Abstract
Alveolar echinococcosis is the most serious helminthic zoonosis of the northern hemisphere affecting humans. The causative agent, Echinococcus multilocularis, occurs in geographically distinct strains that can be distinguished based on sequence variations of mitochondrial genes and nuclear microsatellite targets. This report finds that the human case of Alveolar echinococcosis occurring in Minnesota in 1977 was caused by the N2 haplotype described previously; the N2 haplotype is distinct from European and Asian isolates of the parasite and is restricted to the central region of North America.
Echinococcus multilocularis is a tapeworm whose lifecycle includes a canid definite host and a small mammal intermediate host.1 Accidental ingestion of E. multilocularis eggs by humans (i.e., an aberrant host) can lead to alveolar echinococcosis (AE), the most serious helminthic zoonosis of the northern hemisphere, with an incidence of more than 18,000 cases per year2 and a mortality rate > 90% if left untreated.1,3
The parasite has a wide geographical distribution across the northern hemisphere and it exists in geographically distinct strains that can be distinguished based on sequence variations of mitochondrial genes4 or based on polymorphism of microsatellites.5,6 It is possible to distinguish between isolates obtained from North America and Europe only using specific markers,4,5 as not all markers do not allow such a distinction.6 Given the recent detection of the European isolate of E. multilocularis in Canada,7 questions have been raised on what is the extent of the distribution of this strain in North America and how long these European isolates have been present in this continent. Certainly, the presence of allochthonous strains in North America and the fact that the incidence of human AE cases is different for different strains, impose to put more effort in characterizing the origin of infections in humans. Similarly, the detection of human cases from North American strains would pose serious questions both on the pathogenicity of this autochthonous strain and on the possibility that AE might have been overlooked.
Based on sequence variation of mitochondrial genes, two North American isolates have been described by Nakao and co-workers4: the N1 haplotype that was found in voles from Alaska (St. Lawrence Island), and the N2 haplotype that was identified in samples originating from Indiana (five adult parasites harbored by a red fox) and South Dakota (adult parasite, laboratory strain). In addition to the endemic region of St. Lawrence Island, Alaska,8 only three cases of presumed locally acquired AE in human patients have been described in North America.9–11 The first case was a fisherman from Manitoba, Canada,11 who presented with an abdominal mass in 1928, the second case was a 56-year-old female from Minnesota that presented for abdominal pain in 1977,9 and the third case was an immunosuppressed 49-year-old patient from Alberta, Canada in 2013.10
Yamasaki and co-workers12 sequenced the mitochondrial gene cox1 of a metacestode originating from the human patient diagnosed with AE in Minnesota in 1977. The DNA was extracted from formalin fixed paraffin embedded tissue and the cox1 gene was amplified through polymerase chain reaction. To account for the fact that the specimen had been fixed in formalin, multiple small regions were amplified using multiple primer pairs resulting in amplicons varying in size from 100 to 216 base pairs and a continuous sequence of 1,608 base pairs. The aim of the current report was to compare this sequence with the sequence data published by Nakao and co-workers4 to determine which geographical isolate the human case was most closely related to. The Basic Local Alignment Search Tool (BLAST) program was used to align the sequence of the human isolate in question (AB374425.1; E. multilocularis mitochondrial cox1 gene for cytochrome c oxidase subunit 1, complete cds) and the sequence of the North American, central region isolate (AB461419.1; E. multilocularis mitochondrial cox1 gene for cytochrome c oxidase subunit 1, complete cds, country: USA:Indiana). Figure 1 depicts the nucleotide substitutions of cox1 that differ between Asian, European, and North American haplotypes and shows the substitutions of the N2 haplotype (numbering is based on AB461419.1). The sequence from the Minnesota case and the sequence from Indiana were found to be 100% identical revealing that the human case in 1977 was caused by the N2 haplotype.
Figure 1.
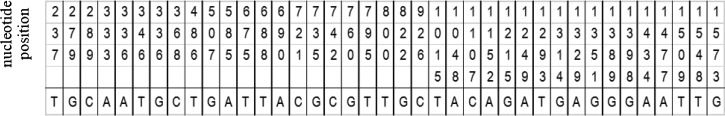
Nucleotide positions of cox1 that differ between Asian, European, and North American haplotypes of Echinococcus multilocularis. Shown is the haplotype of N2; the DNA sequence for cox1 obtained from the metacestode of a human patient in Minnesota is 100% identical. The upper row shows the nucleotide position (the base pairs of the cox1 gene are numbered consecutively) and the lower row shows the nucleotide present in the N2 strain at each respective position.
Therefore, in accordance with the finding by Yamasaki and colleagues12 that reported almost identical sequences between a South Dakota isolate retrieved from a fox and the Minnesota human case, we can now confirm that those sequences belong to what has been defined by Nakao et al. as the N2 Central Region haplotype.
Assessing the strain of human AE cases in North America has become increasingly relevant because European haplotypes have recently been found in wild carnivores7 and domestic dogs in Western and Central Canada (British Columbia, Ontario, and Saskatchewan13,14; Audrey Tataryn, personal communication). The pathogenicity of the European strain of E. multilocularis for humans is not disputed. Given the apparent low number of human AE cases in North America, the pathogenicity of the North American strain has been questioned, although without a proper epidemiological study. This report however shows that the North American, central region isolate indeed can cause disease in humans and highlights the importance, to not only to make precise diagnoses of human cases of Echinococcosis at the species level, but also to determine the strain that is causing the infections and investigate the origin of infection of each single AE case in humans. With the emergence of the pathogenic European strain in wild and domestic hosts, there is the potential for increases in human AE cases in the central region of North America. From a public health perspective, it is then a priority to properly assess the level of risk for increasing numbers of human AE cases. We recommend both a retrospective study of all the human cases that until now have been diagnosed with Echinococcosis without further distinction between E. multilocularis or E. granulosus cases, and the establishment of a surveillance system to monitor the origin of all the new human cases to follow possible trends in cases caused by the different strains.
Footnotes
Authors' addresses: Claudia Klein, University of Calgary, Department of Veterinary Clinical and Diagnostic Sciences, Calgary, Alberta, Canada, E-mail: claudia.klein@ucalgary.ca. Alessandro Massolo, University of Calgary, Department of Ecosystem and Public Health, Calgary, Alberta, Canada, E-mail: amassolo@ucalgary.ca.
References
- 1.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of Echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig PS, McManus DP. Echinococcosis: transmission biology and epidemiology - Preface. Parasitology. 2003;127((Suppl 1)):S1. [PubMed] [Google Scholar]
- 4.Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y, Ito A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int. 2009;58:384–389. doi: 10.1016/j.parint.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Bart JM, Knapp J, Gottstein B, El-Garch F, Giraudoux P, Glowatzki ML, Berthoud H, Maillard S, Piarroux R. EmsB, a tandem repeated multi-loci microsatellite, new tool to investigate the genetic diversity of Echinococcus multilocularis. Infect Genet Evol. 2006;6:3–400. doi: 10.1016/j.meegid.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Haag KL, Zaha A, Araujo AM, Gottstein B. Reduced genetic variability within coding and non-coding regions of the Echinococcus multilocularis genome. Parasitology. 1997;115:521–529. doi: 10.1017/s0031182097001649. [DOI] [PubMed] [Google Scholar]
- 7.Gesy K, Hill JE, Schwantje H, Liccioli S, Jenkins EJ. Establishment of a European-type strain of Echinococcus multilocularis in Canadian wildlife. Parasitology. 2013;140:1133–1137. doi: 10.1017/S0031182013000607. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JF, Rausch RL. Alveolar hydatid-disease: a review of clinical-features of 33 indigenous cases of Echinococcus multilocularis infection in Alaskan Eskimos. Am J Trop Med Hyg. 1980;29:1340–1355. [PubMed] [Google Scholar]
- 9.Gamble WG, Segal M, Schantz PM, Rausch RL. Alveolar hydatid disease in Minnesota. First human case acquired in the contiguous United States. JAMA. 1979;241:904–907. doi: 10.1001/jama.241.9.904. [DOI] [PubMed] [Google Scholar]
- 10.Kowalewska K, Sis B, Massolo A. Rounds LMPG . Faculty of Medicine and Dentistry, University of Alberta; Edmonton, AB: 2013. Biology and ecology of Echinococcus multilocularis: a public health concern at the interface between wildlife, domestic animals and humans? [Google Scholar]
- 11.James E, Boyd W. Echinococcus alveolaris (with the report of a case) Can Med Assoc J. 1937;36:354–356. [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki H, Nakao M, Nakaya K, Schantz PM, Ito A. Genetic analysis of Echinococcus multilocularis originating from a patient with alveolar echinococcosis occurring in Minnesota in 1977. Am J Trop Med Hyg. 2008;79:245–247. [PubMed] [Google Scholar]
- 13.Peregrine AS, Jenkins EJ, Barnes B, Johnson S, Polley L, Barker IK, De Wolf B, Gottstein B. Alveolar hydatid disease (Echinococcus multilocularis) in the liver of a Canadian dog in British Columbia, a newly endemic region. Can Vet J. 2012;53:870–874. [PMC free article] [PubMed] [Google Scholar]
- 14.Skelding A, Brooks A, Stalker M, Mercer N, de Villa E, Gottstein B, Peregrine AS. Hepatic alveolar hydatid disease (Echinococcus multilocularis) in a boxer dog from southern Ontario. Can Vet J. 2014;55:551–553. [PMC free article] [PubMed] [Google Scholar]


