality traits such as impulsivity and aggression are frequently present in suicidal behaviors; therefore, they are considered endophenotype candidates,6 particularly among younger participants.7 This association appears to be independent of comorbid depression; furthermore, evidence from twin studies has demonstrated a genetic contribution to these traits that could explain a portion of the heritability of suicidal behavior.8
Impulsivity is partially inherited and partially influenced by environmental factors during neurodevelopmentally critical periods in childhood and adolescence.9 Family studies have reported a co-segregation of aggression and impulsivity with regard to suicidal behaviors.6 Links have been found between aggressive behavior and serotonergic activity and, recently, interleukin-6 (IL-6) and C-reactive protein (CRP) levels.6,10 Population-based studies have reported positive associations between impulsivity and plasma IL-6 levels.11
Two previous studies reported an association between suicidal behaviors and elevated levels of IL-6 in the cerebrospinal fluid (CSF) and plasma of patients with depression;12,13 however, one study did not find differences between suicide attempters and healthy controls with regard to CSF IL-6 levels.14 Personality traits such as impulsivity might explain these discrepancies as well as portions of the high IL-6 levels previously reported with regard to suicidality.10 Furthermore, Lindqvist et al.12 found that patients who chose violent suicide attempt methods15 had significantly higher CSF IL-6 levels than those of nonviolent attempters.
Because the results regarding elevated IL-6 levels among suicidal patients are inconsistent and previous studies have reported findings concerning cytokine IL-6 with regard to personality traits such as aggression and impulsivity,10,11 we assessed the relationship between personality traits and IL-6 in the plasma and CSF of suicide attempters. Our primary hypothesis was that the personality factor dominated by impulsive traits (that is, extraversion) would be associated with higher IL-6 levels in plasma and CSF. Our secondary aim was to assess whether IL-6 levels were related to the choice of suicide attempt method. We hypothesized that violent suicide attempt methods would be associated with higher IL-6 levels in plasma and CSF.
Materials and methods
Setting
The clinical cohort was composed of patients participating in a study on biological and psychological risk factors for suicidal behavior. They were recruited among patients receiving a clinical follow-up at the Psychiatric Clinic of the Karolinska University Hospital after a suicide attempt.
The Regional Ethical Review Board in Stockholm approved the study protocol (Dnr 93-211), and the patients provided written informed consent to participate.
Participants
To be included in this study, patients had to be aged 18 years or older with a suicide attempt within the last month, an adequate capacity to communicate verbally and able to write in Swedish. Patients were excluded if they had schizophrenia spectrum psychosis, dementia or mental retardation, or if they were engaged in intravenous drug abuse.
Suicide attempt was defined as any nonfatal, self-injurious behavior with the intent to cause death.16 A trained psychiatrist interviewed the participants using the Structured Clinical Interview of Diagnostic and Statistical Manual of Mental Disorders (DSM-III) for Axis I disorders (SCID-I)17 to establish a diagnosis (American Psychiatric Association). Axis II diagnoses were established using the SCID-II interview.
Plasma was collected from 58 suicide attempters (23 men, mean age=39 years, s.d.=12.7 years, range=20–69 years; 35 women, mean age=36 years, s.d.=12 years, range=18–68 years). CSF was collected from 43 suicide attempters (15 men, mean age=45 years, s.d.=12.8 years, range=22–69 years; 28 women, mean age=36 years, s.d.=12.7 years, range=18–68 years) in the same sample (Table 1).
Table 1. Demographic and clinical data concerning the study population.
| Suicide attempters | Plasma IL-6 n=58 | CSF IL-6 n=39 |
|---|---|---|
| Age (years) mean±s.d., range | 37.2±12.2, 18–69 | 38.6±13.6, 18–69 |
| Age (years) mean±s.d., range (male) | 39±12.7, 20–69 | 45.6±13.4, 22–69 |
| Age (years) mean±s.d., range (female) | 36±12, 18–68 | 35.5±12.8, 18–68 |
| Gender (n=male) (% male) | 23, 40% | 12, 31% |
| BMI (kg/m2) mean, range | 24.6, 18.1–32.8 | 24.7, 18.2–32.8 |
| Depression as primary diagnosis | 44/58, 76% | 30/39, 77% |
| Personality disorder | 22/56a, 39% | 15/38a, 39% |
| Alcohol abuse | 14/58, 24% | 7/39, 18% |
| Suicide method violent (% yes) | 11/58, 19% | 7/39, 18% |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; IL, interleukin.
In the plasma cohort, two, and in the CSF cohort, one recording on personality disorder was missing.
The patient characteristics regarding age, gender, body mass index (BMI), primary diagnosis and comorbidity are presented in Table 1. A paired plasma sample was obtained from all of the patients who provided CSF. The paired sampling was conducted on the same day on the basis of the study protocol. Fifty-one of the patients eligible for plasma sampling (88%) and 36 of the patients eligible for CSF sampling (84%) were drug-naive to antidepressants before the suicide attempt. The seven patients (16%) who had been treated with antidepressants before their suicide attempts had a mean washout period of 34 days (s.d.=14.1 days; range=26–62 days).
Somatic comorbidities
The patients were assessed with regard to somatic comorbidities. Two patients suffered from cardiovascular disease, three patients suffered from diabetes, one patient suffered from Crohn's disease, one patient suffered from celiac disease and four patients suffered from chronic pain not otherwise specified.
Blood sampling procedure
The blood samples were collected in EDTA-containing tubes in conjunction with the suicide attempt between 0730 and 0800 hours after a night of fasting and bed rest. The blood samples were collected between 1993 and 1998. The same conditions were applied to all of the samples. The blood was centrifuged within 5 min at room temperature (1000 × g for 10 min). The plasma was collected and stored at −80 °C until the cytokine levels were measured. The samples had not been thawed before the cytokine analysis in 2010.
Lumbar puncture procedure
Twelve milliliters of CSF were collected using a standard lumbar puncture protocol between 0800 and 0900 hours after the patients had been in bed while fasting since midnight. The CSF was immediately centrifuged, and the supernatant was stored at −80 °C until analyzed. The aliquoted CSF samples had not been thawed before the cytokine analysis.
Cytokine analysis in plasma
The samples were analyzed using a high-throughput automated biochip immunoassay system from EvidenceH (Randox Laboratories, Crumlin, UK). Biochip Array technology was used for the simultaneous quantitative detection of multiple analytes from a single sample.
The technology used was based on the Randox Biochip, a solid-state device containing an array of discrete test regions for immobilized antibodies specific to different cytokines and growth factors. A sandwich chemiluminescent immunoassay was employed for the cytokine array. The light signal generated from each test region on the biochip is detected using digital imaging technology, and the concentration of the analyte present in a patient or control sample was calculated using a calibration curve.
All of the values obtained in this study were in the range of the standard calibration curve. Because a high-sensitivity kit was used, each calibration began at 0 pg ml−1. The inter- and intra-assay variations are <10% according to manufacturer. The quality control procedures were implemented in the cytokine profiles that had passed predefined acceptance criteria to guarantee a high degree of precision. The results from the primary analysis were included in a previous study.18
Cytokine analysis in CSF
The samples were analyzed using two MSD Human Proinflammatory-4 II Ultra-Sensitive 96-well plates (K15025C-1). The samples were analyzed according to the manufacturer's recommendations, with the following exceptions: The standard points started at 1000 pg μl−1; and the calibrator, samples and controls were incubated overnight at 4 °C. The duplicates of four patient samples regarding IL-6 showed high variability and were therefore excluded from the analysis. A total of 39 patients are included in the following CSF results and table sections. The inter-assay variation was 7.3%, and the intra-assay variations were 7.9 and 6.6%, respectively. The results from the primary analysis were published in a previous study.14
Assessment of personality traits
The Karolinska Scale of Personality (KSP) is a self-assessment questionnaire initially constructed to measure personality traits in biological research.19 The personality inventory consists of 135 items grouped into 15 subscales. Each item is provided as a statement that can be responded to using a four-point Likert-type scale that ranges from ‘Does not apply at all' to ‘Applies completely.' The KSP raw scores were transformed into T scores (population M=50, s.d.=10) on the basis of an age- and gender-stratified Swedish normative sample.19
In the current study, we used the four-factor structure proposed by Gustavsson et al. that includes (1) neuroticism (including the subscales socialization (negative loading), somatic anxiety, psychic anxiety, muscular tension, psychasthenia, inhibition of aggression, irritability and guilt); (2) psychoticism (including the subscales detachment and suspicion); (3) nonconformity (including the subscales social desirability (negative), indirect aggression and verbal aggression); and (4) extraversion (including the subscales impulsiveness and monotony avoidance).20
The KSP factor structure proposed above was recently validated using a large cohort of suicide attempters. The current patient sample comes from the same population that was used to validate the factor structure.21 The psychometric properties of the KSP and its subscales, with regard to long-term stability and predictive ability, are acceptable.20,22
Classification of suicide attempts
Suicide attempts were classified as violent or nonviolent in line with a previous dichotomization that has shown predictive abilities for future suicide attempts and completed suicides.15 Self-poisoning and single-cut wrists were considered nonviolent attempts, and all others (for example, attempted drowning, shooting, hanging or gassing) were considered violent. See Table 1 for information on the distribution of this variable among the patient sample.
Statistical analyses
The JMP 10 (SAS Institute, Cary, NC, USA) statistical package for Mac OS X was used for all statistical analyses. The plasma and CSF IL-6 data were tested for normality using the Shapiro–Wilk test. To reduce skewness to the right, all of the data were logarithmically transformed for additional analysis. The quantitative population characteristics were described using means, standard errors and quantiles.
The potential effect of confounds with regard to the log of plasma IL-6 was tested using a multivariate linear regression model. The models consisted of age, gender, BMI, depression severity (as measured by the Montgomery–Åsberg Depression Rating Scale (MADRS)),23 a comorbid diagnosis of personality disorder and alcohol abuse. Only age significantly predicted plasma IL-6 in the model (t=3.8, P=0.0004); other P-values ranged between 0.26 and 0.86. Furthermore, adding a diagnosis of somatic comorbidity to a separate model with age and extraversion as predictors did not affect the significance of the model, and somatic comorbidity did not predict plasma IL-6 (P=0.94).
Similarly, the potential confound effect for the log of CSF IL-6 was tested using the same parameters; however, no significance was found for the tested confounds, and the P-values ranged between 0.09 and 0.95. The sample storage time was not significantly correlated with IL-6 levels (P=0.96 for plasma, P=0.43 for CSF).
Tests of parametric correlations were performed using Pearson's r. Tests of nonparametric correlations were performed using Spearman's ρ.
A standard forced multiple regression analysis tested the association between plasma IL-6 levels and KSP personality factors, with the former as the dependent variable and the latter (neuroticism, psychoticism, extraversion and nonconformity) and age as the independent variables. Group differences (violent vs nonviolent suicide attempts) were computed using one-way analyses of variance for the continuous variables.
Alpha was set at 0.05.
Results
Plasma and CSF IL-6 levels
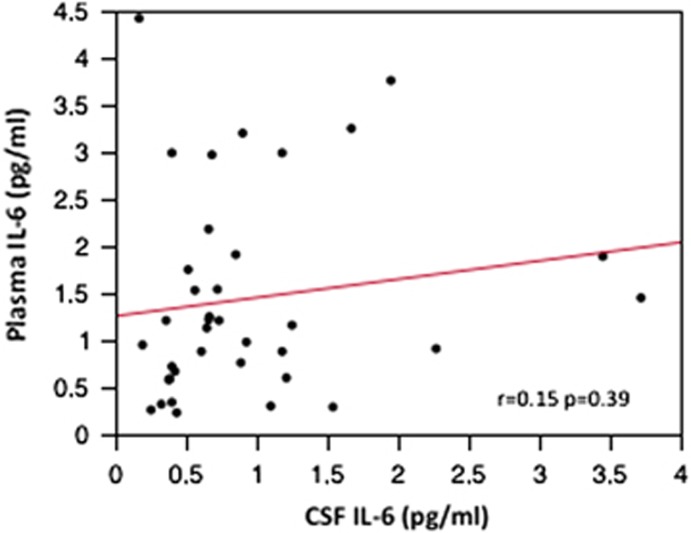
The plasma and CSF IL-6 results from the cytokine analyses are presented in Table 2. The correlation between the untransformed data of these levels in the patient sample was not significant (r=0.15, P=0.39; Figure 1).
Table 2. IL-6 levels in plasma and CSF.
| Suicide attempters | Plasma IL-6 | CSF IL-6 |
|---|---|---|
| n=58 | n=39 | |
| Levels (pg ml−1), mean±s.e.m. | 1.43±0.17 | 1.0±0.15 |
| Range | 0.23–8.15 | 0.17–3.7 |
| Median, quantile (0.1, 0.9) | 1.12, 0.30, 3.0 | 0.67, 0.33, 2.27 |
Abbreviations: CSF, cerebrospinal fluid; IL, interleukin.
Figure 1.
Correlation between plasma and cerebrospinal fluid (CSF) interleukin-6 (IL-6) in suicide attempters.
Plasma and CSF IL-6 levels and KSP personality factors
The plasma IL-6 levels were significantly and positively correlated with the KSP personality factor extraversion (r=0.48, P<0.0001). In contrast, CSF IL-6 levels were not significantly correlated with any personality factor (Table 3).
Table 3. Pearson's r correlation between personality factors and plasma and CSF IL-6 levels (log).
| Plasma IL-6 | CSF IL-6 | Neuroticism | Psychoticism | Extraversion | Nonconformity | |
|---|---|---|---|---|---|---|
| Neuroticism | −0.20 | −0.09 | 1 | 0.45** | 0.01 | 0.08 |
| Psychoticism | −0.05 | −0.04 | 0.45** | 1 | 0.03 | −0.11 |
| Extraversion | 0.48** | 0.25 | 0.01 | 0.03 | 1 | 0.18 |
| Nonconformity | 0.14 | −0.10 | 0.08 | −0.11 | 0.18 | 1 |
Abbreviations: CSF, cerebrospinal fluid; IL, interleukin. **P<0.0001.
A standard linear regression model was used with the four KSP personality factors and age to predict plasma IL-6 levels. The regression model was significant: adjusted R2=0.41, F=8.8, DF=5, P<0.0001. Extraversion and age were independent and significant predictors of plasma IL-6 levels (Table 4).
Table 4. Personality traits as predictors for plasma interleukin-6 (IL-6) in suicide attempters.
| t ratio | P-value | |
|---|---|---|
| Extraversion | 4.38 | 0.0001 |
| Psychoticism | 0.23 | 0.82 |
| Neuroticism | −0.49 | 0.63 |
| Nonconformity | 0.73 | 0.47 |
| Age | 4.22 | 0.0001 |
R2=0.46 (F ratio=8.8, DF=5, P<0.0001).
Plasma and CSF IL-6 levels and KSP extraversion subscales
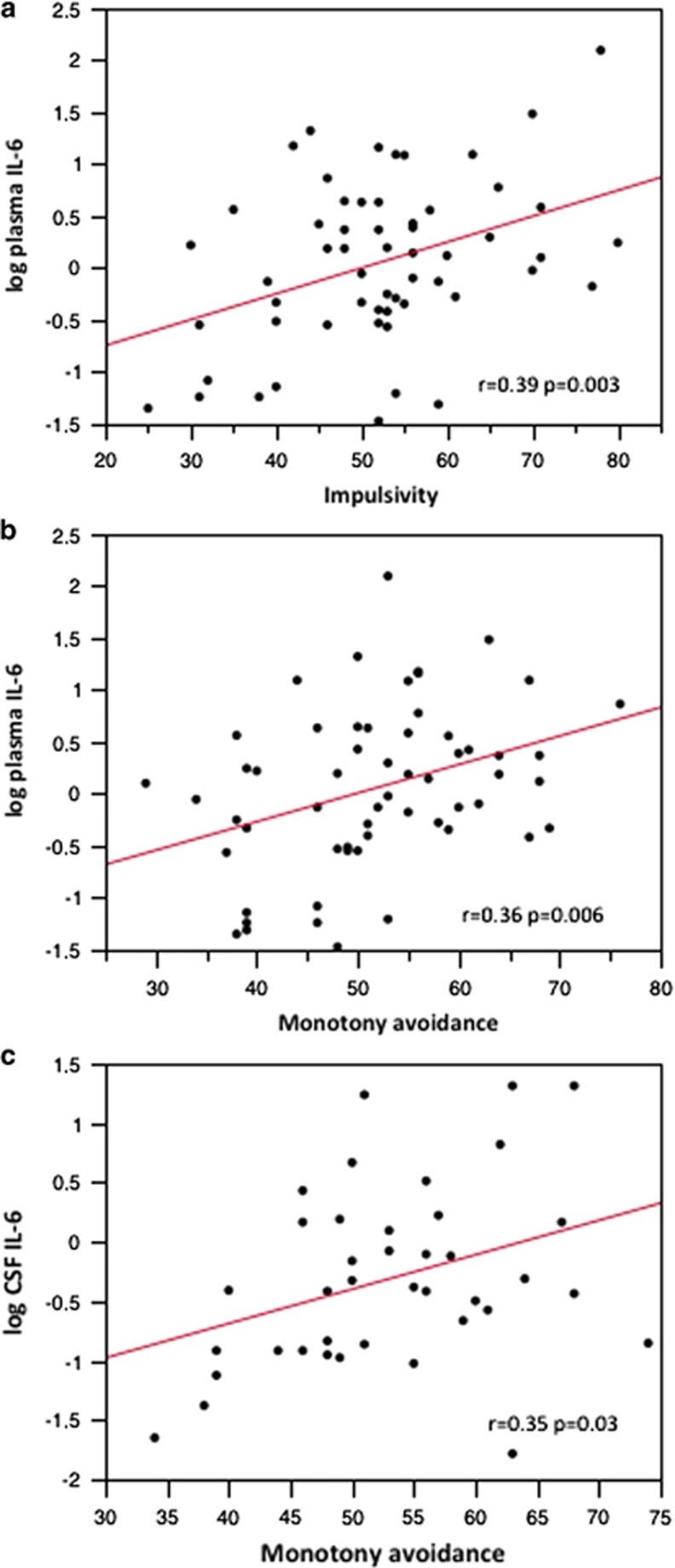
In the next step, a correlational analysis between the KSP subscales for extraversion (impulsivity and monotony avoidance) and the plasma and CSF IL-6 levels was performed. Plasma IL-6 was significantly correlated with impulsivity (r=0.39, P<0.01; Figure 2a) and monotony avoidance (r=0.36, P<0.01; Figure 2b), whereas CSF IL-6 was correlated with monotony avoidance (r=0.35, P<0.05; Figure 2c). The correlation between CSF IL-6 and impulsivity was not significant.
Figure 2.
(a) Correlation between plasma interleukin-6 (IL-6) and impulsivity; (b) correlation between plasma IL-6 and monotony avoidance; (c) correlation between cerebrospinal fluid (CSF) IL-6 and monotony avoidance.
Plasma, CSF IL-6 and personality traits with regard to suicide attempt method
Plasma and CSF IL-6 levels tended to be higher among suicide attempters who had used a violent method than among the nonviolent attempters (F=3.51, P=0.07; F=2.4, P=0.13, respectively). After adjusting for age and gender, the choice of a violent suicide attempt tended to be associated with higher plasma IL-6 levels (P=0.051).
Significant differences were not observed with regard to the extraversion scores between violent and nonviolent suicide attempters (P=0.55).
The regression model with plasma IL-6 as the dependent variable and age, choice of suicide attempt method, extraversion and the interaction between choice of method and extraversion did not reveal a significant interaction term (P=0.13). When the nonsignificant interaction term was removed from the model, it was significant (adjusted R2=0.45, F=16.9, DF=3 and P<0.0001). Extraversion and age significantly predicted plasma IL-6 levels, whereas the choice of method trended toward significance (P=0.07).
Discussion
The current study found that suicide attempters with more impulsive and sensation-seeking personality traits had significantly higher plasma levels of IL-6. High CSF IL-6 levels were associated with the trait monotony avoidance, which reflects the need for excitement, change and the avoidance of routine activities. This personality trait can be regarded as similar to sensation seeking, a trait conceptualized in impulsivity models. Monotony avoidance mediates suicidal behaviors.24
Furthermore, we found a trend such that violent suicide attempters had higher plasma IL-6 levels than nonviolent attempters. In our earlier study, we did not find elevated CSF IL-6 levels in suicide attempters compared with healthy volunteers.14 In the light of the results showing higher IL-6 levels in more impulsive suicide attempters, we hypothesize that IL-6 is more strongly associated with certain aspects of suicidality (for example, impulsivity or the choice of a violent method) than suicidality per se. Our results indicate that extraversion and violent method might be independently associated with plasma IL-6 levels, which matches earlier reports of the plasma inflammatory process and impulsivity and aggression.10,11
Lindqvist et al.12 found that violent suicide attempters had the highest CSF IL-6 levels. In our cohort, 39% of suicide attempters had a personality disorder; this diagnosis is frequently associated with high impulsivity.25 Comorbid personality disorders were not reported in the Lindqvist study, which might partially explain the different findings.
A few studies, most of them using population-based cohorts, have focused on IL-6 as a possible biomarker for personality traits associated with psychiatric and somatic morbidities.11,26,27 Sutin et al.11 reported an association between impulsivity (as measured using the NEO-PI-R five-factor model) and high IL-6 levels. However, part of this association might be ascribed to smoking habits and weight, factors that are known to mediate a proinflammatory state.28 Additional markers of inflammation have been associated with impulsivity: a population-based study reported an association between impulsivity and chronic inflammatory activity as measured by higher white blood cell counts;29 and a recent genetic study reported an association between a polymorphism of the CRP gene that is associated with higher levels of CRP as well as extraversion and impulsivity as measured using the KSP.30 CRP is an acute phase reactant produced by hepatocytes under IL-6 control.31
IL-6 is a cytokine produced in the peripheral and central nervous systems. It exerts pleiotropic effects and is induced by infectious processes and stressful events.32,33 IL-6 is predominantly regarded as a proinflammatory cytokine that acts during the acute phase of the innate immune response, and it is pivotal in the switch to acquired immunity after an immune challenge.34 However, IL-6 might (in certain settings) have antiinflammatory and neuroregulatory properties.33
IL-6 has previously been associated with several types of somatic morbidities such as cardiovascular disease, diabetes, certain cancers and autoimmune diseases. Persistently high peripheral levels of IL-6 have been associated with increased risks for morbidity and mortality.35 Previous studies have frequently reported that certain personality traits (predominantly more impulsive traits) are associated with increased somatic morbidities.11 In addition, low-grade increased inflammatory activity, as reflected by increased CRP and IL-6, is a risk factor for somatic disease.36 This finding might suggest a relationship between certain personality traits and a dysregulated inflammatory status. It is unclear whether previously reported associations reflect a trait- or state-like condition. Inflammatory activity is part of a complex interaction of multiple factors that are associated with increased somatic morbidity, including lifestyle parameters such as obesity and smoking, which are associated with more impulsive traits.
Neuroticism is a known risk factor for depression.37 IL-6 levels were not significantly associated with neuroticism in our cohort of suicide attempters. Depression severity as measured using the MADRS was not associated with IL-6 levels among suicide attempters. Previous associations regarding neuroticism and inflammation are contradictory. In a recent population-based study, the association between IL-6 levels and neuroticism was only prevalent among the patients who were the most socioeconomically deprived; it was not prevalent among those least deprived.38 The results from these studies might not be directly comparable because of their diverse designs, samples and the use of different personality inventories. However, population-based and clinical studies have reported associations between increased inflammatory statuses and personality dimensions/traits such as low conscientiousness and high measures of aggressiveness and hostility.10,11,26,27,39
How might the association between IL-6 levels and impulsivity be explained? The link between impulsive/aggressive traits and central serotonergic dysfunction has been demonstrated via CSF monoamine metabolite, postmortem and imaging studies.8,40, 41, 42 The serotonin metabolite, CSF 5-hydroxyindoleacetic acid, is negatively correlated with CSF IL-6 levels in patients treated with interpheron-alpha, indicating that the central nervous system inflammatory response interacts with serotonin metabolism.43 Previous animal studies have shown that IL-6 administration might influence serotonin metabolism in several brain regions.44
The differences in the results between plasma and CSF IL-6 levels might be because of the smaller number of patients in the CSF group. Because of the smaller CSF sample size, we were only able to detect large effects; thus, caution should be applied when interpreting the negative findings of this study. In general, cytokine variability should be considered when interpreting the results regarding the lack of consistency between the plasma and CSF correlations.45 Evidence exists of IL-6 permeability through the blood–brain barrier;46 in our results, however, the IL-6 levels in plasma and CSF were not correlated, which is consistent with a previous CSF study on IL-6 levels in suicide attempters.12 Furthermore, the true difference between peripheral and central IL-6 production might reflect different sources and mechanistic properties.
Certain limitations should be noted. First, the sample size was moderate and the cross-sectional design excludes the possibility of determining causality. Possible additional confounds include smoking and socioeconomic status, data that could not be collected in a full and structured manner from the charts. Smoking was previously reported as mediating portions of the assayed peripheral IL-6 levels,11 and the lack of information of smoking is a limitation. Furthermore, a long interval existed between the biological sampling and when the assays were conducted. Time might affect the stability of the cytokines given that the cytokines have been known to significantly decay in long-term storage, as one study previously reported.47 In this context, our assayed levels should be interpreted cautiously. However, the storage time in our cohort was not significantly correlated with IL-6 levels, and the plasma and CSF samples had never been thawed before the cytokine analyses.
The strength of this study was its careful clinical assessment of the suicide attempters, the paired measurements of IL-6 in plasma and CSF, and the fact that the patients were drug-naive or medication-free at the time of sampling.
We found a significant positive association between plasma IL-6 levels and impulsivity in a cohort of suicide attempters. If these findings replicated, they suggest that high IL-6 levels might be partially related to impulsive traits in suicide attempters. These results might be useful as a link in endophenotypic studies on impulsivity among suicidal participants and might prompt additional mechanistic or genetic studies. Our study provides additional support for the involvement of immune system activity in brain function, psychiatric morbidity and neuroregulation.
Acknowledgments
The Swedish Research Council (Project Nos.: 5454; K2009-61P-21304-04-4; K2009-61X-21305-01-1), the Thuring Foundation, the Gadelius Foundation, the Swedish Society of Medicine and the Regional Agreement on Medical Training and Clinical Research (ALF) between the Stockholm County Council and Karolinska Institutet provided funding for this study.
The authors declare no conflict of interest.
References
- WHO WHO Suicide prevention (SUPRE)Available at http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/index.html (accessed 26 November 2012).
- Runeson B, Asberg M. Family history of suicide among suicide victims. Am J Psychiatry. 2003;160:1525–1526. doi: 10.1176/appi.ajp.160.8.1525. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Fiske A. Genetic influences on suicide and nonfatal suicidal behavior: twin study findings. Eur Psychiatry J Assoc Eur Psychiatr. 2010;25:264–267. doi: 10.1016/j.eurpsy.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud P. Personality traits as intermediary phenotypes in suicidal behavior: genetic issues. Am J Med Genet C Semin Med Genet. 2005;133C:34–42. doi: 10.1002/ajmg.c.30044. [DOI] [PubMed] [Google Scholar]
- Courtet P, Gottesman II, Jollant F, Gould TD. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies. Transl Psychiatry. 2011;1:e7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–563. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Turecki G. The relationship of impulsive aggressiveness to suicidality and other depression-linked behaviors. Curr Psychiatry Rep. 2007;9:460–466. doi: 10.1007/s11920-007-0062-2. [DOI] [PubMed] [Google Scholar]
- Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. J Psychiatry Neurosci. 2005;30:398–408. [PMC free article] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, Wang P, Baker LA. Heritability and longitudinal stability of impulsivity in adolescence. Behav Genet. 2012;42:378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2013;71:158–165. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. High neuroticism and low conscientiousness are associated with interleukin-6. Psychol Med. 2010;40:1485–1493. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Isung J, Aeinehband S, Mobarrez F, Mårtensson B, Nordström P, Asberg M, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry. 2012;2:e196. doi: 10.1038/tp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träskman L, Asberg M, Bertilsson L, Sjöstrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- O'Carroll PW, Berman AL, Maris RW, Moscicki EK, Tanney BL, Silverman MM. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat Behav. 1996;26:237–252. [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. American Psychiatric Press: Washington, DC, USA; 1990. [Google Scholar]
- Isung J, Mobarrez F, Nordström P, Asberg M, Jokinen J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry. 2012;13:468–473. doi: 10.3109/15622975.2011.624549. [DOI] [PubMed] [Google Scholar]
- Schalling D, Edman G. The Karolinska Scales of Personality (KSP). An Inventory for Assessing Temperament Dimensions Associated with Vulnerability for Psychosocial Deviance. The Department of Psychiatry, The Karolinska Institute: Stockholm, Sweden; 1993. [Google Scholar]
- Gustavsson JP, Weinryb RM, Goransson S, Pedersen NL, Asberg M. Stability and predictive ability of personality traits across 9 years. Pers Indiv Differ. 1997;22:783–791. [Google Scholar]
- Hirvikoski T, Jokinen J. Personality traits in attempted and completed suicide. Eur Psychiatry. 2012;27:536–541. doi: 10.1016/j.eurpsy.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ortet G, Ibanez MI, Llerena A, Torrubia R. The underlying traits of the Karolinska Scales of Personality (KSP) Eur J Psychol Assess. 2002;18:139–148. [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Ortin A, Lake AM, Kleinman M, Gould MS. Sensation seeking as risk factor for suicidal ideation and suicide attempts in adolescence. J Affect Disord. 2012;143:214–222. doi: 10.1016/j.jad.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Mroczek DK, Moynihan J, Chapman BP. Big 5 personality traits and interleukin-6: evidence for ‘healthy Neuroticism' in a US population sample. Brain Behav Immun. 2013;28:83–89. doi: 10.1016/j.bbi.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, van Wijngaarden E, Seplaki CL, Talbot N, Duberstein P, Moynihan J. Openness and conscientiousness predict 34-week patterns of Interleukin-6 in older persons. Brain Behav Immun. 2011;25:667–673. doi: 10.1016/j.bbi.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA. Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis NMCD. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Milaneschi Y, Cannas A, Ferrucci L, Uda M, Schlessinger D, et al. Impulsivity-related traits are associated with higher white blood cell counts. J Behav Med. 2011;35:616–623. doi: 10.1007/s10865-011-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Holm G, Träskman-Bendz L, Brundin L, Ekman A. The +1444C>T polymorphism in the CRP gene: a study on personality traits and suicidal behaviour. Psychiatr Genet. 2013;23:70–76. doi: 10.1097/YPG.0b013e32835d71b6. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman EZ, Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovasc Diabetol. 2010;9:62. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar K, Lloyd SM, McLean JS, Batty GD, Burns H, Cavanagh J, et al. Personality, socio-economic status and inflammation: cross-sectional, population-based study. PLoS One. 2013;8:e58256. doi: 10.1371/journal.pone.0058256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mõttus R, Luciano M, Starr JM, Pollard MC, Deary IJ. Personality traits and inflammation in men and women in their early 70s: the Lothian Birth Cohort 1936 study of healthy aging. Psychosom Med. 2013;75:11–19. doi: 10.1097/PSY.0b013e31827576cc. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Samuelsson M, Nordstrom A-L, Nordstrom P. HPT axis, CSF monoamine metabolites, suicide intent and depression severity in male suicide attempters. J Affect Disord. 2008;111:119–124. doi: 10.1016/j.jad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Moberg T, Nordström P, Forslund K, Kristiansson M, Asberg M, Jokinen J. CSF 5-HIAA and exposure to and expression of interpersonal violence in suicide attempters. J Affect Disord. 2011;132:173–178. doi: 10.1016/j.jad.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Biancotto A, Wank A, Perl S, Cook W, Olnes MJ, Dagur PK, et al. Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS One. 2013;8:e76091. doi: 10.1371/journal.pone.0076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- De Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]