Abstract
Previous work has shown that leptin appears to regulate the plasma levels of hormones such as adrenocorticotropic hormone (ACTH) and cortisol in humans and that it has antidepressant effects in animals. It is unknown whether fluctuations in circulating leptin levels are correlated to changes in human emotions. This study was conducted to determine whether minute-to-minute fluctuations in the plasma concentrations of human leptin were associated with psychological variables. Leptin was sampled every 7 min throughout the day in 10 healthy subjects (five men and five women) studied in a clinical research center, and visual analog scales were applied every hour. We found highly significant correlations between fluctuations in plasma leptin concentrations and three psychological variables: sadness, carbohydrate craving and social withdrawal. We showed that during the course of the day increases in leptin levels are associated with decreased search for starchy foods, decreased feelings of sadness and increased social withdrawal. Our findings support the hypothesis that during the course of the day as leptin levels increase individuals subjectively feel happier (less sad) and have less inclination to interact socially. Conversely, when leptin levels decrease, we show increases in sadness and social cooperation, which might facilitate the search for food. We suggest that increased human leptin levels may promote positive feelings and that decreased leptin levels might modulate inner states that motivate and facilitate the search for nutrients.
Introduction
It has been widely observed that food intake, body weight and mood are associated in humans. Alterations in food intake and body weight are cardinal diagnostic features of mood disorders such as major depression.1 The mechanisms underlying the interactions between body weight, food intake and mood have not been elucidated. Leptin, an adipose tissue hormone, acts on hypothalamic receptors to inhibit food intake and stimulate thermogenesis, thereby regulating energy balance and body weight.2, 3, 4 Plasma leptin concentrations reflect fat mass; however, within individuals leptin levels fluctuate throughout the day in a pulsatile and circadian manner.5, 6, 7, 8 We hypothesized that leptin might affect mood acting as a link between the body's adipose mass and the brain. Here we show that during the course of the day fluctuations in the plasma leptin level are highly correlated with psychological measures of mood, social stress and food (carbohydrate) craving.
Despite the well-established effects of leptin administration in animals to reduce food consumption and decrease body weight, meals do not immediately influence leptin levels in humans. In a study investigating the circadian patterns of secretion of leptin, meal ingestion and meal-induced increases in plasma insulin and glucose did not influence changes in leptin concentrations during the 24-h period.9 Furthermore, when appetite ratings and leptin levels were compared, subjective feelings of hunger were not affected by postprandial plasma leptin concentrations in healthy volunteers.10 Those data indicate that leptin influences energy metabolism but that it does not act as an immediate satiety factor. In addition to its effect on eating behavior, leptin may have a role in the body's hormonal response to stress. We have shown that human leptin levels are highly and inversely correlated to the stress hormones cortisol and adrenocorticotropic hormone (ACTH).5 Our group and others have conducted studies supporting the concept that leptin might modulate the bioactivity of neuropeptides, such as corticotropin-releasing hormone (CRH), a neurohormone that regulates the biological and behavioral responses to stress.11, 12, 13, 14, 15, 16
Whereas several studies have included single hormone or cytokine measures as they relate to symptoms, including leptin17,18 and interleukin-6 (IL-6),19 we have previously shown that both leptin and IL-6 fluctuate substantially throughout the day.6, 7, 8,20,21 Clinical symptoms likewise fluctuate from hour to hour. Single time point measurements are therefore very limited, as they cannot be temporally related to diurnal fluctuations in psychometric variables.
The experiment described here tests the hypothesis that fluctuations in plasma leptin concentrations throughout the day are highly associated with simultaneous fluctuations in psychometric variables such as mood, stress-related behaviors and appetite.
Materials and methods
Ten healthy normal-weight subjects (five male and five female subjects; body mass index=23.4±0.9 kg/m2, age=29.5±2.2 years) were admitted to a clinical research unit at the Clinical Center at the National Institutes of Health. The research was approved by the Institutional Review Board of the National Institutes of Health Clinical Center (Bethesda, MD, USA). Informed consent was obtained before all procedures in writing. We started the experiment after 48 h of acclimatization to the research suite. Subjects had four standardized meals a day: breakfast, served at 0830; lunch, at 1230; dinner, at 1730, and an evening snack, at 2100 hours. Each subject received a total amount of calories per day that was calculated to keep each individual at their admission body weight, with 20% of calories served at breakfast, 35% at lunch, 35% at dinner and 10% at the evening snack. Subjects were exposed to light from 0700 to 2300 hours. The subjects were allowed to walk from their bed to the bathroom and to an adjacent hospital day room but were not allowed to exercise in order to maintain their level of physical activity at a comparable baseline. Blood samples for the determination of leptin were drawn via a venous catheter from each subject at 7-min intervals for 24 h starting at 0800 hours, as previously described.5,6,8 Partial results that did not include psychometric data were previously reported.6 Plasma leptin concentrations were determined using radioimmunoassay as described,5,8 and values were averaged at 1-h intervals. Symptom ratings were obtained from 100-mm visual analog self-rating scales for eight emotional states: sadness, social withdrawal, appetite, carbohydrate craving, concentration, tiredness, self-esteem and physical discomfort.22 Each subject completed the self-ratings hourly from 0900 to 2300 hours, simultaneously with the blood collection. Results were analyzed by linear regression (Pearson correlations), using the software program StatisticaTM (StatSoft, Tulsa, OK, USA). Bonferroni correction was used in the assessment of statistical significance because of multiple comparisons.
Results
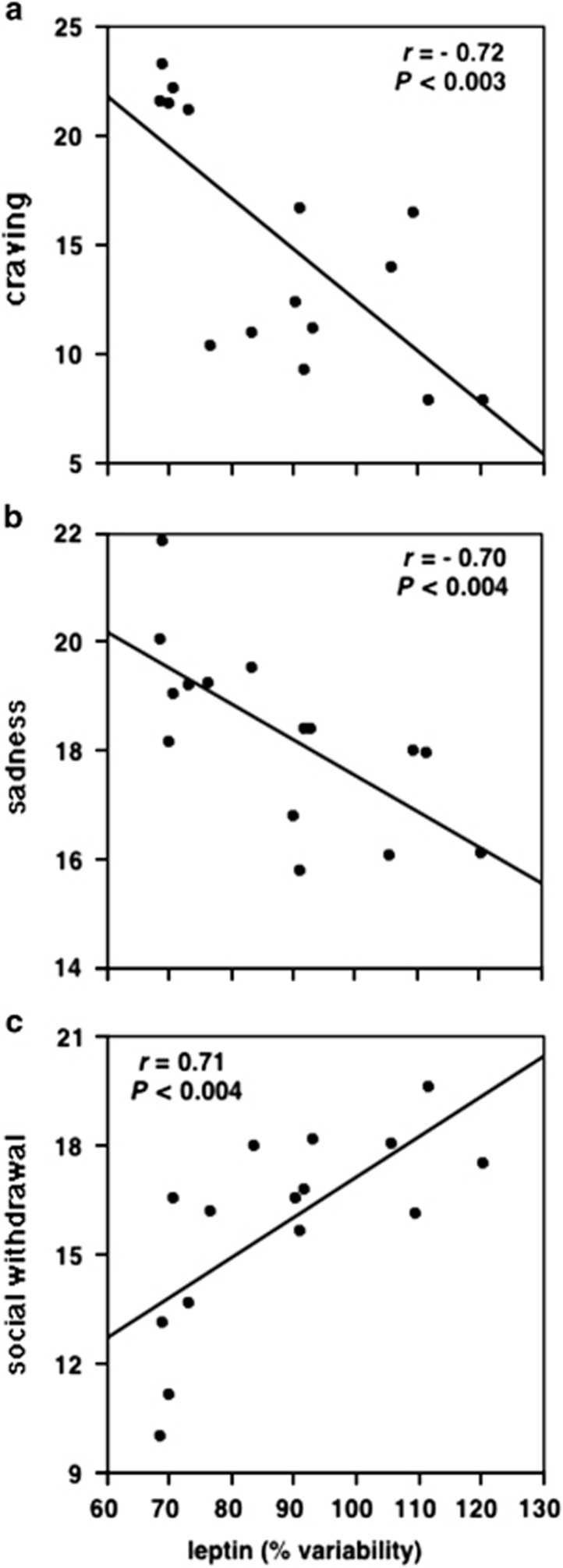
We collected a total of 2070 blood samples and 1200 psychometric variable data points. To assess fluctuation in the plasma leptin concentration, we calculated leptin variability, which has been previously defined as a percentage of individual 24-h average, using the formula: variability at time t = (hormone level at time t/24-h individual average level)x100.5 A higher score in the visual analog scale measurement indicates more pronounced symptoms. Social withdrawal, concentration, self-esteem and physical discomfort were considered as measures analogous to behaviors observed in animal models of stress.23 Using linear regression (Pearson's correlation) we found that craving for sugar, social withdrawal and sadness were highly and significantly correlated with leptin variability. The other subjective psychological states did not show significant correlation with leptin levels. Significance was calculated taking into consideration multiple comparisons (Bonferroni's correction). We found a highly significant negative correlation between leptin variability and craving, indicating that an increase in leptin levels was accompanied by similar decrease in the subjective feelings of carbohydrate craving (Figure 1a). A highly negative correlation was also found when sadness and leptin variability were compared (Figure 1b). On the other hand, social withdrawal had a highly positive correlation with plasma leptin concentrations (Figure 1c). There is a clear increase in leptin values throughout the day, which is followed by a similar pattern in social withdrawal scores. Craving for carbohydrates and sadness have a tendency to diminish towards the end of the day, indicating an hourly negative correlation between leptin and both scores.
Figure 1.
Variability in leptin concentrations is highly correlated with psychological states. (a) Leptin is inversely correlated with subjective feelings of craving (r=0.72; P<0.003). (b) Likewise, there is a highly significant correlation between the subjective ratings of sadness and leptin (r=0.70; P<0.004). (c) There is a positive correlation between ratings of social withdrawal and leptin (r=0.71; P<0.003).
Discussion
To our knowledge, this is the first report that fluctuations in longitudinally sampled human leptin concentrations are temporally correlated with psychometric variables.
A study by Larsson et al.24 examined 64 healthy postmenopausal women and showed a correlation between plasma leptin concentrations and total carbohydrate intake. That study examined the correlation between leptin and carbohydrate intake in a group of subjects whose leptin levels were assessed once and related to actual food intake. In our study we standardized food intake and examined how fluctuations in rapidly sampled plasma leptin concentrations correlated to psychometric variables within subjects. In spite of these very distinct study designs, using group comparisons (Larsson et al.) and within subject variations (this study), it is noteworthy that the results of both studies are consistent with each other, indicating that the psychometric variables we studied may be relevant to actual patterns of ad libitum food intake, as documented by Larsson et al.24
Tsofliou et al.18 used brisk walking and a chocolate-based snack, in an attempt to replicate typical physical activity and eating behaviors, to investigate the effects on appetite and on associations between serum leptin and appetite. Associations between circulating leptin and suppressed appetite or elevated satiety were found following a bout of moderate physical activity, but not at any time point during the snack or the control conditions. The authors concluded that moderate physical activity and snack intake suppress the appetite of obese women acutely. They suggested that associations between circulating leptin and appetite satiety ratings might indicate the involvement of leptin in short-term appetite regulation in response to physical activity-induced factors. Moreover, two other studies looked at the correlation between leptin and subjective variables; however, those were based on single point measurements of leptin17,18 and cannot establish a temporal correlation between variability in diurnal leptin levels and fluctuations in psychometric variables throughout the course of the day.
Despite our previous work showing that leptin replacement specifically affects macro- and micronutient intake in leptin-deficient patients,25,26 in this study we could not detect any correlation between appetite and leptin, which further supports the concept that leptin might not be a short-term satiety factor, at least in baseline levels of energy expenditure. However, the effects of leptin on food intake might explain the negative correlation we found with craving. Other hormones may have a more pronounced role in short-term satiety. This includes gastrointestinal peoptides, such as glucagon-like peptide-1.27
Previous work from our group and others have shown that leptin affects brain function in food-related cognitive tasks.28,29 Leptin also increases gray matter in the cerebellum, anterior cingulate gyrus and inferior parietal lobule.30, 31, 32, 33 In addition to these effects, leptin has potent pro-cognitive effects.34,35 Leptin levels are inversely correlated to rates of dementia and Alzheimer's disease36,37 and it acts on dopaminergic midbrain neurons to promote survival and to protect against apoptosis38 via activation of mitogen-activated protein kinase and phosphoinositide 3-kinase/Akt/nuclear factor kappa B-dependent signaling cascades. Given the functional and structural effects of leptin on the human brain, it is noteworthy, but not unexpected, that leptin might be closely correlated with the diurnal regulation of emotional states, in a manner suggestive of a possible regulatory role by this signal of nutrional status on specific emotions, such as sadness and emotional withdrawal. The possible effects of leptin on human emotions might be understood in terms of evolution. We show that during the course of the day, increases in leptin concentrations are associated with decreased motivation to search for starchy foods, decreased feelings of sadness and increased social withdrawal. This is consistent with the hypothesis that as leptin levels increase individuals subjectively feel happier (less sad) and have less inclination to interact socially in a manner that might facilitate the search for food. Conversely, when leptin levels decrease there are increases in sadness and social cooperation (necessary to obtain food).
Conclusions
We showed that during the course of the day increases in leptin levels are associated with decreased search for starchy foods, decreased feelings of sadness and increased social withdrawal. Our results support the hypothesis that decreased circulating leptin levels might modulate inner states that facilitate the search for food. Sadness and social withdrawal are the key symptoms of major depression, a disorder that has been postulated to involve abnormal CRH activity.1 The effects of leptin in the brain have been well studied in animals, including its potential role as an antidepressant39 that can increase neurogenesis.40,41 Moreover, deletion of leptin receptors in the adult hippocampus induces depression-related behaviors in mice.42 However, the specific central mechanisms by which leptin, a peripherally secreted hormone, might regulate human emotional states throughout the course of the day have not yet been identified. Future studies should determine whether the effects of leptin on the regulation of higher brain functions in humans are directly regulated by leptin receptors or whether such effects are mediated by neuropeptides, such as CRH.
Acknowledgments
This study was supported by grants from the National Institutes of Health: K30HL04526, RR16996, HG002500, RR017611, DK063240, DK58851 (JL), RR017365, MH062777 and RR000865 (M-LW).
Disclaimer
The funders had no role in study design, data collection, analysis, and decision to publish or preparation of the manuscript.
The authors declare no conflict of interest.
References
- Wong M-L, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56:s38–s46. doi: 10.1111/j.1753-4887.1998.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Goodson S, Halford JC. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes Relat Metab Disord. 2001;25:S29–S34. doi: 10.1038/sj.ijo.0801693. [DOI] [PubMed] [Google Scholar]
- Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83:4140–4147. doi: 10.1210/jcem.83.11.5291. [DOI] [PubMed] [Google Scholar]
- Licinio J, Negrão AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci USA. 1998;95:2541–2546. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Ozata M, Negrao AB, Ziotopoulou M, Caglayan S, Suchard M, et al. Synchronicity of frequently sampled TSH and leptin concentrations in healthy adults and leptin deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–3291. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannic JL, Oppert JM, Lahlou N, Basdevant A, Auboiron S, Raison J, et al. Plasma leptin and hunger ratings in healthy humans. Appetite. 1998;30:129–138. doi: 10.1006/appe.1997.0112. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Gardner JD, Rothwell NJ, Luheshi GN. Leptin affects food intake via CRF-receptor-mediated pathways. Nat Neurosci. 1998;1:103. doi: 10.1038/353. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Olama SM, Elsaid TO, El-Arman M. Serum Leptin in Egyptian patients with fibromyalgia syndrome: relation to disease severity. Int J Rheum Dis. 2013;16:583–589. doi: 10.1111/1756-185X.12155. [DOI] [PubMed] [Google Scholar]
- Tsofliou F, Pitsiladis YP, Malkova D, Wallace AM, Lean ME. Moderate physical activity permits acute coupling between serum leptin and appetite-satiety measures in obese women. Int J Obes Relat Metab Disord. 2003;27:1332–1339. doi: 10.1038/sj.ijo.0802406. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96. doi: 10.1111/j.1749-6632.2012.06634.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Larsson H, Elmstahl S, Berglund G, Ahren B. Evidence for leptin regulation of food intake in humans. J Clin Endocrinol Metab. 1998;83:4382–4385. doi: 10.1210/jcem.83.12.5342. [DOI] [PubMed] [Google Scholar]
- Licinio J, Milane M, Thakur S, Whelan F, Yildiz BO, Delibasi T, et al. Effects of leptin on intake of specific micro- and macronutrients in a woman with leptin gene deficiency studied off and on leptin at stable body weight. Appetite. 2007;49:594–599. doi: 10.1016/j.appet.2007.03.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, et al. Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite. 2005;45:75–80. doi: 10.1016/j.appet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynologia Polska. 2010;61:194–206. [PubMed] [Google Scholar]
- Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obes Rev. 2011;12:e315–e323. doi: 10.1111/j.1467-789X.2010.00840.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Paz-Filho G, Wong ML, Kohno M, Licinio J, London ED. Effects of leptin deficiency and replacement on cerebellar response to food-related cues. Cerebellum. 2013;12:59–67. doi: 10.1007/s12311-012-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Berman SM, Chakrapani S, Delibasi T, Monterosso J, Erol HK, et al. Short-term plasticity of gray matter associated with leptin deficiency and replacement. J Clin Endocrinol Metab. 2011;96:E1212–E1220. doi: 10.1210/jc.2011-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- Paz-Filho G, Mastronardi CA, Licinio J.Leptin treatment: facts and expectations Metabolism advance online publication, 3 August 2014; doi:10.1016/j.metabol.2014.07.014 (e-pub ahead of print). pii: S0026-0495(14)00234-0. [DOI] [PubMed]
- Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, et al. Leptin replacement improves cognitive development. PLoS ONE. 2008;3:e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J.Leptin levels and Alzheimer disease JAMA 20103031478author reply -9. [DOI] [PubMed] [Google Scholar]
- Doherty GH, Oldreive C, Harvey J. Neuroprotective actions of leptin on central and peripheral neurons in vitro. Neuroscience. 2008;154:1297–1307. doi: 10.1016/j.neuroscience.2008.04.052. [DOI] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17:790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Huang TY, Garza JC, Chua SC, Lu XY. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. int J Neuropsychopharmacol. 2013;16:857–867. doi: 10.1017/S1461145712000703. [DOI] [PMC free article] [PubMed] [Google Scholar]