Abstract
Antimalarial drugs are a key tool in malaria elimination programs. With the emergence of artemisinin resistance in southeast Asia, an effort to identify molecular markers for surveillance of resistant malaria parasites is underway. Non-synonymous mutations in the kelch propeller domain (K13-propeller) in Plasmodium falciparum have been associated with artemisinin resistance in samples from southeast Asia, but additional studies are needed to characterize this locus in other P. falciparum populations with different levels of artemisinin use. Here, we sequenced the K13-propeller locus in 82 samples from Haiti, where limited government oversight of non-governmental organizations may have resulted in low-level use of artemisinin-based combination therapies. We detected a single-nucleotide polymorphism (SNP) at nucleotide 1,359 in a single isolate. Our results contribute to our understanding of the global genomic diversity of the K13-propeller locus in P. falciparum populations.
Discussions of global malaria elimination programs have been renewed in light of the decline in malaria-associated morbidity and mortality resulting from scaled-up vector- and parasite-targeted interventions over the past decade.1 A key strategy in malaria elimination programs is effective use of antimalarial drugs, particularly artemisinin-based combination therapies (ACTs), which are the first-line therapies in most malaria-endemic countries.1 However, malaria elimination goals face a new threat with the emergence of artemisinin resistance in southeast Asia.2–5 A recent study used both in vivo and in vitro methods to identify non-synonymous single-nucleotide polymorphisms (SNPs) at the PF3D7_1343700 kelch propeller domain (K13-propeller) in Cambodian Plasmodium falciparum associated with artemisinin resistance.6 Subsequent investigations at the K13-propeller locus in other southeast Asian populations, including those of Vietnam, Myanmar, Laos, and Thailand, confirm the association of non-synonymous SNPs at the K13-propeller with artemisinin resistance.7 However, these polymorphisms were not detected in sub-Saharan Africa P. falciparum samples from Gambia, Mali, Ghana, Burkina Faso, Congo, Democratic Republic of Congo, Kenya, Tanzania, Malawi, and Uganda, where little to no artemisinin resistance has been reported.7–9 Furthermore, other non-synonymous SNPs were identified in the sub-Saharan African samples that were not observed in the southeast Asian P. falciparum samples. These findings suggest that polymorphisms in the K13-propeller could vary geographically, and therefore, genetic studies on the K13-propeller in other malaria-endemic countries are needed to assess the use of this locus as a tool to monitor the global emergence and spread of artemisinin resistance.
Haiti is one of two remaining malaria-endemic countries in the Caribbean. It has several favorable elimination characteristics.10 (1) One parasite species (P. falciparum) accounts for the majority, if not all, cases of malaria.11 (2) Malaria transmission is low.12–14 (3) Being on the only malaria-endemic island in the Caribbean, there is likely reduced importation of malaria parasite-carrying vectors from neighboring malaria-endemic regions compared with countries in Africa and South America that are surrounded by other malaria-endemic countries. Another important feature is the absence of chloroquine resistance in the present P. falciparum population in Haiti, which was evidenced by a recent in vivo efficacy study (Okech BA and others, unpublished data) and molecular investigations into the pfcrt and pfmdr genes.15,16 Although pyrimethamine resistance was reported in an in vitro study from 1984,17 a recent molecular study reported only the pfdhfr S108N low-level pyrimethamine resistance mutation and no sulfadoxine resistance-associated mutations in the pfdhps in Haiti.18 Currently, chloroquine remains the first-line treatment for malaria in Haiti. However, ACTs are likely candidates to replace chloroquine in the future should chloroquine resistance emerge in Haiti.
There is evidence that ACTs have been used by international health non-government organizations in Haiti16 (Ministry of Public Health and the Population [MSPP], personal communication) and antimalarial treatment studies.19 Because of limited oversight by the MSPP in Haiti, it is not known how widespread ACT use is in Haiti. It is, therefore, important to generate baseline data on genetic markers associated with artemisinin resistance before ACTs become widely used in Haiti. We originally targeted the pfatpase6 gene, because it carried an SNP (S769N) that was associated with lowered sensitivity to artemether in an earlier investigation in French Guiana20; however, we did not detect the S769N mutation in our preliminary sample (data not shown). With more recent studies unable to confirm the association of the S769N mutation with artemisinin resistance in other populations21,22 and the recent identification of the K13-propeller as a strong candidate for artemisinin resistance, we decided to refocus our investigation on K13-propeller polymorphisms. To contribute to the growing knowledge about the global genetic diversity of the K13-propeller and establish a baseline for future surveillance of potential artemisinin resistance in Haiti, we sequenced an 810-base pair region of the K13-propeller from P. falciparum samples collected in Haiti between 2010 and 2013.
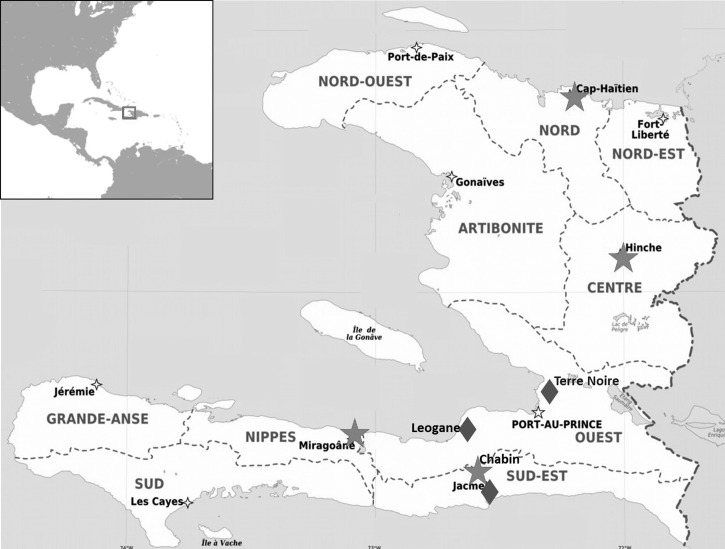
Blood spot samples were collected from seven study sites in Haiti: Terre Noire, Leogane, Jacmel, Chabin, Hinche, North Cap Haitian, and Nippes (Figure 1). Details about the collection of these samples have been discussed previously.15,18 Terre Noire is considered an urban region, whereas Leogane, Jacmel, Chabin, Hinche, North Cap Haitian, and Nippes are more rural. Although malaria transmission in Haiti is low overall, there have been reports of transmission hotspots in the Southeast Department (which includes Jacmel and Chabin), particularly near coastal areas.23,24 DNA was extracted using the QIAamp DNA Investigator Kit and eluted with 60 μL elution buffer (Qiagen Inc., Valencia, CA). Nested polymerase chain reaction (PCR) amplification of an 810-base pair region of K13-propeller (nucleotides 1,298–2,108) was carried out as described in the work by Ariey and others6 with the following modified reagent concentrations for both the primary and nested reactions: 1× GoTaq Flexi Buffer, 2.5 mM MgCl2, 0.2 mM each nucleotide, 0.25 μM each primer in the primary PCR, and 0.625 U Go Taq Hot Start Polymerase (Promega, Madison, WI). Amplicons were sequenced with the nested primers using Big Dye Master Mix and run on an ABI Genetic Analyzer (Applied Biosystems, Foster City, CA). DNA sequences were aligned to the 3D7 reference sequences (National Center for Biotechnology Information Reference Sequence XM_001350122.1) using Sequencher 4.10.1 (Gene Codes Corp, Ann Arbor, MI).
Figure 1.
A map of the study sites in Haiti. Most samples were collected from sites in the west (Ouest) and southeast (Sud-Est) departments represented by the blue diamonds. Smaller samples sets are represented by red stars.
Of 94 samples initially targeted for sequencing of the K13-propeller, 82 samples were successfully sequenced: 38 samples from Terre Noire, 18 samples from Leogane, 15 samples from Jacmel, 1 sample from Chabin, 4 samples from Hinche, 4 samples from North Cap Haitian, and 2 samples from Nippes. One SNP, T to A at nucleotide 1,359 (glycine at amino acid 453), was detected in a single mixed infection sample (i.e., both mutant and wild-type alleles were observed) and confirmed two times. The 1,359 SNP results in a synonymous substitution, and therefore, it likely plays no role in artemisinin resistance. No other K13-propeller polymorphisms were detected in any of the other samples. This sample was collected from Leogane in January of 2012 during the high-transmission season in Haiti. Although Haiti holds a chloroquine treatment policy in Haiti, it is not clear whether unregulated ACT administration is occurring in Leogane.
The absence of known artemisinin resistance polymorphisms in the K13-propeller in our P. falciparum samples in Haiti is not surprising, because the level of artemisinin use is likely too low to exert selective pressure on the P. falciparum population. Low genetic variation observed at the K13-propeller mirrors the results of investigations into other antimalarial resistance-associated loci in Haiti, including dhfr, dhps, pfcrt, and pfmdr,15,18 supporting the idea that Haiti's P. falciparum population has low genetic variation at antimalarial resistance-associated genes.
In summary, these data serve as baseline information for monitoring artemisinin resistance should artemisinin-based therapies become widespread in Haiti. Given the diversity of the K13-propeller locus observed in P. falciparum samples from sub-Saharan Africa, it is possible that other pathways and polymorphisms could lead to artemisinin resistance. Continued investigations of the K13-propeller locus and additional genome-wide association studies to identify novel resistance loci in other malaria-endemic countries of varying transmission intensities and levels of ACT use are needed to elucidate the mechanisms behind artemisinin resistance. This information is crucial to ensure that appropriate genetic markers are included in molecular surveillance of artemisinin resistance globally.
ACKNOWLEDGMENTS
The authors thank the following organizations and people for their support: Haiti Ministry of Public Health and the Population (MSPP), Government of Haiti, Christianville Foundation (Gressier, Haiti), Community Coalition for Haiti, Fish Ministries Haiti, Family Health Ministries, University of Florida, the US Department of Defense, and Hospital St. Croix staff, including Carol, Farrah, and Gillian.
Footnotes
Financial support: This material is based on work supported by a Department of Defense Global Emerging Infections Surveillance and Response System (DoD-GEIS) Grant C0607_12_UN (to B.A.O.) and United Negro College Fund/Merck Graduate Science Research Dissertation Fellowship (to T.E.C.).
Authors' addresses: Tamar E. Carter, Alexis Boulter, and Connie J. Mulligan, Genetics Institute, University of Florida, Gainesville, FL, E-mails: tamarec@ufl.edu, aboulter@ufl.edu, and cmulligan@ad.ufl.edu. Alexandre Existe, National Public Health Laboratory, Ministry of Health and Population, Port-au-Prince, Haiti, E-mail: alexandre.existe@gmail.com. Jean R. Romain, Hospital St. Croix, Leogane, Haiti, E-mail: roosevald2008@yahoo.fr. Jean Yves St. Victor, Blanchard Clinic, Family Health Ministries, Terre Noire, Haiti, E-mail: jeanyvessaintvictor@yahoo.fr. Bernard A. Okech, Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mail: bokech@ufl.edu.
References
- 1.World Health Organization . World Malaria Report 2013. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Lindegardh N, Tarning J, Imwong M, Jacob CG, Rasmussen C, Perin J, Ringwald P, Nyunt MM. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS ONE. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, Dorsey G, Rosenthal PJ. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS ONE. 2014;9:e105690. doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, Tagbor H, Williams J, Bojang K, Njie F, Desai M, Kariuki S, Gutman J, Mathanga DP, Martensson A, Ngasala B, Conrad MD, Rosenthal PJ, Tshefu AK, Moormann AM, Vulule JM, Doumbo OK, Ter Kuile FO, Meshnick SR, Bailey JA, Juliano JJ. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2015;211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts L. Elimination meets reality in Hispaniola. Science. 2010;328:850–851. doi: 10.1126/science.328.5980.850. [DOI] [PubMed] [Google Scholar]
- 11.Weppelmann TA, Carter TE, Chen Z, von Fricken ME, Victor YS, Existe A, Okech BA. High frequency of the erythroid silent Duffy antigen genotype and lack of Plasmodium vivax infections in Haiti. Malar J. 2013;12:30. doi: 10.1186/1475-2875-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townes D, Existe A, Boncy J, Magloire R, Vely JF, Amsalu R, De Tavernier M, Muigai J, Hoibak S, Albert M, McMorrow M, Slutsker L, Kachur SP, Chang M. Malaria survey in post-earthquake Haiti–2010. Am J Trop Med Hyg. 2012;86:29–31. doi: 10.4269/ajtmh.2012.11-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold BF, Priest JW, Hamlin KL, Moss DM, Colford JM, Jr, Lammie PJ. Serological measures of malaria transmission in Haiti: comparison of longitudinal and cross-sectional methods. PLoS ONE. 2014;9:e93684. doi: 10.1371/journal.pone.0093684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Fricken ME, Weppelmann TA, Lam B, Eaton WT, Schick L, Masse R, Beau De Rochars MV, Existe A, Larkin J, 3rd, Okech BA. Age-specific malaria seroprevalence rates: a cross-sectional analysis of malaria transmission in the Ouest and Sud-Est departments of Haiti. Malar J. 2014;13:361. doi: 10.1186/1475-2875-13-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbadry MA, Existe A, Victor YS, Memnon G, Fukuda M, Dame JB, Yowell CA, Okech BA. Survey of Plasmodium falciparum multidrug resistance-1 and chloroquine resistance transporter alleles in Haiti. Malar J. 2013;12:426. doi: 10.1186/1475-2875-12-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuberger A, Zhong K, Kain KC, Schwartz E. Lack of evidence for chloroquine-resistant Plasmodium falciparum malaria, Leogane, Haiti. Emerg Infect Dis. 2012;18:1487–1489. doi: 10.3201/eid1809.120605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen-Dinh P, Zevallos-Ipenza A, Magloire R. Plasmodium alciparum in Haiti: susceptibility to pyrimethamine and sulfadoxine-pyrimethamine. Bull World Health Organ. 1984;62:623–626. [PMC free article] [PubMed] [Google Scholar]
- 18.Carter TE, Warner M, Mulligan CJ, Existe A, Victor YS, Memnon G, Boncy J, Oscar R, Fukuda MM, Okech BA. Evaluation of dihydrofolate reductase and dihydropteroate synthetase genotypes that confer resistance to sulphadoxine-pyrimethamine in Plasmodium falciparum in Haiti. Malar J. 2012;11:275. doi: 10.1186/1475-2875-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornthwaite J, Shah H, Thornthwaite B, Respess H. The 16 day administration of artemisinin for the long-term cure of malaria in Haiti (P3134) J Immunol. 2013;190(186):14. [Google Scholar]
- 20.Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 21.Cui L, Wang Z, Jiang H, Parker D, Wang H, Su XZ. Lack of association of the S769N mutation in Plasmodium falciparum SERCA (PfATP6) with resistance to artemisinins. Antimicrob Agents Chemother. 2012;56:2546–2552. doi: 10.1128/AAC.05943-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao M, Wang Z, Yang Z, Yuan L, Parker DM, Putaporntip C, Jongwutiwes S, Xangsayarath P, Pongvongsa T, Moji H, Dinh Tuong T, Abe T, Nakazawa S, Kyaw MP, Yan G, Sirichaisinthop J, Sattabongkot J, Mu J, Su XZ, Kaneko O, Cui L. Genetic diversity and lack of artemisinin selection signature on the Plasmodium falciparum ATP6 in the Greater Mekong Subregion. PLoS ONE. 2013;8:e59192. doi: 10.1371/journal.pone.0059192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raccurt CP, Ciceron M, Dossil R, Boncy J. Prevalence of Plasmodium falciparum during the rainy season (June–December) in the southeast district of Haiti. Med Sante Trop. 2012;22:435–439. doi: 10.1684/mst.2013.0124. [DOI] [PubMed] [Google Scholar]
- 24.Raccurt CP, Ciceron M, Existe A, Boncy J, Brasseur P, Lemoine F. Gametocyte carriage in asymptomatic Plasmodium falciparum infections in Haiti (2010–2013) Bull Soc Pathol Exot. 2014 doi: 10.1007/s13149-014-0367-4. in press. [DOI] [PubMed] [Google Scholar]