Abstract
Liposomal amphotericin B is an effective and safe alternative for the treatment of visceral leishmaniasis in the Indian subcontinent. In this study, we used a higher-dose regimen of an indigenously manufactured liposomal amphotericin B (FUNGISOME; L-AmBL), which was intended to improve the efficacy in terms of long-lasting cure rate. Thirty men and thirty women between 12 and 60 years old with parasitologically confirmed visceral leishmaniasis were enrolled in two cohorts of 15 patients each. Subjects in cohort I were administered one dose (10 mg/kg body weight) of L-AmBL intravenously. After the safety at this dose was confirmed in cohort I, patients were recruited in cohort II. They received one infusion of an escalated dose (15 mg/kg body weight). The safety of these two doses was evaluated over a period of 30 days, and efficacy was assessed for initial cure at day 30 and definitive cure at 6 months. FUNGISOME was found to be safe, with an initial cure rate of 100% at day 30 and a definitive cure rate of 93.3% at the 6-month follow-up in both the cohorts.
Introduction
Visceral leishmaniasis (VL) has a global yearly incidence of 200,000–400,000 cases and accounts for a loss of approximately 2.4 million disability-adjusted life years (DALYs) each year, of which 400,000 DALYs are estimated in the southeast Asia region.1 In India between 2004 and 2008, the estimated number of cases was 146,700–282,800.2 According to a World Health Organization (WHO) report, in the Indian subcontinent, the median total expenditure by a patient on VL treatment has been estimated to be 1.2–1.4 times the annual per capita income.3
Before miltefosine was recommended for the VL elimination program for India, Nepal, and Bangladesh,4 amphotericin B deoxycholate was the preferred drug by the National Vector Borne Disease Control Program (NVBDCP) in India as a first-line drug in areas with sodium stibogluconate (SSG) sensitivity < 90%. The recommended schedule is 1 mg/kg body weight by intravenous infusion given daily or on alternative days for 15–20 infusions. Prolonged duration of treatment, frequent adverse reactions, and need for monitoring renal functions and electrolytes are well-recognized drawbacks. Studies conducted with amphotericin B in India showed that 1 mg/kg given for 15–20 days had an efficacy of 96–100%.5–8
Lipid formulations of amphotericin B are rapidly concentrated into organs, such as the liver and spleen, providing targeted delivery of the drug and minimizing exposure of the free drug to other organs. Thus, tolerance is greatly improved, and adverse events (AEs), including nephrotoxicity, are minimized, which enables delivery of large doses of the drug over short periods of time. Among the lipid formulations, liposomal amphotericin B (AmBisome; L-AmB Gilead Sciences, Foster City, CA) has been extensively used for the treatment of VL with excellent results.8–12 In a recent trial, a single dose of 10 mg/kg L-AmB achieved a cure rate of > 95% in the Indian subcontinent, which led to a paradigm shift in the approach to the treatment of VL.9 After this trial, this regimen has been recommended as the most preferred regimen for VL in south Asia by the WHO.3 The benefits of this treatment are excellent efficacy, assured compliance, substantially reduced hospitalization time, and ability to treat large numbers of VL patients.
FUNGISOME (L-AmBL) is a liposomal formulation of amphotericin B in saline developed by an Indian company Lifecare Innovations (Gurgaon, Haryana, India). It is approved by the Drugs Controller General of India (DCGI) and has been available in the Indian market since 2003. Every lipid formulation of amphotericin B has different safety and efficacy, even with the same doses, and thus, it is necessary to test every new formulation as a new drug.8,13 Currently, in the treatment of VL, L-AmBL is used in an extended regimen (7 days), requiring a corresponding length of hospitalization with associated costs and a risk of non-compliance.14 In a recent study, VL patients were treated with 5 or 7.5 mg/kg body weight L-AmBL in a single dose or 10 mg/kg body weight L-AmBL in a 5-mg/kg double dose. At 1-month follow-up, all of the patients showed 100% initial cure. However, total doses of 5, 7.5, and 10 mg/kg showed 60%, 50%, and 90% cure rates, respectively, at 6 months post-treatment.15 Encouraged by the excellent efficacy of a single dose of L-AmB, we chose to test a higher single dose of this indigenously produced L-AmBL for the treatment of VL, which might provide an alternative to L-AmB.
The primary objective of this study was to use a higher-dose regimen of L-AmBL with an intention to improve the efficacy in terms of long-lasting cure rate for the treatment of VL in India.
Methodology
This prospective, open-label, sequential study was carried out at our field site (Kala-Azar Medical Research Center; Muzaffarpur, Bihar, India) and approved by the Ethics Committee of the Kala-Azar Medical Research Center. The study was conducted in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use—Guideline for Good Clinical Practice (ICH-GCP) and ethical principles that have their origin in the Declaration of Helsinki.
The study also complied with Schedule Y, Guidelines for Clinical Trials on Pharmaceutical Products in India–GCP Guidelines issued by the Central Drugs Standard Control Organization and the Indian Council of Medical Research Ethical Guidelines. The study was registered on Clinical Trial Registry, India (CTRI; www.ctri.nic.in; registration number: CTRI/2011/11/002145).
Inclusion criteria.
Parasitologically confirmed patients with VL between 12 and 60 years old were enrolled after informed consent. For children less than 18 years old, written informed consent was taken from parents or guardian.
Exclusion criteria.
Patients were excluded in the study if they had hemoglobin of < 5 g/dL, serum creatinine or blood urea nitrogen (BUN) > 1.5 times the upper limit of normal, a platelet count < 40,000/m3, serum bilirubin > 2 mg/dL, prothrombin time of more than 5 seconds above the control levels, history of VL treatment in the last 45 days, hepatitis B or C, human immunodeficiency virus (HIV), active tuberculosis or another serious illness or associated disease known to alter liver/kidney functions, or known hypersensitivity/allergy to the study drug or their constituents or were women who were pregnant or lactating.
Number of patients.
No formal sample size computation was done for this study. The total number of patients enrolled in the study was 30 (i.e., 15 patients in each cohort). Patients who met eligibility criteria were enrolled in the study in two cohorts consecutively.
Study drug.
A commercially available product (L-AmBL; FUNGISOME; Lifecare Innovations Pvt. Ltd, Gurgaon, Haryana, India) was used as the study drug. The sealed bottles of L-AmBL were stored at 2–8°C and subjected to sonication before administration. Sonicated L-AmBL was stored for up to 24 hours at 2–8°C and shaken well before use. Partially used vials were not stored for future patient use and were segregated for disposal.
Dose escalation strategy.
Patients in cohort I were administered a single dose of 10 mg/kg body weight L-AmBL intravenously. Recruitment of cohort II was started when dose-limiting toxicities (DLTs) occurred in no more than two patients in cohort I. Patients in cohort II received a single dose of 15 mg/kg body weight L-AmBL. DLT was defined as doubling of the serum creatinine level from baseline and exceeding 2.0 mg/dL, an absolute level of more than 2.5 mg/dL, any AE of grade 3 or higher except for hematologic toxicity, for which the criterion was a decrease of 33% or more from the baseline values for hemoglobin, total leukocyte count, and platelet count, or any other serious AEs (SAEs).
Treatment protocol
Treatment was started within 6 days of the baseline screening evaluation. Subjects were hospitalized for the duration of time that the dose was infused and discharged after 24 hours depending on the subject's condition or at the discretion of the investigator, with detailed reporting instructions in case of vomiting or any AE. The discharged subjects were instructed to report back on days 8 and 30.
Cohort I of the study received a single dose of L-AmBL intravenously at 10 mg/kg body weight, and cohort II of the study received a single dose of L-AmBL intravenously at 15 mg/kg body weight. Subjects administered L-AmBL intravenously received 500 mg paracetamol and 25 mg pheniramine maleate as pre-medication at least 30 minutes before injection of the study drug. The study day infusion was preceded by an initial test dose of 1 mg L-AmBL diluted in 20 mL saline infused for 30 minutes. The subject was watched for an additional 30 minutes, and if the subject did not develop any hypersensitivity reaction, the rest of the dose was administered at the rate of 100 mL/hour.
Safety assessment.
Along with vital parameters and spleen size, all patients completed the following tests at baseline, on the day of discharge, and on days 8 and 30: complete blood count (CBC), liver function test, renal function test, serum electrolyte test, and urine analysis. An electrocardiogram (EKG) was obtained on day 0, at the time of discharge, and on day 8. Grading of toxicity was done according to the Common Toxicity Criteria for Adverse Events.16 Patients were withdrawn from the study if they had intolerable toxicity, showed a lack of efficacy, died, or withdrew consent.
Efficacy assessment.
Efficacy was measured in terms of initial cure at day 30 and definitive cure at 6 months.
Initial cure was defined as negative spleen or bone marrow parasitology plus improvement in clinical signs and symptoms by day 30 defined as defervescence of fever to below 99°F, reduction in spleen size, improvement in laboratory parameters, or weight gain with respect to the pre-treatment value. Parasite density was graded by microscopy in a blinded fashion using a conventional logarithmic scale of 0 (no parasites per 1,000 oil immersion fields) to +6 (> 100 amastigotes per field).17 In case any subject had an aspirate score of +1 at day 30, a reaspiration was performed after 4 weeks of assessment.
Definitive cure was defined as the absence of signs or symptoms of relapse at 6 months in those with initial cure. If, at any point during follow-up or at the 6-month visit, a relapse was suspected, parasitological confirmation by examination of splenic smear was done to document relapse.
Treatment failure was defined as either the lack of initial cure or relapse within 6 months of follow-up. Patients who relapsed were given a rescue therapy of amphotericin B (0.75 mg/kg administered for 15 daily infusions).
Statistical analysis
Analysis population.
The study data were analyzed using SAS, version 9.1.3. The safety data were coded using MedDRA, version 15.0. Data were analyzed using an intent-to-treat (ITT) population.
General methodology.
The continuous data were summarized using numbers of subjects (n), mean, SD, median, minimum and maximum; categorical data were summarized using frequencies (n) and percentages and tabulated for each treatment group.
This study was sponsored by Lifecare Innovations Private Limited and supported by a grant from the Department of Science and Technology, Government of India. The sponsor of the study had no role in study design, execution, collection, and interpretation of data; preparation of the manuscript; and decision to publish it.
Results
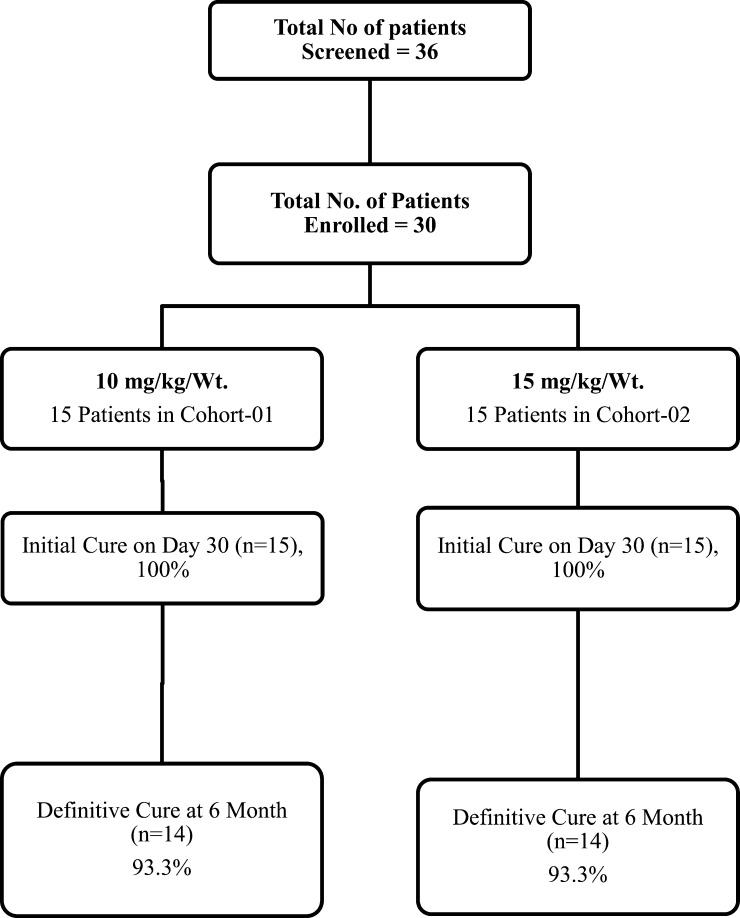
All of the patients completed the study and follow-up. There were no study withdrawals. Disposition of patients is shown in Figure 1 . Baseline and day 30 clinical and laboratory characteristics of cohorts I and II are shown in Table 1.
Figure 1.
Disposition of patients.
Table 1.
Hematological and biochemical parameters of two treatment groups
| Group I (N = 15) | Group II (N = 15) | |||||
|---|---|---|---|---|---|---|
| Baseline test mean (SD) | At 30 days test mean (SD) | P value | Baseline test mean (SD) | At 30 days test mean (SD) | P value | |
| Age (years) | 26.1 (10.52) | 29 (11.14) | ||||
| Male sex n (%) | 7 (46.7) | 8 (53.3) | ||||
| Weight (kg) | 38.9 (6.69) | 42.1 (6.45) | 0.002 | 38.9 (6.69) | 42.1 (6.45) | < 0.001 |
| Spleen (cm below costal margin) | 4.1 (2.09) | 0.2 (0.41) | < 0.001 | 4.3 (2.89) | 0.5 (0.99) | < 0.001 |
| Hemoglobin (g/dL) | 7.88 (1.119) | 9.65 (0.988) | < 0.001 | 8.43 (1.796) | 9.47 (1.124) | 0.020 |
| Platelets (/μL) | 112,266.7 (45,857.65) | 247,133.3 (56,496.35) | < 0.001 | 130,466.7 (46,321.65) | 226,666.7 (71,454.95) | 0.001 |
| White blood cells (/μL) | 3,213.3 (1,247.78) | 7,726.7 (2,528.54) | < 0.001 | 3,053.3 (1,779.19) | 6,406.7 (1,211.53) | < 0.001 |
| Alanine aminotransferase (ALT; IU/mL) | 27.1 (10.26) | 32.2 (10.99) | 0.258 | 43.8 (28.75) | 30.1 (12.97) | 0.070 |
| Aspartate aminotransferase (AST; IU/mL) | 62.5 (26.44) | 52.3 (15.34) | 0.383 | 70.5 (26.95) | 44.6 (15.1) | 0.004 |
| BUN (mg/dL) | 8.97 (2.399) | 10.53 (2.801) | 0.038 | 9.49 (3.157) | 12.26 (4.179) | 0.079 |
| Creatinine (mg/dL) | 0.742 (0.1147) | 0.704 (0.1541) | 0.423 | 0.776 (0.1418) | 0.784 (0.1504) | 0.842 |
Safety.
In all 50 patients, AEs were reported in 28 patients in cohort I and 22 patients in cohort II. Most patients (90%) experienced infusion-related chills and rigor (Table 2). DLTs were observed in two (13.3%) subjects in cohort I. One subject experienced a decrease of 33% or more of platelets from the baseline values, and the same patient also had another SAE in the form of acute pulmonary edema after the infusion; however, he recovered on continued follow-up. Another subject experienced nephrotoxicity with a rise in serum creatinine from 0.88 to 4.03 mg/dL on day 4 after infusion that returned to normal by day 11. In cohort II, one (6.7%) subject experienced DLT in the form of a decrease in platelet count and nephrotoxicity with serum creatinine of 2.21% mg on day 2 of infusion from the baseline value of 0.92% mg, which returned back to normal on day 8.
Table 2.
Summary of AEs
| Body system n (%) | Grade CTC | Treatment group | Overall (N = 30) | |
|---|---|---|---|---|
| Cohort I: 10 mg/kg (N = 15) | Cohort II: 15 mg/kg (N = 15) | |||
| Total number of patients with a single AE | 15 (100) | 14 (93.3) | 29 (96.7) | |
| Gastrointestinal disorders | ||||
| Diarrhea | 1 | 2 (13.3) | 4 (26.7) | 6 (20.0) |
| Vomiting | 1 | 5 (33.3) | 3 (20.0) | 8 (26.7) |
| General disorders and administration site conditions | ||||
| Chills | 1 | 15 (100.0) | 12 (90.0) | 27 (90.0) |
| Investigations | ||||
| Nephrotoxicity (blood creatinine increased)* | 1 (6.7) | 1 (6.7) | 2 (6.7) | |
| Hematology (platelet count decreased) | 2 | 1 (6.7)† | 1 (6.7) | 2 (6.7) |
All AEs were mild (common toxicity criteria grades 1 and 2) except where noted.
Grade 3 dose-limiting nephrotoxicity in cohort I and grade 2 dose-limiting nephrotoxicity in cohort II.
The same patient also had an SAE in the form of pulmonary edema.
Efficacy.
After infusion of the drug, there was prompt resolution of fever, regression of spleen, and recovery of hematological parameters in all patients in both cohorts (Table 1). An initial cure of 100% was achieved in both cohorts; however, during follow-up, one patient relapsed at month 6 in cohort I and month 4 in cohort II, resulting in a definitive cure of 93.3% in both (Figure 1).
Discussion
Miltefosine was chosen for the elimination program in India, Nepal, and Bangladesh for its ease of use and applicability in the control program.4 However, the need for prolonged therapy (28 days), potential risk of teratogenicity, and vulnerability to develop resistance are some of the drawbacks of this oral drug, which are perpetuated by its dwindling efficacy in the Indian subcontinent.18–20 There is a need for a better alternative, but the inventory of antileishmanial drugs is very small. Pentavalent antimonials cannot be used because of extensive resistance in the region,21,22 and paromomycin has yet to be released for use in the program and carries the drawback of 21 intramuscular injections.
Lipid formulations of amphotericin B become the obvious choice for use in the control program. After we showed the efficacy of a single dose of L-AmB in the treatment of Indian VL,8 a recent trial in Bangladesh has shown a similar high efficacy (97%) of a single dose of 10 mg/kg L-AmB when given at a rural public hospital, suggesting that it is feasible to give this therapy at primary healthcare centers.23
The indigenously manufactured liposomal amphotericin B used in this study has been on the horizon for several years, but its clinical development has been slow. In a previous study using this preparation, a total dose of 15–21 mg/kg had shown an efficacy of 90–100% and was found safe against VL cases, irrespective of their prior treatment status.24 This phase 2 study shows that a single dose of L-AmBL at 10 mg/kg has excellent efficacy for the treatment of VL. The efficacy of the higher dose (15 mg/kg) was found to be similar to that with the lower dose. The regimens were well-tolerated; only two patients in cohort I had SAEs, and both resolved without sequelae. One patient in cohort I had grade 3 elevation of serum creatinine levels, which resolved on day 11. Most patients in our study experienced chills, which were grade 1 in severity. Infusion reactions were also common with a single-dose L-AmB infusion in a previous study, although in a smaller proportion of patients.8 Although transient rises in creatinine can occur, chronic renal toxicity from liposomal amphotericin B is rare, even with daily doses of up to 15 mg/kg.25 Dose requirement of amphotericin B varies on different continents, but in the Indian subcontinent, even 3.75–7.5 mg/kg total dose cured 89–93% of patients11,12,26; these findings indicate use of 10 or 15 mg/kg liposomal amphotericin B as a single dose. Dose requirement in other regions, like the Mediterranean basin or Africa, is much higher. A single dose of liposomal amphotericin, which is efficacious, ensures compliance, and does not need monitoring or prolonged hospitalization, offers significant advantages over prevailing regimens in the Elimination Program. However, the use of sonication before drug administration in our study needs to be eliminated in future development, because it will be difficult to sonicate when using LAmBL at the Primary Health Centers (PHCs) level.
Although the market price of L-AmB remains quite high, it is available at a preferential price of US$18.00 per 50-mg vial for developing countries. The pricing of new liposomal amphotericin B formulation L-AmBL has to be competitive and cheaper than L-AmB. In addition to the high market price, requirement of a cold chain remains an impediment for all liposomal amphotericin B preparations, and L-AmBL is no exception. In a post-marketing study in India, of 109 patients treated with L-AmBL (irrespective of the indication), none showed nephrotoxicity.27 L-AmB, however, has shown nephrotoxicity, which is defined as 1.5 and 2 times baseline serum creatinine in 47% and 21% of neutropenic patients, respectively. Nephrotoxicity was observed in 32% of patients treated with AmBisome for invasive fungal infection.28 However, in patients with VL treated with L-AmB at 10 mg/kg in a single dose, increased anemia or thrombocytopenia or evidence of nephrotoxicity or hepatotoxicity occurred in 2% of patients.9 In this study, one (6.7%) patient in each group developed transient nephrotoxicity, and one patient in the 10-mg group developed reversible thrombocytopenia and acute pulmonary edema; however, the real spectrum of AEs can only be assessed in a study with a larger sample size.
However, the findings of this preliminary study suggest that a single dose of L-AmBL could be used as an alternative to L-AmB in the treatment of VL. These results document an improved treatment regimen compared with the prior report and further refine our approach to single-dose liposomal amphotericin B treatment. The added advantage of the drug is that it is manufactured in India. Additional phase 3 studies are needed to strongly establish the efficacy and safety of single-dose administration of L-AmBL in the treatment of VL.
ACKNOWLEDGMENTS
The authors thank the Department of Science and Technology, Government of India for supporting this study. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Shyam Sundar, Anup Singh, Madhukar Rai, and Jaya Chakravarty, Department of Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, E-mails: drshyamsundar@hotmail.com, dranupbhu@gmail.com, rai_madhukar@sify.com, and tapadar@gmail.com.
References
- 1.Searo W. Regional Strategic Framework for Elimination of Kala-Azar from South-East Asia Region (2005–2015) New Delhi, India: World Health Organization; Regional Office for South-East Asia: 2005. [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Leishmaniasis Control Team WHO. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Control of the Leishmaniasis. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. 2010. http://whqlibdoc.who.int/trs/WHO_ TRS_949_eng.pdf Available at. Accessed June 29, 2011.
- 4.Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res. 2006;123:195–196. [PubMed] [Google Scholar]
- 5.Thakur CP, Sinha GP, Pandey AK, Barat D, Singh RK. Daily versus alternate-day regimen of amphotericin B in the treatment of kala-azar: a randomized comparison. Bull World Health Organ. 1994;72:931–936. [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur CP, Narayan S. A comparative evaluation of amphotericin B and sodium antimony gluconate, as first-line drugs in the treatment of Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2004;98:129–138. doi: 10.1179/000349804225003154. [DOI] [PubMed] [Google Scholar]
- 7.Thakur CP, Singh RK, Hassan SM, Kumar R, Narain S, Kumar A. Amphotericin B deoxycholate treatment of visceral leishmaniasis with newer modes of administration and precautions: a study of 938 cases. Trans R Soc Trop Med Hyg. 1999;93:319–323. doi: 10.1016/s0035-9203(99)90037-8. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S, Mehta H, Suresh AV, Singh SP, Rai M, Murray HW. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004;38:377–383. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 10.Berman JD, Badaro R, Thakur CP, Wasunna KM, Behbehani K, Davidson R, Kuzoe F, Pang L, Weerasuriya K, Bryceson AD. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull World Health Organ. 1998;76:25–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Sundar S, Jha TK, Thakur CP, Mishra M, Singh VR, Buffels R. Low-dose liposomal amphotericin B in refractory Indian visceral leishmaniasis: a multicenter study. Am J Trop Med Hyg. 2002;66:143–146. doi: 10.4269/ajtmh.2002.66.143. [DOI] [PubMed] [Google Scholar]
- 12.Sundar S, Jha TK, Thakur CP, Mishra M, Singh VP, Buffels R. Single-dose liposomal amphotericin B in the treatment of visceral leishmaniasis in India: a multicenter study. Clin Infect Dis. 2003;37:800–804. doi: 10.1086/377542. [DOI] [PubMed] [Google Scholar]
- 13.Sundar S, Mehta H, Chhabra A, Singh V, Chauhan V, Desjeux P, Rai M. Amphotericin B colloidal dispersion for the treatment of Indian visceral leishmaniasis. Clin Infect Dis. 2006;42:608–613. doi: 10.1086/500138. [DOI] [PubMed] [Google Scholar]
- 14.Karande SC, Boby KF, Lahiri KR, Jain MK, Kshirsagar NA, Gokhale PC, Pandya SK. Successful treatment of antimony-resistant visceral leishmaniasis with liposomal Amphotericin B in a child. Trop Doct. 1995;25:80–81. doi: 10.1177/004947559502500209. [DOI] [PubMed] [Google Scholar]
- 15.Mondal S, Bhattacharya P, Rahaman M, Ali N, Goswami RP. A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a short-course liposomal amphotericin B therapy. PLoS Negl Trop Dis. 2010;4:e764. doi: 10.1371/journal.pntd.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Program CTE . Common Toxicity Criteria. 2002. http://ctep.cancer.gov/reporting/index.html Available at. Accessed October 7, 2009. [Google Scholar]
- 17.Chulay JD, Bryceson AD. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983;32:475–479. doi: 10.4269/ajtmh.1983.32.475. [DOI] [PubMed] [Google Scholar]
- 18.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, Boelaert M, Dujardin JC, Chakravarty J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55:543–550. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 19.Rahman M, Ahmed BN, Faiz MA, Chowdhury MZ, Islam QT, Sayeedur R, Rahman MR, Hossain M, Bangali AM, Ahmad Z, Islam MN, Mascie-Taylor CG, Berman J, Arana B. Phase IV trial of miltefosine in adults and children for treatment of visceral leishmaniasis (kala-azar) in Bangladesh. Am J Trop Med Hyg. 2011;85:66–69. doi: 10.4269/ajtmh.2011.10-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis. 2013;56:1530–1538. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- 21.Rijal S, Chappuis F, Singh R, Bovier PA, Acharya P, Karki BM, Das ML, Desjeux P, Loutan L, Koirala S. Treatment of visceral leishmaniasis in south-eastern Nepal: decreasing efficacy of sodium stibogluconate and need for a policy to limit further decline. Trans R Soc Trop Med Hyg. 2003;97:350–354. doi: 10.1016/s0035-9203(03)90167-2. [DOI] [PubMed] [Google Scholar]
- 22.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 23.Mondal DAJ, Hasnain MG, Hossain MS, Ghosh D, Huda MM, Nabi SG, Sundar S, Matlashewski G, Arana D. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. Lancet Glob Health. 2014;2:e51–e57. doi: 10.1016/S2214-109X(13)70118-9. [DOI] [PubMed] [Google Scholar]
- 24.Bodhe PV, Kotwani RN, Kirodian BG, Pathare AV, Pandey AK, Thakur CP, Kshirsagar NA. Dose-ranging studies on liposomal amphotericin B (L-AMP-LRC-1) in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93:314–318. doi: 10.1016/s0035-9203(99)90036-6. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M, Barrett J, Anaissie EJ. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother. 2001;45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundar S, Agrawal G, Rai M, Makharia MK, Murray HW. Treatment of Indian visceral leishmaniasis with single or daily infusions of low dose liposomal amphotericin B: randomised trial. BMJ. 2001;323:419–422. doi: 10.1136/bmj.323.7310.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanath S, Gogtay NJ, Kshirsagar NA. Post-marketing study to assess the safety, tolerability and effectiveness of FungisomeTM: an Indian liposomal amphotericin B preparation. J Postgrad Med. 2005;51:58–63. [PubMed] [Google Scholar]
- 28.Astellas Pharma US, Inc. Ambisome (Amphotericin B) Liposome for Injection: Prescribing Information. 2012. http://www.astellas.us/docs/ambisome.pd Available at. Accessed August 20, 2013.