Abstract
Both familial and sporadic forms of Alzheimer disease (AD) present memory impairments. It has been proposed that these impairments are related to inflammation in relevant brain areas such as the hippocampus. Whether peripherally triggered and neuron-driven brain inflammation produce similar and equally reversible alterations is a matter of discussion. Here we studied the effects of ibuprofen administration on a familial AD mouse model overexpressing GSK-3β that presents severe brain inflammation. We compared these effects with those observed in a peripherally triggered brain inflammation model based on chronic lipopolysaccharide (LPS) administration. Both proinflammatory stimuli produced equivalent reversible morphological alterations in granule neurons; however, GSK-3β had a much more prominent role in newborn neuron connectivity, causing alterations that were not reversed by ibuprofen. Although both insults triggered similar behavioral impairments, ibuprofen rescued this defect in LPS-treated mice but did not produce any improvement in GSK-3β-overexpressing animals. This observation could be attributable to the different microglial phenotype induced by ibuprofen treatment. These data may be clinically relevant for AD therapies, as GSK-3β appears to determine the efficacy of ibuprofen treatment.
Introduction
New neurons are continuously added to two discrete brain regions throughout life, namely the subventricular zone of the lateral ventricles, and the subgranular zone of the hippocampal dentate gyrus (DG). Adult hippocampal neurogenesis (AHN) is involved in hippocampal-dependent learning and is crucial for several processes, such as pattern separation.1
Numerous extrinsic and intrinsic stimuli are known to modulate the rate of AHN, among these inflammation is one of the most important negative regulators.2,3 In fact, several proinflammatory cytokines block newborn neuron maturation and the recruitment of these cells into behaviorally relevant circuits.4 However, the interaction between microglia and newborn neurons has been reported to be multifaceted, as both neuroprotective and detrimental effects have been demonstrated.5,6
Brain inflammation is a hallmark of numerous psychiatric and neurodegenerative diseases, such as Alzheimer disease (AD).7 In fact, brain inflammation is present both in familial and sporadic forms of AD, and it has been proposed to be one of the most important risk factors for the latter.8 The involvement of microglia in AD pathogenesis has been addressed in animal models and in human patients; however, the contribution of these cells to this process remains unclear.9 Data from AD animal models have revealed symptom amelioration after the administration of nonsteroidal antiinflammatory drugs (NSAIDs). These compounds have thus been proposed as potential therapeutic tools to prevent and treat AD progression. In addition, seminal clinical trials point to a reduced incidence of AD after chronic treatment with NSAIDs.10, 11, 12, 13 However, contradictory data impede the acceptance of these drugs as an effective and safe treatment for AD.7 Here we used an AD murine model that overexpresses GSK-3β under the control of the neuronal promoter CamKII (GSK-3-OE mice).14, 15, 16 This animal displays most of the pathological features present in the hippocampus of AD patients, including severe hippocampal apoptosis (linked to microgliosis and astrogliosis17,18) and severe alterations in the maturation of newborn granule neurons.18 In addition, granule neurons of GSK-3-OE mice show morphological alterations that resemble those of AD patients.17 Since we have observed indirect effects derived from GSK-3β overexpression, such as a dramatic brain proinflammatory phenotype,16 here we hypothesize that some of the early morphological alterations observed in the granule neurons of GSK-3-OE mice are related to the proinflammatory microenvironment, in which newborn neurons grow. Using several types of retroviruses, here we studied the impact of chronic, peripheral infusion of lipopolysaccharide (LPS) to newborn neurons of different ages on various maturational aspects, such as morphology and connectivity.
With the aim to study the potential therapeutic effects of the NSAID ibuprofen on AHN, behavioral pattern separation, and microglial activation, we also evaluated the benefits of ibuprofen treatment in mice peripherally treated with LPS and in mice overexpressing GSK-3β. Our results shed further light on microglial activation and could be relevant for the treatment of various pathologies involving brain inflammation.
Materials and methods
A detailed methodological description of the experimental design, stereotaxic surgery, killing, immunohistochemistry, volume estimation of the DG, cell counts, morphometric analysis, number and size of PSD95-GFP+ clusters, measurement of mossy fiber terminal area, electron microscopy, behavioral tests and human subjects are provided in Supplementary Experimental Procedures.
Animals
Six-week-old female C57BL/6Jcc mice were obtained from Harlan Laboratories (Bresso, Italy). Animals were subjected to a 2-week habituation period before experiments began. They were housed in a specific pathogen-free colony facility in accordance with European Community Guidelines (directive 86/609/EEC) and handled following European and local animal care protocols. In addition, the murine model of AD conditionally overexpressing GSK-3β under the control of the neuronal CamKII promoter (GSK-3-OE mice) was generated as previously described.16
Treatment with LPS from Escherichia coli
LPS was administered subcutaneously via Alzet osmotic pumps (Durect, Cupertino, CA, USA) following the experimental design previously described. Various pump models were used, depending on the duration of the treatment. For 2-week treatments, #1002# osmotic pumps were used, whereas, for 4- and 8-week treatments, the #1004# model was used. Pumps were filled with a solution of LPS (Sigma, from E. coli 055:B5, St Louis, MO, USA) diluted in 0.1 M phosphate-buffered saline. To obtain a continuous LPS delivery of 300 μg kg−1 per day, the LPS concentration was adjusted, depending on the flow specified by the manufacturer.
Ibuprofen treatments
Ibuprofen was administered in food pellets (375 mg kg−1, Harlan Laboratories). On the basis of average food consumption and body weight of the mice, the dose of ibuprofen was calculated to be 37.5 mg kg−1 per day.
Retrovirus stock preparation
We used two retroviral stocks that varied in the genes encoded: CAG-GFP encoding for GFP19 and PSD95-GFP for GFP fused with PSD95.20 The PSD95-GFP retrovirus allowed postsynaptic cluster visualization (green channel). Moreover, anti-GFP immunohistochemistry (red channel) allowed visualization of the whole dendritic tree.20 The plasmids used for the production of GFP-expressing retroviruses were a generous gift from Professor Fred H. Gage, while those used to produce the PSD95-GFP virus were kindly provided by Professor Carlos Lois. Retroviral stocks were concentrated to working titers of 1 × 107–2 × 108 p.f.u. ml−1 by ultracentrifugation.19
Cytokine antibody array
Right hippocampi were lysed, and protein concentration was estimated using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The mouse cytokine array (Array III; Ray Biotech, Norcross, GA, USA) consisted of 62 soluble signaling factors and cytokine antibodies spotted in duplicate onto a PVDF membrane. The membranes were blocked with 10% bovine serum albumin in phosphate-buffered saline and subsequently incubated with samples overnight at 4 °C. The membranes were washed with buffer supplied by manufacturer, exposed to 500-fold diluted biotin-conjugated anti-cytokine antibodies at room temperature for 2 h. They were then washed, incubated with a 1000-fold diluted HRP-conjugated streptavidin for 1 h, and immersed for 1 min in peroxidase substrate solution. For each spot, the net density gray level was normalized by subtracting the background gray levels from the total raw density gray levels using Chemidoc XRS system ImageQuant LAS 4000 mini (BioRad, Hercules, CA, USA) and ImageJ analysis software.
Statistical analysis
Data were analyzed by a one-way analysis of variance test for each statistical comparison. A post hoc DMS test was used when more than two experimental groups were compared. For the comparison of qualitative variables, a Pearson's χ2 test was applied. All statistics were analyzed using the SPSS 17.0.1 software (SPSS, 1989; Apache Software Foundation, Chicago, IL, USA). In the case of retroviral experiments, all the experimental groups in which cells were of the same age were analyzed together (for each variable or zone of study) using a one-way analysis of variance, and post hoc comparisons were made. For the sake of clarity and brevity, individual statistical values of retroviral experiments are presented as Supplementary Tables 1–4.
Results
For the purpose of clarity and brevity, only F- and P-values for global analysis of variance comparisons are shown in the Results section. Individual P-values and post hoc comparisons are given in Supplementary Tables T1–T4.
Both LPS administration and GSK-3β overexpression produced brain inflammation
We compared the brain proinflammatory phenotype produced by GSK-3β overexpression in neurons with a model of chronic inflammation based on peripheral LPS administration.
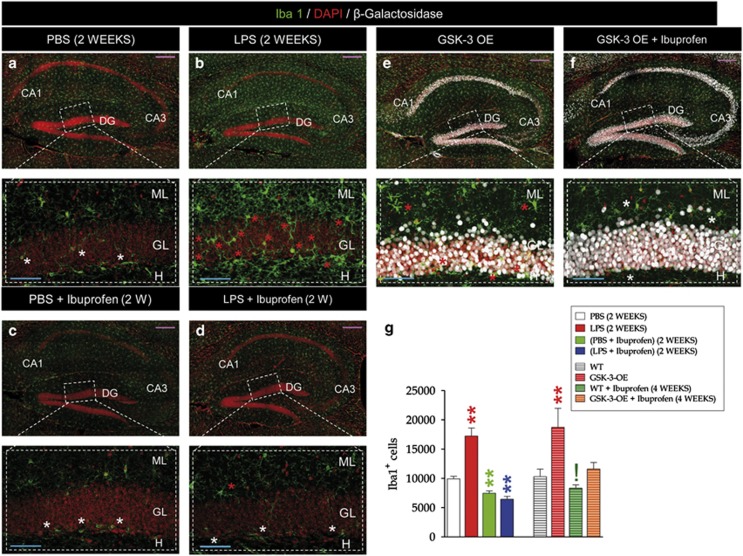
LPS-treated (Figures 1a and b) and GSK-3-OE (Figure 1e) mice showed a marked brain proinflammatory phenotype, characterized by an increased number of activated Iba1+ microglial cells (Figure 1g), a similar phenomenon to what has been described in the brains of AD patients7 (see Supplementary Figure S2C and D). Ibuprofen treatment reduced the number of Iba1+ cells in all the experimental groups (Figures 1c, d, f and g).
Figure 1.
Both peripheral LPS administration and neuronal GSK-3β overexpression produced brain inflammation. (a–f) Representative images of microglial Iba1+ cells in different experimental conditions and their respective high magnification images. (g) Number of Iba1+ cells in the dentate gyrus. Both LPS-treated (a–b) and GSK-3-OE (e) mice showed a marked brain proinflammatory phenotype and an increased number of activated Iba1+ microglial cells. Ibuprofen treatment reduced the number of Iba1+ cells in all the experimental groups (c, d, f, g). (**, 0.001<P<0.01) (!, 0.05<P<0.1). White asterisks indicate microglial cells showing a resting morphological phenotype. Red asterisks indicate microglial cells showing an activated morphological phenotype. DG, dentate gyrus; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; WT, wild type.
GSK-3-OE mice have been reported to show a drastic depletion of proliferative clusters in the subgranular zone and a marked disorganization of this zone.21 To analyze the ultrastructural changes in the subgranular zone produced by peripheral administration of LPS, ultrathin sections were examined under an electron microscope (Supplementary Figure S3). Although no change in the number of proliferative clusters was observed after LPS treatment (Supplementary Figure S3E), the number of precursor cells per cluster decreased (2 weeks: F1,239=5.468; P-value=0.02; 8 weeks: F1,129=4.019; P-value=0.047; Supplementary Figure S3F). LPS increased the presence of microglial cells within proliferative clusters (Supplementary Figure S3G; 8 weeks: F1,123=6.87; P-value=0.01) and caused a dramatic disorganization of the subgranular zone, reflected by the higher percentage of precursor cells located outside this zone (Supplementary Figure S3H; 2 weeks: F1,237=13.571; P-value<0.001; 8 weeks: F1,123=7.141; P-value=0.009). These alterations resemble those previously described in GSK-3-OE mice.21
LPS administration for 2 weeks increased the number of apoptotic Fractin+ cells, a phenomenon also observed in untreated GSK-3-OE mice (F3,30=9.88; P-value<0.001; Supplementary Figure S4I). Both increases were fully counteracted by ibuprofen treatment, although this drug produced an additional decrease in the rate of apoptosis in LPS-treated mice (F3,19=59.82; P-value<0.001). This decrease was not observed in GSK-3-OE animals. No significant changes in the total number of mature granule neurons (Supplementary Figures S4A and G) or in the DG volume (Supplementary Figure S4H) were observed after any of the treatments, although GSK-3-OE mice tended to show a decrease in the number of mature granule neurons (F3,30=1.635; P-value=0.098) and in DG volume (F3,18=3.009; P-value=0.063).
LPS altered newborn neuron morphology and maturation in a similar way to neuronal GSK-3β overexpression
GFP- or PSD95:GFP-expressing retroviruses were delivered to the hippocampus, and LPS was administered following the experimental design shown in Supplementary Figure S1. To analyze newborn neuron morphology at different cell ages or after different treatments or recovery periods, mice were killed at various time points.
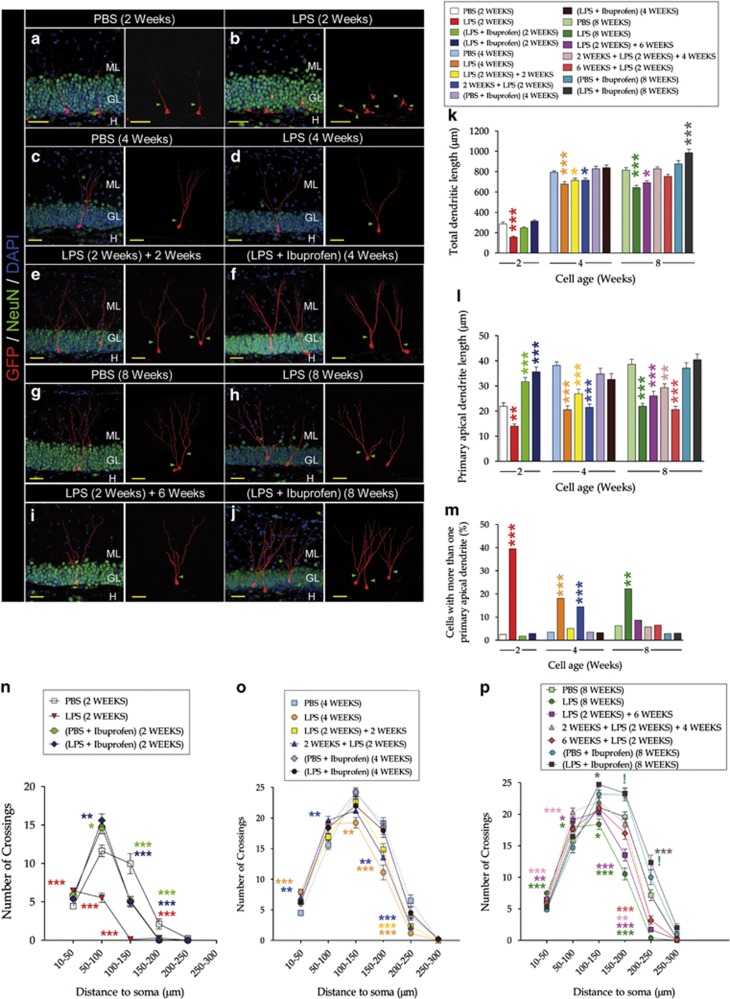
LPS administration at cell birth and during 2 (Figures 2a and b), 4 (Figures 2c and d) or 8 (Figures 2g–i) weeks caused a significant reduction of the total dendritic length of newborn neurons (Figures 2a–k). These alterations were not reversed 2 (Figure 2e) or 6 (Figure 2i) weeks after LPS withdrawal. In contrast, when LPS was administered 6 weeks after cell birth, no decrease in this parameter was observed. Ibuprofen reversed the dendritic atrophy produced by LPS administration (Figures 2f and j). LPS significantly and permanently reduced the length of apical primary dendrites (Figure 2l) and increased newborn neuron migration into the GL (Supplementary Figure S5F). Similar alterations have been previously described in GSK-3-OE mice.17 Ibuprofen administration reversed alteration in the length of apical primary dendrites (Figure 2l). In addition, LPS increased the percentage of newborn neurons with more than one primary apical dendrite (Figure 2m). This increase was restored 2 or 6 weeks after LPS withdrawal, and was abolished by ibuprofen treatment (Figure 2m). F- and P-values for individual comparisons are shown in Supplementary Table T1.
Figure 2.
LPS produced morphological alterations in newborn granule neurons. GFP- or PSD95:GFP-expressing retroviruses were delivered into the hippocampus, and LPS was administered during different periods, following the experimental design shown in Supplementary Figure S1. Mice were killed at a range of time points to analyze the morphology of 2-, 4- and 8-week-old newborn neurons. (a–j) Representative pictures showing retrovirus-labeled neurons of different ages belonging to the diverse experimental groups. (k) Quantification of total dendritic length. LPS administration at cell birth caused a significant reduction in total dendritic length. These alterations were not reversed (e) 2 or (i) 6 weeks after LPS withdrawal. In contrast, when LPS was administered 6 weeks after cell birth, no decrease in total dendritic length occurred. Ibuprofen reversed the dendritic atrophy produced by LPS administration. (l) Quantification of primary apical dendrite length. LPS significantly and permanently reduced the length of apical primary dendrites. Ibuprofen prevented the appearance of these alterations. (m) Percentage of newborn granule neurons with more than one primary apical dendrite. LPS significantly increased this percentage. The increase was restored 2 or 6 weeks after LPS withdrawal, and abolished by ibuprofen treatment. (n–p) Sholl's analysis of (n) 2-, (o) 4- and (p) 8-week-old newborn neurons. Sholl's analysis revealed that LPS altered dendritic tree morphology and branching, significantly increasing proximal branching, while a mostly unbranched distal dendritic tree was observed. These alterations were detected regardless of cell age, and also 2 and 6 weeks after LPS withdrawal. Ibuprofen treatment increased distal branching in 4- and 8-week-old neurons. Yellow scale bar, 50 μm. Green triangles, primary apical dendrite. (*, 0.01<P<0.05) (**, 0.001<P<0.01) (***P<0.001) (!, 0.05<P<0.1). GL, granule layer; H, hilus; LPS, lipopolysaccharide; ML, molecular layer; PBS, phosphate-buffered saline.
Sholl's analysis revealed that LPS altered the dendritic tree morphology of 2-, 4- and 8-week-old neurons (Figures 2n–p). This drug increased proximal branching, whereas a mostly unbranched distal dendritic tree was detected. These alterations took place regardless of cell age and recovery period. Ibuprofen treatment prevented the appearance of this aberrant morphology in 4- and 8-week-old neurons (Figures 2o and p). Individual F- and P-values are shown in Supplementary Table T2.
Newborn neuron maturation was analyzed by quantifying the percentage of neurons that expressed either the mature neuron marker NeuN or the transiently expressed neuroblast marker doublecortin (DCX). Slight variations in the percentage of NeuN+ cells were observed only in 2- and 4-week-old neurons, thus pointing to changes in maturation dynamics without affecting the final maturation process (Supplementary Figure S5E). In addition, LPS treatment produced a transient increase in the percentage of 4-week-old neurons that were DCX+. This phenomenon was prevented by ibuprofen treatment (Supplementary Figure S5A–D).
LPS administration altered postsynaptic cluster density and size. This effect was not reversed by ibuprofen treatment.
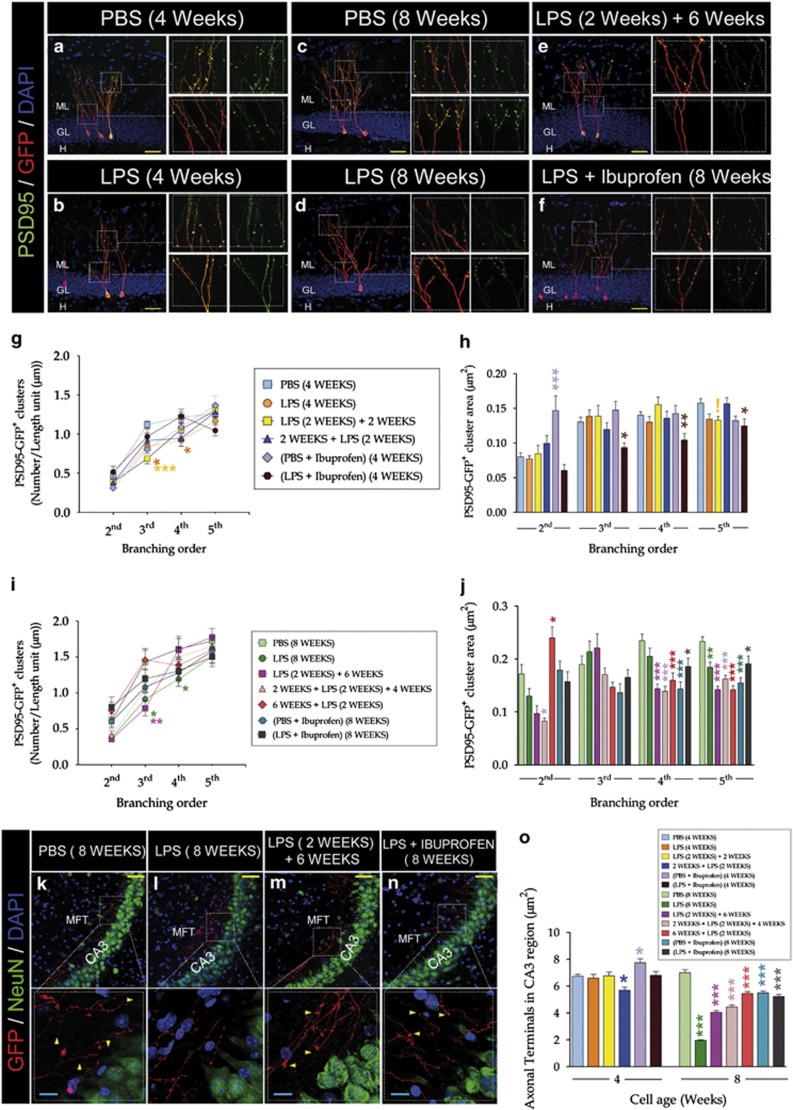
LPS slightly reduced the postsynaptic cluster density of 4- (Figures 3a, b and g) and 8- (Figures 3c and e, i and j) week-old neurons. No relevant changes in postsynaptic cluster area were detected in 4-week-old neurons (Figure 3h). However, LPS significantly and permanently decreased the distal postsynaptic cluster area of 8-week-old granule neurons (Figure 3j). Ibuprofen did not restore this reduction (Figures 3f, h and j). Individual F- and P-values are shown in Supplementary Tables T3 and T4.
Figure 3.
LPS altered postsynaptic cluster density and area, and mossy fiber terminal area in the CA3 region. PSD95:GFP-expressing retroviruses were delivered into the hippocampus, and LPS was administered during different periods, following the experimental design shown in Supplementary Figure S1. Mice were killed 4 and 8 weeks after retroviral injections to analyze newborn neuron connectivity. (a–f) Representative pictures showing retrovirus-labeled neurons of different ages belonging to the diverse experimental conditions. (g, i) Quantification of the postsynaptic cluster density of (g) 4- and (i) 8-week-old neurons. LPS slightly reduced the postsynaptic cluster density of 4- and 8-week-old neurons. (h, j) Quantification of the postsynaptic cluster area of (h) 4- and (j) 8-week-old neurons. No relevant changes in postsynaptic cluster area were observed in 4-week-old neurons (h); however, a significant and permanent decrease of distal postsynaptic cluster area was observed in 8-week-old neurons (j). This effect was not reversed by ibuprofen treatment. (k–n) Representative images showing mossy fiber terminals (MFTs) of retrovirus-labeled neurons of different ages belonging to the diverse experimental groups. (o) Quantification of mossy fiber terminal area in the CA3 region. LPS administration produced only minor changes in the mossy fiber terminal area of 4-week-old neurons but significantly and permanently decreased the presynaptic terminal area in 8-week-old ones, regardless of the cell age at which it was applied and the recovery period. ibuprofen did not counteract the aforementioned effect. Yellow scale bar, 50 μm. Blue scale bar, 10 μm. Yellow triangles, mossy fiber terminal. (*, 0.01<P<0.05) (**, 0.001<P<0.01) (***P<0.001) (!, 0.05<P<0.1). GL, granule layer; h, hilus; LPS, lipopolysaccharide; ML, molecular layer; PBS, phosphate-buffered saline.
LPS administration altered mossy fiber terminal area in the CA3 region. This effect was not reversed by ibuprofen treatment.
LPS administration produced changes in the MFT area of 4-week-old neurons (Figure 3o; F5,1967=6.15; P-value<0.001). In addition, LPS significantly and permanently decreased 8-week-old neuron MFTs regardless of the cell age at which it was applied and the recovery period (Figures 3k–m and o) (F6,2042=126.65; P-value<0.001). Ibuprofen did not counteract the aforementioned effect (Figures 3n and o).
Cellular effects of ibuprofen in a murine model overexpressing GSK-3β
As previously demonstrated, GSK-3-OE mice displayed most of the cellular alterations observed in the granule neurons of AD patients17 (see Supplementary Figures S2A and B), as well as marked brain inflammation.16 To examine whether ibuprofen prevents these alterations, the morphology and connectivity of granule neurons were examined after an 8-week treatment with this drug.
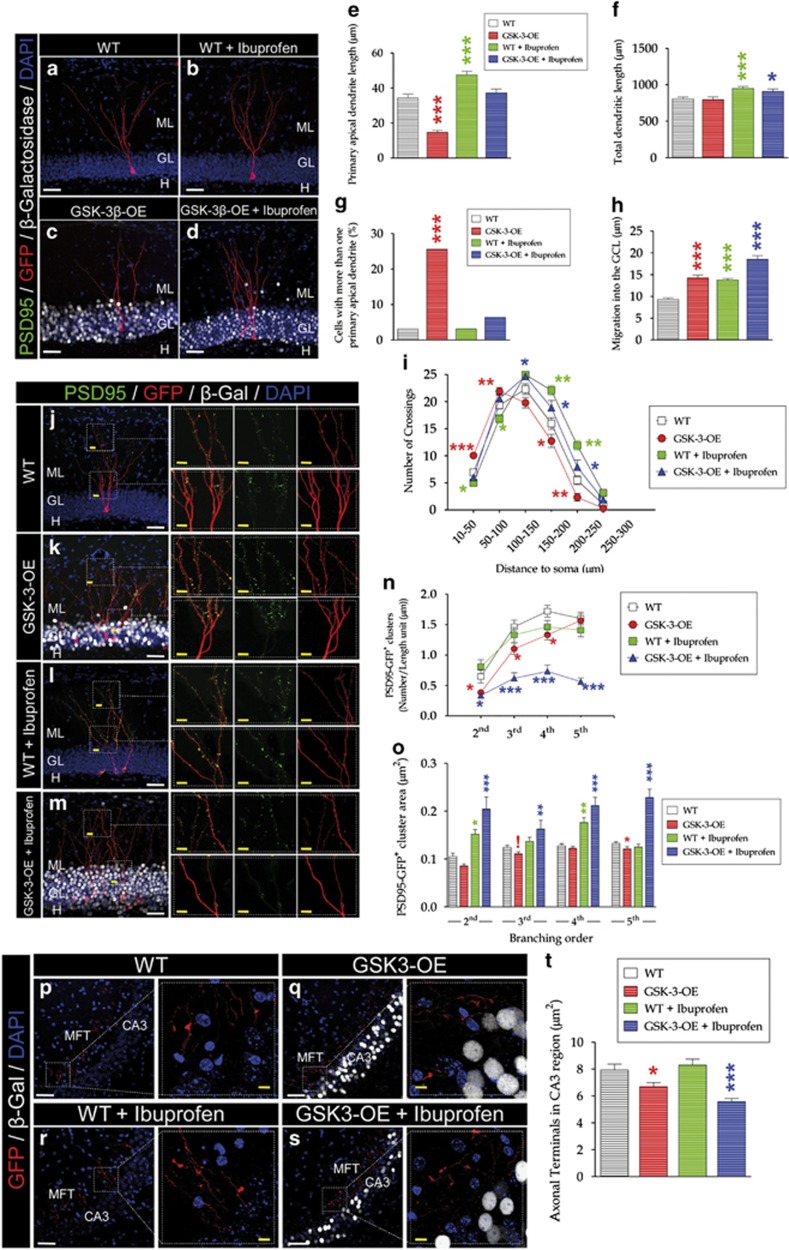
In GSK-3-OE mice, ibuprofen increased total dendritic and primary apical dendrite length and reduced the percentage of cells with more than one primary apical dendrite (Figures 4a–h). These mice also showed increased cell migration into the GL, an effect that was not reversed by ibuprofen treatment (Figure 4h; individual F- and P-values are shown in Supplementary Table T1). The altered morphology of the granule neurons of this model was completely normalized after ibuprofen treatment, as shown in Sholl's analysis diagram (Figure 4i; individual F- and P-values are shown in Supplementary Table T2).
Figure 4.
Cellular effects exerted by ibuprofen in a murine model of Alzheimer disease (AD) overexpressing GSK-3β. To study whether ibuprofen treatment reverses the cellular alterations shown by this murine model of AD, the morphology and connectivity of retrovirus-labeled granule neurons were examined after an 8-week treatment. (a–d) Representative images showing retrovirus-labeled neurons of different ages belonging to the distinct experimental groups and genotypes. (e) Quantification of primary apical dendrite length. (f) Quantification of total dendritic length. (g) Percentage of newborn neurons with more than one primary apical dendrite. Ibuprofen significantly increased total dendritic (f) and primary apical dendrite length (e) and reduced the percentage of cells with more than one primary apical dendrite (g) in GSK-3β-overexpressing (GSK-3-OE) mice. (h) Cell migration into the granule cell layer. GSK-3-OE mice also showed increased cell migration into this layer. This effect was not reversed after ibuprofen treatment. (i) Sholl's analysis. The altered morphology observed in the granule neurons of GSK-3-OE mice17 was completely normalized after ibuprofen treatment. (j–m) Representative images showing retrovirus-labeled neurons belonging to the different genotypes and treatments. (n) Quantification of postsynaptic cluster density. GSK-3-OE mice showed a reduced number of PSD95-GFP+ clusters, as previously described.17 Ibuprofen produced an enormous reduction of PSD95-GFP+ clusters in these mice, whereas it produced no change in WT counterparts. (o) Quantification of postsynaptic cluster area. Ibuprofen drastically increased the PSD95-GFP+ cluster area in GSK-3-OE mice. (p–s) Representative images showing mossy fiber terminals of retrovirus-labeled neurons belonging to the different genotypes and treatments. (t) Quantification of the mossy fiber terminal area in the CA3 region. GSK-3-OE mice showed a reduced terminal button area, which was further depleted after ibuprofen treatment. MFT, mossy fiber terminal; WT, wild type.
GSK-3-OE mice showed a reduced PSD95-GFP+ cluster density, as previously demonstrated.17 Surprisingly, ibuprofen led to an enormous additional reduction of this parameter in these animals, whereas it produced no change in WT mice (Figures 4j–n). These observations thus support the predominant role that GSK-3β had at the synapse.17 In addition, ibuprofen drastically increased the PSD95-GFP+ cluster area (Figures 4l, m and o) in GSK-3-OE mice. Individual F- and P-values are shown in Supplementary Tables T3 and T4. Regarding MFTs, GSK-3-OE mice showed a decreased terminal button area (Figures 4p, q and t), which was further reduced after ibuprofen treatment (Figures 3s and t; F3,692=16.34; P-value<0.001).
LPS- and GSK-3β-driven inflammation produced different brain cytokine expression and distinct microglial activation phenotype in response to ibuprofen treatment.
A brain expression array of 62 cytokines confirmed that peripheral LPS administration and GSK-3 overexpression produced different microglial activation phenotypes (Supplementary Figures S6 and S7). The increased expression of IL-12 and IL-10 serve as a first indicator for M1 vs M2 phenotypes respectively,22 thus suggesting the existence of a M1 microglial phenotype in the case of LPS treatment and an alternative M2 microglial phenotype in the case of neuronal GSK-3β overexpression. In addition, LPS treatment increased proinflammatory cytokine levels (IL-1, TNF, IFN, among others), while GSK-3 overexpression increased the levels not only of these cytokines but also of pro-proliferative factors (IL-10, BCL-2, among others). Ibuprofen produced a rapid shift to a neuroprotective phenotype of cytokine secretion in LPS-treated mice; however, this change did not occur in GSK-3-OE mice. This observation suggests a reduced plasticity of the microglial/endothelial/vascular system in these mice. A schematic model is shown in Supplementary Figure S7.
Both LPS administration and GSK-3β overexpression impaired pattern separation and increased anxiety-like behavior. Differential responses to ibuprofen treatment.
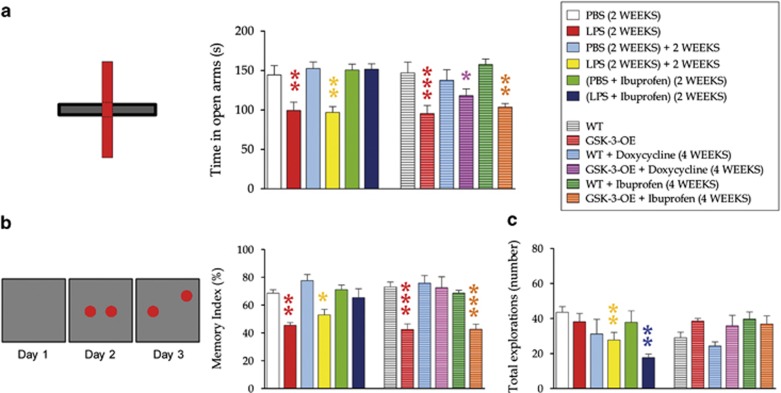
Anxiety-like behavior was evaluated using the elevated plus maze paradigm. LPS-treated animals spent less time in open arms, thus indicating increased anxiety-like behavior, which was still observable 2 weeks after withdrawal of the treatment (F5,41=11.12; P-value<0.001; Figure 5a). GSK-3-OE mice spent less time in open arms than WT mice, but these differences were abolished after doxycycline treatment (which switched off transgene expression). Surprisingly, although ibuprofen reversed the alterations produced by LPS treatment, it did not lead to any change in GSK-3-OE mice, thereby suggesting that GSK-3β has a prominent role in anxiety-like behavior (F5,34=15.38; P-value<0.001).
Figure 5.
Anxiety-like behavior and behavioral pattern separation impairment produced by LPS treatment and GSK-3β overexpression. (a) Anxiety-like behavior was evaluated in the elevated plus maze paradigm. Animals were subjected to a 5-min single trial. Data are presented as the total time spent standing or walking on the open arms. LPS significantly reduced the time the animals spent on open arms, thus indicating increased anxiety-like behavior, which was still observable 2 weeks after LPS withdrawal. GSK-3-OE mice also spent less time on open arms than WT mice. These differences were abolished by doxycycline treatment (which switched off the transgene expression). Although ibuprofen completely reversed the alterations produced by LPS treatment, it did not cause any change in GSK-3-OE mice. This observation points to a prominent role for GSK-3β in anxiety-like behavior. (b–c) To evaluate their behavioral pattern separation capacity, animals were tested in the novel location preference test. The trial was performed on three consecutive days. Each day animals were subjected to a single 10-min trial (see schematic diagram). The first day, they were placed inside the arena and were allowed to explore it for habituation. During the second day (sample phase), two identical objects were placed symmetrically in the central part of the arena. On the third day (test phase), one of the objects (novel located object) was moved to a peripheral position, whereas the other one remained unaltered. Index memory (time exploring novel located object/time exploring novel+unaltered object) is shown in the graphs. In addition, the total number of explorations is indicated. The LPS dose administered did not significantly alter the general exploratory behavior (c). However, it produced a drastic and permanent reduction of memory index (b). GSK-3-OE mice also showed a reduced memory index, which was fully restored after Doxycycline treatment. Although ibuprofen completely reversed the alterations produced by LPS treatment, it did not cause any change in GSK-3-OE mice. This finding supports the previously demonstrated prominent role of GSK-3β in hippocampal-dependent learning. (*, 0.01<P<0.05) (**, 0.001<P<0.01) (***P<0.001). LPS, lipopolysaccharide; PBS, phosphate-buffered saline; WT, wild type.
To address behavioral pattern separation capacity, animals were evaluated by means of the novel location preference test (Figure 5b). The LPS dose administered did not significantly alter exploratory behavior (Figure 5c). However, this drug caused a drastic and long-lasting impairment of pattern separation (Figure 5b), as the time exploring the newly located object decreased as compared with phosphate-buffered saline-treated mice (F5,42=10.28; P-value<0.001). Interestingly, GSK-3-OE mice also showed impaired behavioral pattern separation; however, this capacity was fully restored after doxycycline treatment. Although ibuprofen reversed the alterations produced by LPS treatment, it did not have any effect on GSK-3-OE mice, thus supporting the prominent contribution of this kinase to hippocampal-dependent learning (F5,38=14.205; P-value<0.001).
Discussion
Newborn neurons are continuously added to the hippocampal DG throughout life. Progenitor cells divide and give rise to transient amplifying precursors, which actively proliferate and differentiate into mature granule neurons, advancing through various stages of maturation. During differentiation, newborn neurons show a progressively increased dendritic tree complexity and project their axons towards the CA3 region.19 Growing evidence suggests that newborn neurons are crucial for hippocampal functioning, not only when mature but also during the transient period in which they are young and excitable.23
Numerous neurodegenerative diseases, such as AD, characterized by cognitive impairments, are also accompanied by alterations in AHN.24 The AD murine model overexpressing GSK-3β under the control of the neuronal promoter CamKII (GSK-3-OE mice) displays alterations in granule neuron morphology reminiscent of the same cells in AD patients17 (more than one apical primary dendrite and unbranched distal dendrites), increased apoptosis and a marked brain proinflammatory phenotype.18 In that study, we observed two types of effects (direct and indirect) derived from GSK-3β overexpression in these mice. Since the expression of CamKII promoter is known to begin around the fourth week after cell birth,25 and morphological alterations were observed much earlier, we proposed that the indirect effects derived from GSK-3β overexpression were mediated by the local proinflammatory environment.17
It has been extensively demonstrated that the inflammatory microenvironment associated with microglial activation strongly influences newborn neuron survival, maturation and recruitment into behaviorally relevant circuits.4 To test the extent to which a low-grade, chronic, proinflammatory environment, similar to that described in the AD patient brain, affects newborn neuron maturation, we peripherally administered LPS from E. coli endotoxin for different lengths of time to C57BL/6 mice, and then examined the morphology and connectivity of retrovirus-labeled newborn neurons. Chronic peripheral LPS treatment produced morphological abnormalities in granule neurons that were similar to those described in GSK-3-OE mice and, more relevantly, to those found in AD patients. Unexpectedly, most of these morphological alterations were irreversible (such as primary dendrite shortening and distal dendrite atrophy). Interestingly, distal dendrite atrophy appeared to be a common feature produced by inflammatory processes of diverse origin26 and occurred regardless of the cell age at which LPS was administered. To the best of our knowledge, this is the first evidence that peripherally triggered brain inflammation during adulthood produces such long-lasting effects on newborn neuron morphology. Jurgens et al.26 demonstrated that influenza infection leads to morphological alterations in granule neurons. Although neuron age was not taken into account in that study, Sholl's analysis of Golgi-stained neurons revealed a marked distal dendrite retraction. Another common feature systematically reported in several animal models of brain inflammation is the aberrant migration of newborn neurons.3,4,27 Here we have demonstrated the irreversibility of this process (at least after 6 weeks) and the independence of the cell age at which the proinflammatory stimulus was applied. In addition, ibuprofen treatment did not reverse the aberrant migration of newborn granule neurons, which has been proposed to be neuroprotective under certain neurodegenerative conditions.3
Brain inflammation, a hallmark of the AD brain and present in both familial and sporadic forms of the disease, is considered to be one of the most important risk factors for sporadic AD development.8 In this regard, NSAIDs have attracted considerable interest during recent decades, since it has been demonstrated that rheumatoid arthritis patients on long-term NSAID treatment show a decreased incidence of AD.7 This observation is supported by other retrospective analyses.10, 11, 12, 13 Numerous data obtained from AD animal models indicate that NSAIDs could be used to treat and prevent AD;7,28 however, prospective clinical trials have failed to demonstrate such beneficial effects in humans.29 As contradictory data regarding Tau- and amyloid-β-related pathology have also been provided using various animal models,9,30,31 here we aimed to study the effects of the nonselective COX-1/COX-2 inhibitor ibuprofen on GSK-3-OE mice. Ibuprofen treatment completely reversed the aberrant granule neuron morphology described in these mice.17 This finding thus supports the notion that morphological alterations are an indirect consequence of GSK-3β overexpression and are mediated by a local proinflammatory microenvironment. Although no negative effects on basal transmission have been reported after ibuprofen treatment, the presence of neuronal COX-2 at the synapse32 points to the active role of this molecule in synaptic transmission and may account for the deleterious effect of its inhibition on the proper functioning of postsynaptic densities (PSDs).28
Regarding connectivity alterations, here we addressed both PSDs and MFTs. LPS treatment produced slight variations in the number of PSDs located in the central domain of the dendritic tree, consistent with the proximal alterations described in Influenza infection.26 However, LPS treatment caused a drastic reduction of PSD area in the distal portions of the dendritic tree, which presumably receive the main afferent connections of these cells (perforant pathway from entorhinal cortex). Although ibuprofen partly rescued the alterations in PSD number, it was unable to normalize the PSD area. This observation thus supports the essential role of microglia in spine pruning and synaptic maturation,33,34 as the observed reduction of PSD area might reveal a decrease in the average PSD size or, alternatively, an increased elimination of small synaptic contacts, which are most often engulfed by microglial cells.33
Interestingly, there was no previous evidence of alterations of newborn neuron MFT areas after LPS treatment. Taken together, the reduction in MFT area produced by LPS and GSK-3β overexpression, as well as the additional decrease after ibuprofen treatment in both cases, supports the general idea that inflammation directly affects the maturation of newborn neurons and the functional integration of these cells into hippocampal circuits.3,4
One of the most novel and striking results of this work is the dramatic reduction in the PSD number of GSK-3-OE mice treated with ibuprofen. In contrast, this drug led to a marked increase in PSD area (in contrast to the lack of effect observed in LPS-treated mice), which can be interpreted as a compensatory mechanism to synaptic loss. Alternatively, it can be hypothesized that a different type of microglial activation takes place as a result of ibuprofen treatment in these mice, resulting in an increase in the engulfment and elimination of small, more labile33 PSDs. This observation supports the multifaceted interaction between microglia and newborn neuron maturation.35 Interestingly, GSK-3β triggers synapse elimination.36,37 Under such adverse conditions, it is therefore reasonable that COX-1 or COX-2 inactivation may have additional deleterious effects on synapse number.28
The differences found between LPS-stimulated and GSK-3-OE mice treated with ibuprofen points to the presence of distinct microglial activation phenotypes in these two scenarios. Under physiological conditions, microglia show a resting phenotype (ramified morphology with many fine processes and small cell body38). In response to proinflammatory stimuli, microglial cells alternate between two types of activation phenotypes, named M1 and M2. The former, also known as ‘classical' microglial activation, involves the secretion of proinflammatory mediators such as IL-1α/β, TNF-α, IFN-γ, MCP-1, IL-12 and IL-6.39, 40, 41 Aging,42,43 infection,3,9 chronic neurodegeneration44 and ischemia39 promote the M1 phenotype. In contrast, M2 or ‘alternative' microglial activation, is characterized by the expression of antiinflammatory cytokines such as IL-4, IL-10 or IL-13. M2 is considered to be neuroprotective,3 and a dynamic shift to this phenotype has been observed after proinflammatory stimulus cessation,39 NSAID treatment44 and during low-grade chronic inflammation, as occuring in the early stages of AD.45 Consistent with the abundant literature, after LPS treatment we found a clear M1 phenotype, which was abolished (and substituted by a M2 phenotype) by ibuprofen treatment. However, one of the most novel and striking findings of this study is that GSK-3β overexpression-driven brain inflammation generated a completely different pattern of cytokine expression. In this regard, not only proinflammatory (IL-1, TNF-α, KC, IFN-γ, among others) but also antiinflammatory and pro-survival (IL-10, BCL-2) factor levels were increased in the GSK-3-OE mouse brain. A similar hybrid phenotype has been described in other AD animal models.39,46 Despite the hybrid phenotype of microglia in GSK-3-OE animals, the effect of ibuprofen was attenuated compared with its effect on LPS-treated mice. Proinflammatory cytokine levels did not differ significantly between ibuprofen-treated and nontreated GSK-3-OE mice. This is a relevant observation, and it suggests that the dysregulation of GSK-3β activity induces the presence of a less sensitive microglial/vascular/endothelial system that is not prone to shift to a pure neuroprotective phenotype, as GSK-3β per se is a strong proinflammatory stimulus.47 In this regard, it has been proposed that both physiological aging and AD make microglia less responsive and senescent,42 possibly because of continuous microglial priming.
It is expected that the putative benefits exerted by ibuprofen treatment derive in cognitive improvement. As previously demonstrated, brain inflammation dramatically impairs hippocampal-dependent learning,48 specifically behavioral pattern separation.4,27,49 In addition, human studies50 and animal models of AD7,31 also demonstrate a clear negative relationship between inflammation and cognitive skills. Here we have shown that chronic low doses of peripherally administered LPS impair behavioral pattern separation and increase anxiety-like behavior, without markedly affecting general exploratory behavior. These impairments were still observable 2 weeks after LPS withdrawal, in line with findings by Ormenod et al.,49 who demonstrated long-lasting spatial memory disruption 5–6 weeks after acute LPS administration. In our study, ibuprofen treatment did not have any effect on the behavior of phosphate-buffered saline-treated mice, but completely reversed anxiety-like behavior and restored behavioral pattern separation in LPS-treated animals. In this regard, microglial COX-1 inhibition is sufficient to rescue LPS-triggered acute cognitive impairments.51 This observation suggests that the effects of ibuprofen on hippocampal-dependent learning are related to its capacity to inhibit COX-1 activity. GSK-3-OE mice show impaired hippocampal-dependent learning.16,17 However, our study provides the first evidence of increased anxiety-like behavior and impaired hippocampal-dependent behavioral pattern separation, both highly dependent on AHN.52 These two behaviors were completely normalized by doxycycline treatment (which switched off GSK-3β overexpression); however, neither was reversed by ibuprofen treatment. Although a causal relationship cannot be assured, we propose that the absence of positive effects exerted by ibuprofen on newborn neuron connectivity could account for the lack of behavioral improvement, as AD is thought to be primarily a synaptopathy, in which cognitive impairments are intimately linked to synaptic loss.53 We consider that these data may be of great clinical relevance and that they should be taken into account for designing efficient strategies to palliate the progression of this disease. Doxycycline switches off GSK-3β overexpression and has been demonstrated to be effective in restoring the morphology and connectivity of granule neurons, and normalizing diverse behaviors.17 Thus an integrative strategy including both antiinflammatory and GSK-3-β inhibitors may provide a novel approach to pharmacological interventions for AD.
Acknowledgments
This study was funded by grants from the Spanish Ministry of Health (SAF 2006-02424, BFU-2008-03980, BFU-2010-21507), the Comunidad de Madrid (SAL/0202/2006), the Fundación M. Botín, the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED, ISCIII) and the Fundación R. Areces. We thank ME Vera, E Hevia and J Palacín for help regarding animal protocols; E Langa for help with biochemical determinations; R Cuadros for help with electron microscopy; E García for help producing retroviral vectors; the Cajal Institute Behavioral Unit for help with the elevated plus maze device; N de la Torre for help with manuscript edition; FH Gage for providing the plasmids used for the production of GFP-expressing retroviruses; and C Lois for providing the plasmids used for the production of the PSD95-GFP virus. Human control samples were generously provided by the Biobanco del Hospital Universitario Reina Sofia (Córdoba, Spain). We thank Dr R Sánchez Sánchez for facilitating access to human control samples. AD samples were generously provided by the Banco de Tejidos de la Fundación CIEN (Madrid, Spain).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Rosi S. Modulation of adult-born neurons in the inflamed hippocampus. Front Cell Neurosci. 2013;7:145. doi: 10.3389/fncel.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, et al. Trauma-induced alterations in cognition and Arc expression are reduced by previous exposure to 56Fe irradiation. Hippocampus. 2012;22:544–554. doi: 10.1002/hipo.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer's Disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Bras J, Hardy J. Snapshot: genetics of Alzheimer's disease. Cell. 2013;155:968–e961. doi: 10.1016/j.cell.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiol Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Jenkins BG, Carreras I, Kaymakcalan S, Cormier K, Kowall NW, et al. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp Neurol. 2010;223:377–384. doi: 10.1016/j.expneurol.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe GA, Grayson DA, Creasey HM, Waite LM, Casey BJ, Bennett HP, et al. Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol. 2000;57:1586–1591. doi: 10.1001/archneur.57.11.1586. [DOI] [PubMed] [Google Scholar]
- in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA. Nonsteroidal anti-inflammatory drug use and Alzheimer's disease risk: the MIRAGE Study. BMC Geriatr. 2005;5:2. doi: 10.1186/1471-2318-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Engel T, Hernandez F, Avila J, Lucas JJ. Full reversal of Alzheimer's disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Fuster-Matanzo A, Teixeira CM, Jurado-Arjona J, Ulloa F, Defelipe J, et al. GSK-3beta overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Mol Psychiatry. 2013;18:451–460. doi: 10.1038/mp.2013.4. [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martín M, Sirerol-Piquer MS, García-Verdugo JM, Avila J, Hernández F. Dual effects of increased glycogen synthase kinase-3beta activity on adult neurogenesis. Hum Mol Genet. 2013;22:1300–1315. doi: 10.1093/hmg/dds533. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Lin CW, Lois C. Sequential development of synapses in dendritic domains during adult neurogenesis. Proc Natl Acad Sci USA. 2008;105:16803–16808. doi: 10.1073/pnas.0807970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirerol-Piquer M, Gomez-Ramos P, Hernández F, Perez M, Morán MA, Fuster-Matanzo A, et al. GSK3beta overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus. 2011;21:910–922. doi: 10.1002/hipo.20805. [DOI] [PubMed] [Google Scholar]
- Gertig U, Hanisch UK. Microglial diversity by responses and responders. Front Cell Neurosci. 2014;8:101. doi: 10.3389/fncel.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J. Young and excitable: new neurons in memory networks. Nat Neurosci. 2007;10:273–275. doi: 10.1038/nn0307-273. [DOI] [PubMed] [Google Scholar]
- Chatrchyan S, Khachatryan V, Sirunyan AM, Tumasyan A, Adam W, Bergauer T, et al. Search for B(s)(0) —> mu+ mu- and B(0) —> mu+ mu- decays in pp collisions at sqrt[s]=7 TeV. Phys Rev Lett. 2011;107:191802. doi: 10.1103/PhysRevLett.107.191802. [DOI] [PubMed] [Google Scholar]
- Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe-Chomette P, Zeidan S, Belarbi N, Van Der Meer G, Oury JF, El-Ghoneimi A. Female covered urethral duplication with urogenital sinus. Urology. 2012;79:e3–e5. doi: 10.1016/j.urology.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Choi S.H, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann A, Hahn S, Schilling S, Hoffmann T, Demuth HU, Bulic B, et al. No improvement after chronic ibuprofen treatment in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging. 2012;33:e839–e850. doi: 10.1016/j.neurobiolaging.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Ho Kim K, Min Son S, Mook-Jung I. Contributions of microglia to structural synaptic plasticity. J Exp Neurosci. 2013;7:6. doi: 10.4137/JEN.S11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T. Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the 'Dr. Jekyll and Mr. Hyde concept' of Alzheimer's disease or the yin and yang of neuroplasticity. Prog Neurobiol. 2003;71:83–248. doi: 10.1016/j.pneurobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Ruiz CR, Lebson L, Selenica ML, Rizer J, Hunt JB, Jr, et al. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol Aging. 2013;34:1610–1620. doi: 10.1016/j.neurobiolaging.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer's disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Guillot-Sestier MV, Town T. Brain injury, neuroinflammation and Alzheimer's disease. Front Aging Neurosci. 2013;5:26. doi: 10.3389/fnagi.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C.A, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD. PPARγ activation prevents impairments in spatial memory and neurogenesis following transient illness. Brain Behav Immun. 2013;29:28–38. doi: 10.1016/j.bbi.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Skelly DT, Murray CL, Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33:15248–15258. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. BDNF in the Dentate Gyrus Is Required for Consolidation of "Pattern-Separated" Memories. Cell Rep. 2013;5:759–768. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rábano A, Avila J, et al. The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer's disease. Brain. 2013;136:1913–1928. doi: 10.1093/brain/awt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.