Abstract
Dengue is a potentially fatal acute febrile illness caused by the mosquito-borne dengue viruses (DENV-1 to -4). To estimate DENV seroincidence in school-aged children, a 1-year prospective cohort study was conducted in Patillas, Puerto Rico; 10- to 18-year-olds (N = 345) were randomly selected from 13 public schools. At enrollment, 49.8% of the entire cohort had DENV immunoglobulin G (IgG) anti-DENV antibodies, and there were individuals with neutralizing antibodies specific to each of the four DENV. The mean age of participants with incident DENV infection was 13.4 years. The 1-year seroincidence rate was 5.6%, and 61.1% of infections were inapparent. Having IgG anti-DENV at enrollment was associated with seroincidence (risk ratio = 6.8). Acute febrile illnesses during the study period were captured by a fever diary and an enhanced and passive surveillance system in the municipios of Patillas and Guayama. In summary, at enrollment, nearly one-half of the participants had a prior DENV infection, with the highest incidence in the 10- to 11-year-olds, of which most were inapparent infections, and symptomatic infections were considered mild.
Introduction
Dengue is a febrile illness caused by any one of four mosquito-borne dengue viruses (DENV-1 to -4), with the outcome of infection ranging from inapparent infection to fatal illness. Nearly 40% of the world's population lives in areas where the primary mosquito vectors—Aedes aegypti and Ae. albopictus—are prevalent and transmit DENV.1 According to estimates from 2010 from Bhatt and others, globally, over 2.5 billion people are estimated to live at risk of DENV infection, with 390 million infections per year, of which 96 million people develop symptoms.2–5
Since the emergence of dengue in the US territory of Puerto Rico in the early 1960s, specific dengue prevalence on the island has been well-documented. The majority of information has come from data obtained through the Puerto Rico passive dengue surveillance system (PDSS) and several seroepidemiologic studies.6–15 These studies have shown that the highest incidence of disease is among 10- to 19-year-olds and that disease incidence varies by year and municipality. However, PDSS is not capable of detecting inapparent DENV infection, and none of the previous seroepidemiologic studies described seroincidence of inapparent DENV infections among school-aged children and adolescents.
In June of 2005, to provide a better epidemiologic understanding of dengue in a defined population, an enhanced dengue surveillance system (EDSS) was implemented in Patillas, a municipality with historically high rates of dengue.15 Within this comprehensive surveillance system, we implemented a 1-year prospective cohort study to determine the seroprevalence and seroincidence of symptomatic and inapparent DENV infections among school-aged children and adolescents residing in Patillas.
Materials and Methods
Study site and population.
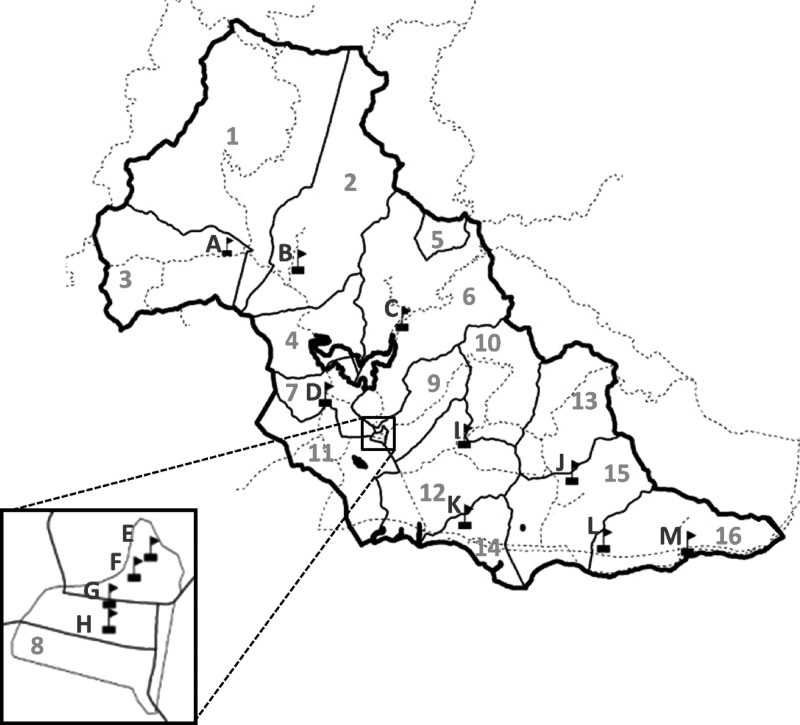
Dengue is endemic in Puerto Rico, where cases are detected every month of the year and transmission peaks during the rainy season (June to November).15 This study was conducted between May of 2007 and May of 2008 in the southeastern coastal municipality of Patillas, Puerto Rico (18°002′1.1″ N, 66°00′52.0″ W). Patillas is a semi-rural municipality with a population of 20,152 and an estimated 10- to 19-year-old population of 3,569 (US Census from 2010). Patillas has three private medical clinics and one principal ambulatory health center, which is the operational site for EDSS.9,15 The nearest acute care hospitals are located in adjacent municipalities. The location of Patillas neighborhoods and public schools are shown in Figure 1.
Figure 1.
Map of the participating schools and neighborhoods of Patillas, Puerto Rico in 2007 and 2008. Black boxes with flags represent participating schools. The heavy black solid line represents the borders of the municipality of Patillas. The light solid black lines represent the border for each Patillas neighborhood. The dotted black lines represent major roads. The letters represent the school designation, and the numbers represent neighborhood designations. Neighborhood 8 (Inset) is the urban center of Patillas, where four of the participating public schools (E–H) are located.
Study design.
We conducted a 1-year prospective cohort study of children and adolescents (10- to 18-year-olds) attending public school and residing in Patillas. Participants were tested for serologic evidence of DENV infection at the beginning and end of the study by the presence of immunoglobulin G (IgG) anti-DENV as detected by enzyme-linked immunosorbent assay (ELISA). Microneutralization test (MNT) was used to differentiate monotypic from heterotypic DENV immune profiles at the beginning and end of the study. Participants were monitored for occurrence of acute febrile illness (AFI) by EDSS and PDSS, parent/participant phone interviews conducted at 6 and 12 months, and review of hospital records.
Sample selection.
One-stage cluster sampling was used to select students stratified by grades 5–11 (as a proxy for age) from all 13 Patillas public schools. Homeroom classes were used as the primary sampling unit and chosen by simple random selection within each grade without replacement. All students (N = 812) from selected homerooms (N = 40) between the ages of 10 and 18 years who were residents of Patillas were invited to participate.
Recruitment and enrollment.
Orientation presentations were given in Spanish to selected students and their parents/guardians. After obtaining signed student assent and parental consent, a serum specimen was collected, and an enrollment questionnaire was administered that captured information on demographic characteristics, recent febrile illness, past arboviral illness diagnoses, and vaccination history. A fever notebook memory aid and instructions on how to record the occurrence of all AFIs experienced during the study period were given to each participant. Each participant was also given a study identification card with instructions for treating physicians to report suspected dengue or AFIs to EDSS or PDSS.
AFI monitoring.
AFIs during the study period were captured by EDSS/PDSS database, hospital record review, and telephone interviews at 6 and 12 months. Diagnostic testing and submission of serum samples to PDSS relies on healthcare provider-initiated requests. All specimens submitted to PDSS are accompanied by a Dengue Case Investigation Report (DCIR) from suspected dengue case-patients to the Centers for Disease Control and Prevention (CDC) Dengue Branch Laboratories. Submission of specimens to EDSS is initiated through the recruitment of patients of all ages presenting with an AFI at the Centro de Servicios Primarios de Salud de Patillas (CSPSP), which is the principal health center and emergency department for Patillas and hosts PDSS. CSPSP is located in the urban center of Patillas and includes an emergency department, an appointment only continuity clinic, and a non-appointment walk-in extended hours clinic. The majority (87%) of Patillas residents receive their primary health care from CSPSP. AFI was defined as documented fever of 38°C or higher at presentation to the emergency department or history of fever that had persisted for 2–7 days without an identified source. Patients who met the case definition for AFI were enrolled in the EDSS by onsite CDC staff to identify and collect clinical information by the completion of the DCIR.
Telephone interviews captured information on interim vaccinations, illnesses, and hospitalizations. Hospital medical record abstractions (using a standardized form) conducted after 12 months were used to capture information on illnesses, clinical course, and outcome in hospitalized participants. Participants not completing the 12-month telephone interview were interviewed using a standardized questionnaire during the 12-month blood specimen collection.
Specimen handling and testing.
A 10-mL venous blood specimen was collected from each participant at the beginning of the study and the 12-month follow-up. Specimens were stored at 4°C until the serum was separated and tested for IgM antibody against DENV (IgM anti-DENV); remaining serum was stored at −20°C. Additional testing for IgG anti-DENV was performed simultaneously on the enrollment and 12-month specimens.
Anti-DENV IgM Antibody Capture (MAC) ELISA was performed as described by Martin and others.16 A positive-to-negative (P/N) ratio ≥ 2.0 was considered a positive result.
IgG Anti-DENV Indirect ELISA was performed as described by Miagostovich and others.17 Enrollment and 12-month specimens were pre-tested at a 1:100 dilution, and those found positive (optical density at 405 nm [OD405] ≥ 0.15) were titrated together to determine the endpoint titer using fourfold dilutions starting from 1:40 and ending at 1:655,360.
MNT, as described by Vorndam and Beltran,18 was performed on paired specimens from study participants whose enrollment anti-DENV IgG ELISA was positive at a 1:100 dilution or specimen pairs from participants with an IgG anti-DENV ELISA titer > 1:40 in any specimen. Briefly, 96-well plates were seeded with Vero cells (ATCC) suspended in Enhanced-M199 media at a concentration of 2 × 105 cells/mL and incubated at 37°C in 10% CO2 for 2 days. Serum specimens were diluted in 30% fetal bovine serum in phosphate-buffered saline, inactivated at 56°C for 30 minutes, and diluted two-fold from 1:80 to 1:2,560. Serum samples and DENV laboratory strains (DENV-1 [Hawaii], DENV-2 [New Guinea-C], DENV-3 [H87], and DENV-4 [H241]) were incubated for 2 hours at room temperature, then added to 96-well Vero plates, and incubated for 5 days at 37°C in a 10% CO2 incubator. DENV laboratory strains were used at the multiplicities of infection (MOIs; DENV-1 MOI = 0.1, DENV-2 MOI = 0.01, DENV-3 MOI = 0.1, and DENV-4 MOI = 0.02) that provided an OD value > 1 for virus control. After incubation, the 96-well plates were fixed in a 1:1 mixture of acetone and methanol for 20 minutes, and the ELISA was performed as previously described.18 Plates were read on a BioTek plate reader at 405 nm. When the virus control reached an OD value of 1.0–1.3, the plate was read, and the sample was compared with the virus control. A positive result is based on a value below 2 SDs of OD from virus control OD. When the MNT results was negative at 1:80 and the anti-DENV IgG ELISA was positive, the MNT was repeated starting at a lower dilution of 1:20. Specimens with results above the maximum titer tested were reported as > 2,560, and specimens below the minimum titer tested were reported as < 80.
Dengue diagnostic testing.
Serum specimens submitted to EDSS or PDSS from study participants presenting with AFI or suspected dengue were tested for evidence of current or acute DENV infection with reverse-transcription polymerase chain reaction (RT-PCR) and anti-DENV IgM antibodies.9,15
Laboratory definitions.
DENV infection, single serotype (i.e., a monotypic neutralization pattern) was a neutralization titer ≥ 1:80 (or ≥ 1:20 in a retested specimen) to only one DENV in a single specimen. DENV infection, serotype undetermined (i.e., a heterotypic neutralization pattern) was a neutralization titer ≥ 1:80 (or ≥ 1:20 in a retested specimen) to two or more DENVs in a single specimen. Predominant DENV was the DENV serotype with a four-fold-higher neutralization titer compared with the other remaining DENV serotypes. Incident DENV infection was a change from negative to positive or a more than or equal to four-fold rise in anti-DENV IgG ELISA titers between paired specimens. Incident primary DENV infection was an anti-DENV IgG ELISA seroconversion from negative to positive in paired specimens. Incident secondary DENV infection was a four-fold or greater rise in anti-DENV IgG ELISA titer in paired specimens.
Clinical definitions.
AFI was any self-reported acute illness with subjective fever or a recorded fever of ≥ 38.0°C lasting ≤ 7 days. Dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) were defined according to the 1997 World of Health Organization (WHO) guidelines.19 DF with hemorrhagic manifestations was a self-reported AFI that fulfilled criteria for DF with evidence of hemorrhage (i.e., petechiae, gum bleeding, nose bleeding, hematuria, menorrhagia, hematemesis, or melena). Undifferentiated febrile illness (UFI) was self-reported AFI not fulfilling the criteria for DF, DF with hemorrhagic manifestations, DHF, or DSS. Inapparent DENV infection was an incident DENV infection with no reported clinical signs or symptoms.
Statistical analyses.
When inferring parameters of the population (i.e., the schoolchildren surveyed), we followed standard survey methodology and incorporated sampling design and finite population correction when computing point estimates, SEs, and confidence intervals (CIs). Design-specific survey calibration was used to improve rate, proportion, ratio, and CI estimates using ancillary information from the Patillas public school population (i.e., joint age and sex distribution for the relevant grades and schools) to adjust for non-response.20 Variables were not analyzed if data were not provided for ≥ 20% of participants. Comparisons of proportions were made using the χ2 test with the Rao–Scott correction. Logistic regression was used to model potential risk factors (e.g., age, sex, and prior DENV exposure) for incident DENV infections. Statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC) and Stata (version 11; StataCorp, LP, College Station, TX).
Human subjects review.
The study protocol was reviewed and approved by the institutional review board committees of the CDC and the University of Puerto Rico School of Medicine.
Results
In May of 2007, of 2,085 students attending grades 5–11 of Patillas public schools, 812 (38.9%) were invited to participate the study, of which 345 (42.5%) were enrolled. The mean age of enrolled students was 13.7 years, and 188 (48.0%) were female. Of 345 participants, 317 (91.7%) denied ever having dengue, 0 reported any other arbovirus diagnoses, and only 4 (0.9%) reported yellow fever vaccination. All participants had a negative anti-DENV IgM ELISA at enrollment.
At enrollment, nearly one-half of the participants had anti-DENV IgG, and the mean age was 14.0 years (Table 1). There was no difference in seroprevalence by gender. However, prevalence was higher among older children, with anti-DENV IgG prevalence increasing by 1.2-fold with each 1-year increase in age. Boys and older students participated at disproportionately lower rates; however, we used standard calibration weighting to minimize this effect and calculate more generalizable estimates (data not shown).
Table 1.
Prevalence of previous DENV infection among 10- to 18-year-olds at enrollment in Patillas, Puerto Rico in 2007
| Population characteristics | IgG anti-DENV* at study enrollment | Total | |
|---|---|---|---|
| Yes | No | ||
| Mean age in years (SE)† | 14.0 (0.13) | 13.4 (0.12) | 13.7 (0.04) |
| Age in years, n (%)‡ | |||
| 10–11 | 52/120 (42.7) | 68/120 (57.3) | |
| 12–13 | 55/120 (45.2) | 65/120 (54.8) | |
| 14–15 | 33/56 (57.7) | 23/56 (42.3) | |
| 16–18 | 30/49 (59.2) | 19/49 (40.8) | |
| Male, n (%)§ | 73/157 (48.9) | 84/157 (51.1) | |
| Total, n | 170/345¶ | 175/345 | |
Calibration weighting was applied to the calculation of all proportions, logistic regression odds ratios (ORs), and P values.
IgG anti-DENV detected by an IgG ELISA.
Adjusted Wald test, F(1,0.32) = 6.45; P value = 0.02.
Logistic regression (age in years used as a continuous variable), OR = 1.15; P value = 0.02. Rao–Scott F(2.64,84.55) = 1.51; P value = 0.22.
Rao–Scott F(1,32.0) = 0.11; P value = 0.74.
In total, 49.8% of total participants (95% CI = 43.6–56.0%).
Of 170 participants with anti-DENV IgG at enrollment, 46 (27.5%; 95% CI = 13.6–27.4%) had a past DENV infection, single serotype; 98 (52.9%; 95% CI = 46.5–59.3%) had a past DENV infection, serotype undetermined; and the remaining 26 (19.6%; 95% CI = 13.6–27.4%) did not have MNT performed (Table 2). One participant showed a monotypic neutralization pattern in the enrollment specimen but did not have a detectable anti-DENV IgG by ELISA in the same specimen.
Table 2.
Patterns of neutralization antibody to DENV in 10- to 18-year-olds with IgG anti-DENV at enrollment in Puerto Rico in 2007
| Anti-DENV neutralization patterns* at enrollment | Age in years, n (%) | Total | |||
|---|---|---|---|---|---|
| 10–11 | 12–13 | 14–15 | 16–18 | ||
| Negative for anti-DENV IgG† | 8 (15.4) | 8 (16.5) | 7 (32.7) | 3 (15.4) | 26 (19.6)‡ |
| Past DENV infection, single serotype | 15 (28.5) | 14 (25.7) | 9 (26.1) | 8 (30.6) | 46 (27.5)§ |
| DENV-1¶ | 0 | 1 (12.2) | 0 | 2 (19.5) | 3 (7.4)∥ |
| DENV-2¶ | 1 (4.9) | 6 (42.2) | 2 (18.1) | 1 (9.8) | 10 (19.9)** |
| DENV-3¶ | 11 (75.3) | 5 (37.3) | 5 (47.5) | 3 (51.1) | 24 (53.8)†† |
| DENV-4¶ | 3 (19.7) | 2 (8.3) | 2 (34.3) | 2 (19.6) | 9 (18.9)‡‡ |
| Past DENV infection, serotype undetermined | 29 (56.1) | 33 (57.8) | 17 (41.3) | 19 (53.9) | 98 (52.9)§§ |
| Total | 52 | 55 | 33 | 30 | 170 |
Calibration weighting was applied to the calculation of all proportions.
MNT was used to detect anti-DENV neutralization antibodies in enrollment specimens with a positive IgG anti-DENV ELISA result.
Enrollment specimens with a negative single-dilution (1:100) IgG anti-DENV ELISA result did not receive additional testing.
95% CI = 13.6–27.4%.
95% CI = 13.6–27.4%.
Predominant serotype; numbers in parentheses represent the percentages of the total for that past single-serotype DENV infection pattern by age group.
95% CI = 3.2–16.3%.
95% CI = 10.5–34.3%.
95% CI = 40.4–66.6%.
95% CI = 10.2–32.4%.
95% CI = 46.5–59.3%.
Of 308 participants who had paired specimens collected, 19 had incident DENV infection for an overall seroincidence rate of 5.6% (Table 3). There was no difference in age or gender between participants with or without incident DENV infection (gender comparison Rao–Scott F[1,320] = 0.8; P value = 0.38). Of 19 participants with incident DENV infection, 3 (13.1%; 95% CI = 3.7–37.3%) had serologic evidence of an incident primary DENV infection, and 16 (86.9%; 95% CI = 62.7–96.3%) had evidence of an incident secondary DENV infection. None of these 19 participants reported yellow fever vaccination or diagnosis with another flavivirus infection during the study period. Of three participants with a primary incident DENV infection, neutralization antibody patterns in paired specimens indicated that two were DENV-2 infections and one was a DENV-3 infection. The infecting DENV could not be determined in 16 participants with a secondary incident DENV infection. All participants were negative for anti-DENV IgM in the 12-month specimen (Supplemental Table 1).
Table 3.
Incident DENV infection rates among 10- to 18-year-olds during the study period in Patillas, Puerto Rico in 2007 and 2008
| Characteristics at enrollment | Incident DENV infections, n (%) | Total | |
|---|---|---|---|
| Yes | No | ||
| Mean age in years (SE)* | 13.4 (2.2) | 13.2 (0.06) | 13.7 (0.05) |
| Age in years, n (%) | |||
| 10–11† | 8 (7.5) | 100 (92.4) | 108 |
| 12–13 | 6 (6.1) | 100 (93.9) | 106 |
| 14–15 | 2 (2.5) | 46 (97.5) | 48 |
| 16–18 | 3 (5.6) | 43 (94.4) | 46 |
| Anti-DENV IgG, n (%)‡ | |||
| Fourfold rise in anti-DENV IgG titer | 16 (9.9) | 136 (90.1) | 152 |
| Negative to positive anti-DENV IgG | 3 (1.4) | 153 (98.5) | 156 |
| Total, n | 19§ | 289 | 308 |
Calibration weighting was applied to the calculation of proportions, logistic regression odds ratios (ORs), SEs, 95% CIs, and P values. Incident DENV infection was defined as a change from negative to positive or a greater than or equal to fourfold rise in IgG anti-DENV titers in paired specimens.
Adjusted Wald test, F(1,32.0) = 0.56; P value = 0.46. Logistic regression (age in years used as a continuous variable), OR = 0.93; P value = 0.47.
F(2.25,72.10) = 0.73; P value = 0.5.
Rao–Scott F(1,32.0) = 20.2; P value = 0.002.
Overall incident DENV infection rate = 5.64% (95% CI = 3.2–8.1).
Participants with anti-DENV IgG detected at enrollment were almost seven times more likely to have an incident DENV infection than participants with no detectable IgG anti-DENV at enrollment (Table 4). However, this pattern of association was only demonstrable for participants with a monotypic neutralization pattern at enrollment (Table 4).
Table 4.
Association of enrollment anti-DENV IgG and neutralization antibodies with incident DENV infection among 10- to 18-year-olds during the study period in Patillas, Puerto Rico in 2007 and 2008
| Enrollment specimen serology patterns | Total | Number of incident DENV infections (%) | Risk ratio | 95% CI | Rao–Scott χ2 P value |
|---|---|---|---|---|---|
| IgG anti-DENV antibodies | |||||
| No | 156 | 3 (1.5) | 1.0 | – | – |
| Yes | 152 | 16 (9.9) | 6.8 | 1.7–27.0 | < 0.008 |
| Anti-DENV neutralization antibody pattern* | |||||
| No IgG anti-DENV or neutralization antibodies | 164 | 5 (2.4) | 1.0 | – | – |
| Past DENV infection, primary DENV infections | 46 | 7 (16.2) | 6.8 | 1.7–27.0 | 0.009 |
| Past DENV infection, secondary DENV infections | 98 | 7 (6.2) | 2.6 | 0.7–9.7 | 0.14 |
Calibration weighting was applied to the calculation of all proportions, risk ratios, 95% CIs, and P values.
MNT was performed only on paired specimens with a positive anti-DENV IgG in either the enrollment or 12-month specimen.
Rates of enrollment IgG anti-DENV (mean = 49.3%; SE = 1.2; 95% CI = 46.8–51.9%) and incident DENV infection (mean = 6.1%; SE = 0.3; 95% CI = 5.4–6.7%) varied by neighborhood. Rates of enrollment IgG anti-DENV (mean = 50.8%; SE = 2.1; 95% CI = 46.4–55.2%) and incident DENV infection (mean = 6.0%; SE = 0.5 95% CI = 5.0–7.1%) also varied by school.
Of 308 participants who had paired specimens collected, 300 (97.0%) completed the enrollment and 12-month questionnaire, and 8 (3.0%) completed only the 12-month questionnaire. Of these 308 participants, 135 (43.6%; 95% CI = 38.6–48.7%) reported at least one AFI.
Of 19 participants with incident DENV infection, 9 (39.8%; 95% CI = 23.1–57.4%) reported by phone interview having at least one AFI: 5 with UFI and 4 with DF. Of nine participants with at least one AFI, one (10.0%; 95% CI = 0.9–57.3%) had an incident primary DENV infection, and eight (90.0%; 95% CI = 42.7–99.1%) had an incident secondary DENV infection. None of these AFIs were reported to EDSS or PDSS. The remaining 10 participants with incident DENV infection (61.1%; 95% CI = 43.3–76.9%) did not report any AFIs by interview, and AFIs in these participants were not reported to EDSS or PDSS. Of these participants, two (13.8%; 95% CI = 3.0–45.5%) had an incident primary DENV infection, and eight (86.2%; 95% CI = 54.5–97.0%) had an incident secondary DENV infection.
There was no association between secondary DENV infection status and symptomatic incident DENV infection. The symptomatic-to-inapparent infection ratio was 1:1.6 (95% CI = 1:0.7–1:2.0). No DHF, DSS, dengue-related hospitalization, or deaths were reported among participants.
Discussion
In this single-year prospective cohort study, we showed that, in an area of Puerto Rico with consistently high incidence of reported dengue cases that was experiencing an epidemic during the study, roughly 1 in 20 children and adolescents was infected with DENV.7,8,21 We also found that children and adolescents with pre-existing immunity against DENV were at a higher risk for subsequent DENV infection during the study. Finally, we found an incident DENV infection rate and a symptomatic-to-inapparent DENV infection ratio in our cohort that were similar to those in other endemic dengue areas.
We estimated the overall seroincidence of DENV infection to be 5.6% in a largely immune cohort of children and adolescents during an island-wide epidemic.9 Our estimate was considerably lower than the estimates from two prior prospective seroincidence studies conducted in Puerto Rico during the 1969 and 1982 epidemics.6,11 These studies found child and adolescent seroincidence rates of 60–70%6 and 37%.11 The higher seroincidence rates in these studies might be explained by the introduction of new DENV serotypes into a largely non-immune population. For example, in 1969, Puerto Rico experienced an epidemic of predominantly DENV-2, a DENV serotype that had not been detected previously in Puerto Rico.22 In 1982, after DENV-1, -2, and -3 had already been detected in Puerto Rico, DENV-4 was introduced and became the dominant DENV serotype during the epidemic of the same year.23 Even considering these epidemiologic patterns, differences observed between these prior studies and our study may also be explained by the different testing methods used and their performance in these studies; some studies used only anti-DENV IgG ELISA or hemagglutination inhibition assay to determine seroincidence but did not necessarily perform neutralization assays to assess specific anti-DENV antibody reactivity. Additionally, in our study, we found that there was a lower incidence of DENV infections in subjects with past DENV infection with undetermined serotype, perhaps because of the presence of protective antibodies against all four serotypes (Table 4).
Although southeast Asia and Central America are thought to have a higher DENV seroincidence than the Caribbean, our 1-year seroincidence rate was comparable with these regions, suggesting that DENV seroincidence in Puerto Rico corresponds to baseline seroincidence in other endemic areas.24–32 For example, studies conducted in southeast Asia have found seroincidence rates ranging from 2.2% to 18%.24–28,30,32 However, many of these studies included a somewhat younger cohort of children (1–16 years old). Our seroincidence rate was also comparable with the rates found in prospective cohort studies conducted in Central and South America (5.8–11.1%).29,31,33 The similarity between our 1-year DENV seroincidence rates and other study populations suggests that Patillas and likely, Puerto Rico can also experience higher rates of DENV transmission.
The symptomatic-to-inapparent DENV infection ratio observed in our study (1:1.6) was similar to that seen in school-aged children from some DENV-endemic areas (Thailand in 1998–2002 and 2004–2007; 1:0.9–1:2.9) but not others (Nicaragua in 2001–2008; 1:3–1:18).27–29,31 The reason for these differences is multifactorial and likely related to host population immune profile, DENV transmission patterns, DENV serotype/strain differences, and intensity of AFI monitoring.34 For example, it seems that AFI detection methods used in studies may affect estimation of symptomatic-to-inapparent DENV infection ratios, because studies that used active AFI monitoring methods (e.g., school absence monitoring with student and parent follow-up) tended to report higher symptomatic-to-inapparent DENV infection ratios (1:0.9–1:2.9) than studies using passive AFI monitoring methods (e.g., instructing participants to visit the study clinic when ill/no active follow-up; ratio = 1:1.3–1:18).25,27,28,31 This suggests that studies with more robust case detection methods are detecting more illness occurrences and produce more accurate symptomatic-to-inapparent DENV infection ratio estimates. Although intrinsic DENV factors, host characteristics, and the interaction between pre-existing neutralization antibodies and the infecting DENVs may have influenced the inapparent DENV infection detected in our cohort, we believe that our study had comparable ratios of inapparent to symptomatic infections with those reported by others, because we used both active AFI methods (phone interviews) and passive methods (fever diary and EDSS + PDSS).34
This study was subject to several limitations. First, the participation rate was low (42.5%), and boys and older students participated at disproportionately lower rates (data not shown). Despite student and parent orientations, we observed that students and parents were reluctant to participate because of fear of blood draws and unfamiliarity with study participation. We used standard calibration weighting to minimize this effect and calculate more generalizable estimates. Second, relying on phone interviews and reports of illness from two passive surveillance systems may have decreased our ability to detect all dengue illness in our study population. Third, we may have underestimated incidence, because we relied on collecting specimens within a 12-month period. Anti-DENV IgG titers may have waned during this period if the participant experienced a secondary DENV infection early in the study and hence, had no measurable change in immune status at the 12-month blood collection. Fourth, we were unable to determine the infecting DENV serotype for many incident infections, which precluded examination of the relationship between circulating DENV serotypes and symptomatic infections.
In conclusion, the 1-year DENV infection rate in our group of school-aged children seemed to be similar to the rates of other dengue-endemic areas that are thought to have much higher levels of DENV transmission than the Caribbean. Similar to other endemic areas, we also found that nearly one-half of school-aged children had already acquired at least one DENV infection by the time that they were 10- to 11-years-old. Notably, we also found that DENV transmission in Patillas tended to cluster spatially, putting some participants more at risk than others for repeat infection. Although we were able to add to our understanding of symptomatic and inapparent DENV infection in a group of school-aged children in Puerto Rico, more studies will be needed to better understand the annual variation in DENV infection patterns and the determinants of symptomatic and inapparent DENV infection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the invaluable participation of the students and parents of Patillas and the indispensable support and assistance provided by the teachers, the principals, and superintendent Dr. Norma Marrero Costa of the Patillas public schools. We are also appreciative of the important contributions of the following people: Dr. Harold Margolis, Dr. Mary Ramos, Dr. Carmen Perez-Guerra, Oscar Padro, Mr. Brian Irizarry, Kurt Wimberger, Dr. Patricia Cano, Dr. Ileana Maldonado, the Laboratorio Genesis staff, and the Centers for Disease Control and Prevention Dengue Branch staff who volunteered for various study activities.
Footnotes
Financial support: This work was supported, in part, under Cooperative Research and Development Agreement (CRDA) CID-01-138-02 with Sanofi Pasteur, Inc.
Authors' addresses: D. Fermín Argüello, Kay M. Tomashek, Luz Quiñones, Manuela Beltran, Luz Acosta, Luis M. Santiago, and Elizabeth Hunsperger, National Centers for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases, Dengue Branch, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mails: dfmanito@gmail.com, kay.tomashek@nih.gov, luzquinones@hotmail.com, mvb6@cdc.gov, lda9@cdc.gov, and enh4@cdc.gov. Brad J. Biggerstaff, Office of the Director, National Centers for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mail: bkb5@cdc.gov. Enid J. Garcia-Rivera, University of Puerto Rico School of Medicine, San Juan, Puerto Rico, E-mail: enid.garcia3@upr.edu. Wellington Sun, Food and Drug Administration, Silver Spring, MD, E-mail: Wellington.Sun@fda.hhs.gov. Laurence Pollissard-Gadroy, Sanofi Aventis, Paris, France, E-mail: laurencehmp@gmail.com. Christine Luxemburger, Sanofi Pasteur, Lyon, France, E-mail: Christine.Luxemburger@sanofipasteur.com.
References
- 1.World Health Organization (WHO) Scientific Working Group on Dengue: Meeting Report. Geneva: WHO; 2006. [Google Scholar]
- 2.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB. More dengue, more questions. Emerg Infect Dis. 2005;11:740–741. doi: 10.3201/eid1105.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Likosky WH, Calisher CH, Michelson AL, Correa-Coronas R, Henderson BE, Feldman RA. An epidemiologic study of dengue type 2 in Puerto Rico, 1969. Am J Epidemiol. 1973;97:264–275. doi: 10.1093/oxfordjournals.aje.a121508. [DOI] [PubMed] [Google Scholar]
- 7.Rigau-Perez JG, Vorndam AV, Clark GG. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994–1995. Am J Trop Med Hyg. 2001;64:67–74. doi: 10.4269/ajtmh.2001.64.67. [DOI] [PubMed] [Google Scholar]
- 8.Rigau-Perez JG, Ayala-Lopez A, Garcia-Rivera EJ, Hudson SM, Vorndam V, Reiter P, Cano MP, Clark GG. The reappearance of dengue-3 and a subsequent dengue-4 and dengue-1 epidemic in Puerto Rico in 1998. Am J Trop Med Hyg. 2002;67:355–362. doi: 10.4269/ajtmh.2002.67.355. [DOI] [PubMed] [Google Scholar]
- 9.Tomashek KM, Rivera A, Muñoz-Jordan JL, Hunsperger E, Santiago L, Padro O, Garcia E, Sun W. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am J Trop Med Hyg. 2009;81:467–474. [PubMed] [Google Scholar]
- 10.Neff JM, Morris L, Gonzalez-Alcover R, Coleman PH, Lyss SB, Negron H. Dengue fever in a Puerto Rican community. Am J Epidemiol. 1967;86:162–184. doi: 10.1093/oxfordjournals.aje.a120722. [DOI] [PubMed] [Google Scholar]
- 11.Waterman SH, Novak RJ, Sather GE, Bailey RE, Rios I, Gubler DJ. Dengue transmission in two Puerto Rican communities in 1982. Am J Trop Med Hyg. 1985;34:625–632. doi: 10.4269/ajtmh.1985.34.625. [DOI] [PubMed] [Google Scholar]
- 12.Morens DM, Rigau-Perez JG, Lopez-Correa RH, Moore CG, Ruiz-Tiben EE, Sather GE, Chiriboga J, Eliason DA, Casta-Velez A, Woodall JP. Dengue in Puerto Rico, 1977: public health response to characterize and control an epidemic of multiple serotypes. Am J Trop Med Hyg. 1986;35:197–211. doi: 10.4269/ajtmh.1986.35.197. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Figueroa L, Rigau-Perez JG, Suarez EL, Reiter P. Risk factors for dengue infection during an outbreak in Yanes, Puerto Rico in 1991. Am J Trop Med Hyg. 1995;52:496–502. doi: 10.4269/ajtmh.1995.52.496. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary DR, Rigau-Perez JG, Hayes EB, Vorndam AV, Clark GG, Gubler DJ. Assessment of dengue risk in relief workers in Puerto Rico after Hurricane Georges, 1998. Am J Trop Med Hyg. 2002;66:35–39. doi: 10.4269/ajtmh.2002.66.35. [DOI] [PubMed] [Google Scholar]
- 15.Ramos MM, Arguello DF, Luxemburger C, Quinones L, Munoz JL, Beatty M, Lang J, Tomashek KM. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance—June 2005–May 2006. Am J Trop Med Hyg. 2008;79:123–127. [PubMed] [Google Scholar]
- 16.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol. 1999;14:183–189. doi: 10.1016/s1386-6532(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 18.Vorndam V, Beltran M. Enzyme-linked immunosorbent assay-format microneutralization test for dengue viruses. Am J Trop Med Hyg. 2002;66:208–212. doi: 10.4269/ajtmh.2002.66.208. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) Dengue Haemorrhagic Fever: Diagnosis, Treatment, and Control. Geneva: WHO; 1997. [Google Scholar]
- 20.Särndal C-E, Lundström S. Estimation in Surveys with Nonresponse. West Sussex, England: Wiley; 2005. [Google Scholar]
- 21.Rigau-Perez JG, Ayala-Lopez A, Vorndam AV, Clark GG. Dengue activity in Puerto Rico during an interepidemic period (1995–1997) Am J Trop Med Hyg. 2001;64:75–83. doi: 10.4269/ajtmh.2001.64.75. [DOI] [PubMed] [Google Scholar]
- 22.Chappell WA, Calisher CH, Toole RF, Maness KC, Sasso DR, Henderson BE. Comparison of three methods used to isolate dengue virus type 2. Appl Microbiol. 1971;22:1100–1103. doi: 10.1128/am.22.6.1100-1103.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Mosquito cell-cultures and specific monoclonal-antibodies in surveillance for dengue viruses. Am J Trop Med Hyg. 1984;33:158–165. doi: 10.4269/ajtmh.1984.33.158. [DOI] [PubMed] [Google Scholar]
- 24.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 25.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 26.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 27.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 28.Yoon IK, Rothman AL, Tannitisupawong D, Srikiatkhachorn A, Jarman RG, Aldstadt J, Nisalak A, Mammen MP, Jr, Thammapalo S, Green S, Libraty DH, Gibbons RV, Getis A, Endy T, Jones JW, Koenraadt CJ, Morrison AC, Fansiri T, Pimgate C, Scott TW. Underrecognized mildly symptomatic viremic dengue virus infections in rural thai schools and villages. J Infect Dis. 2012;206:389–398. doi: 10.1093/infdis/jis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 30.Thai KTD, Nga TTT, Van Nam N, Phuong HL, Giao PT, Hung LQ, Binh TQ, van Doornum GJJ, de Vries PJ. Incidence of primary dengue virus infections in southern Vietnamese children and reactivity against other flaviviruses. Trop Med Int Health. 2007;12:1553–1557. doi: 10.1111/j.1365-3156.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 31.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborio S, Hammond SN, Nunez A, Aviles W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TKT, Luxemburger C, Nguyen TT, Pollissard-Gadroy L, Vu TQH, Pham VB, Nguyen NR, Wartel TA, Lang J. A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans R Soc Trop Med Hyg. 2010;104:592–600. doi: 10.1016/j.trstmh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Restrepo BN, Piedrahita LD, Agudelo IY, Parra-Henao G, Osorio JE. Frequency and clinical features of dengue infection in a schoolchildren cohort from medellin, Colombia. J Trop Med. 2012;2012:120496. doi: 10.1155/2012/120496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.