Abstract
Artemisinin-based combination therapies (ACTs) are currently considered the first-line treatments for uncomplicated Plasmodium falciparum malaria. Among these, artemether-lumefantrine (AL) has been the most widely prescribed ACT in sub-Saharan Africa. Recent clinical trials conducted in sub-Saharan Africa have shown that dihydroartemisinin-piperaquine (DP), a most recent ACT, may have a longer post-treatment prophylactic period and post-treatment infection period (duration of gametocyte carriage) than AL. Using epidemiological and clinical data on the efficacy of AL and DP, we developed and parameterized a mathematical transmission model that we used to compare the population-level impact of AL and DP for reducing P. falciparum malaria transmission in sub-Saharan Africa. Our results showed that DP is likely to more effectively reduce malaria incidence of clinical episodes than AL. However in low P. falciparum transmission areas, DP and AL are likely to be equally effective in reducing malaria prevalence. The predictions of our model were shown to be robust to the empirical uncertainty summarizing the epidemiological parameters. DP should be considered as a replacement for AL as first-line treatment of uncomplicated malaria in highly endemic P. falciparum communities. To optimize the effectiveness of ACTs, it is necessary to tailor treatment policies to the transmission intensity in different settings.
Introduction
Sub-Saharan Africa continues to bear the highest burden of malaria worldwide, with 85% of the estimated 243–500 million annual cases of clinical malaria and 90% of the over 863 thousand to 1 million malaria-associated deaths annually.1,2 Over the past decade, malaria morbidity and mortality have substantially declined in several areas across sub-Saharan Africa.3 This decline has been attributed at least in part to widespread distributions of insecticide-treated bed nets and the introduction of artemisinin-based combination therapies (ACTs).3–6
The ACTs combine a short-lived but highly potent artemisinin-derivative drug, which delivers a rapid reduction of parasitemia with a longer acting, but slow active partner drug. These drug combinations are associated with improved efficacy over monotherapies and decreased chance of malaria parasite becoming resistant to either drug.7,8 As a result of the ability of the artemisinin component to rapidly reduce para sitemia, early treatment of uncomplicated malaria with ACTs may prevent progression to severe disease, thereby reducing the number of severe cases and the malaria mortality rate.9 The ACTs may also reduce overall malaria transmission by decreasing human infectivity to mosquitoes10,11 and by extending the prophylactic period after treatment.8
A variety of ACTs exists, such as artemether-lumefantrine (AL) and dihydroartemisinin-piperaquine (DP), which vary in their efficacy profile against uncomplicated malaria, tolerability, and their ability to reduce infectivity to mosquitoes.8,11–14 The difference in efficacy between these ACTs may have important implications not only for the treatment of individual patients, but also for the population-level impact on malaria transmission.11,13 The balance among these factors, which may themselves vary between communities, will determine whether AL or DP is optimal in different settings. The AL remains the most widely used ACT in Africa.8,12 However, DP, a newer ACT, may appear equally efficacious as AL but with simpler dosing and a longer prophylactic period because of the extended half-life of piperaquine.8,15,16 Comparative efficacy studies in multiple settings have consistently reported a longer duration to recurrent infection in individuals treated with DP as compared with AL.13,14 At the population level, DP has drawbacks in terms of its relative effectiveness in reducing malaria transmission, compared with AL. A recent clinical trial conducted among Kenyan children has shown that despite the longer post-treatment prophylactic period of DP compared with AL, individuals treated with DP may have a longer infectious period and resultant higher malaria transmission potential to mosquitoes after treatment than those treated with AL.11 This variation may be caused by differences in the ability of either the artemisinin component or partner drug to reduce gametocytes, the transmissible stage of malaria. These pharmacodynamics differences raise important public health questions regarding the trade-off benefit between the longer post-treatment prophylactic period of DP and the shorter post-treatment infectious period of AL.
Here, we compared the population-level impact of AL and DP treatments on reducing Plasmodium falciparum malaria transmission in sub-Saharan Africa. For this purpose, we developed a mathematical model of P. falciparum malaria transmission and treatment in endemic communities using epidemiological and clinical findings on the efficacy of AL and DP.8,11,13,14,17 We used this model to evaluate the potential reduction in prevalence and incidence of clinical episodes of malaria, comparing AL or DP as first-line treatment of malaria in different P. falciparum transmission intensity settings. To evaluate the effect of empirical uncertainty in the data surrounding epidemiological parameters on the predictions of our model, we used a Monte Carlo sampling approach. Our modeling framework can be used to inform public policies on optimizing the effectiveness of combination therapies for malaria control.
Methods
Model description.
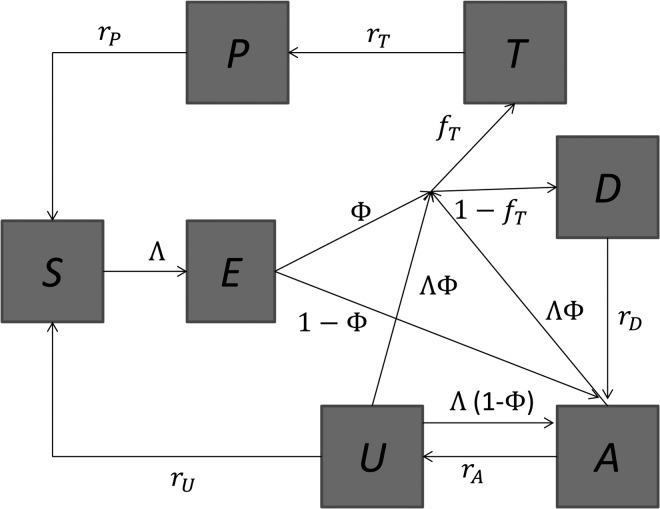
We modeled parasite transmission in human and mosquito populations using a deterministic compartmental structure. The human population is divided into two age groups, children and adults, and we assumed that children and adults have different exposure to mosquito bites, susceptibility, infectivity, and population size. An individual in the human population may be in one of seven infectious states—susceptible (S), latent (E), untreated clinical disease (D), asymptomatic patent infection (A), sub-patent infection (U), treated but still infectious (T), and protected by a prophylaxis period after treatment (P). Upon infection, individuals pass through the latent stage and then either develop clinical disease (with a probability φ) or develop patent (detectable under microscopy) or asymptomatic infection (with a probability 1–φ). From patent asymptomatic infection (A), individuals move to a sub-patent stage (U) at a rate rA. Sub-patent-infected individuals return to the susceptible class at rate rU. We distinguished between patent and sub-patent infection because of their differential contribution to disease transmission and to avoid underestimating the contribution of asymptomatic infection to disease transmission. Moreover, sub-patent infection can be an important component of the infectious reservoir.18 Following infection, individuals may recover to the susceptible state. Individuals move between these states as shown in Figure 1. Transmission among mosquitoes was incorporated dynamically and responds to changes in the prevalence of infectious humans. Mosquitoes are born into a susceptible state SM. If they become infected they enter an infectious state IM, after an incubation period (tincub). The probability that an infected mosquito survives the incubation period and becomes infectious is given by 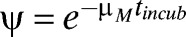 , where μM is the mosquito natural mortality rate. The birth/death rate μM is assumed to be constant. Mosquito infection is life-long. The intensity of malaria transmission was represented as the entomological inoculation rate (EIR), defined as the product of the human biting rate of mosquitoes and the proportion of mosquitoes that are infectious. Detailed description of the transmission model is given in the Supplemental Material.
, where μM is the mosquito natural mortality rate. The birth/death rate μM is assumed to be constant. Mosquito infection is life-long. The intensity of malaria transmission was represented as the entomological inoculation rate (EIR), defined as the product of the human biting rate of mosquitoes and the proportion of mosquitoes that are infectious. Detailed description of the transmission model is given in the Supplemental Material.
Figure 1.
Flow diagram for the human component of the model. S = susceptible; E = latent infection; T = treated clinical disease; D = untreated clinical disease; P = prophylaxis; A = asymptomatic patent infection; U = asymptomatic sub-patent infection.
To compare the impact on malaria transmission of AL and DP as first-line treatments for malaria in Africa, we modeled the pharmacodynamics of AL and DP as informed from a clinical trial conducted among children in Kenya.11 The DP was assumed to have a longer post-treatment prophylactic period than AL, a longer post-treatment infections period (duration of gametocyte carriage) than AL, and a greater post-treatment probability of infecting mosquitoes than AL.11 Comprehensive summary of the model parameters and their values is given in Supplemental Table 1. The impact on malaria transmission was measured as reduction in prevalence and incidence of clinical episodes of malaria at endemic equilibrium.
Uncertainty analysis.
To determine the robustness of model predictions accounting for empirical sources of uncertainty in parameters, we conducted uncertainty analysis, and simulated the effect of the uncertainty surrounding epidemiological parameters. The range of values for each epidemiological parameter was obtained from published literature (see Supplemental Table 1). Parameters were randomly sampled 100,000 times from the range of their values and used in Monte Carlo simulations to calculate variation in infection prevalence and incidence of clinical cases. To identify the contribution of each model parameter to the variability of the outcome measure, we calculated the partial rank correlation coefficients (PRCCs).19 The PRCCs quantify the degree of monotonicity between a specific input parameter and an outcome measure predicted by the model.
Results
We evaluated the potential differential effectiveness between DP and AL as first-line treatment of malaria in different P. falciparum malaria transmission settings in sub-Saharan Africa. The EIR, which is the average number of infectious bites received by a person in a year (ibpy), was used as a measure of malaria transmission intensity. Malaria prevalence was defined for any level of parasitemia rather than exclusively clinical disease, whereas we define incidence in terms of clinical malaria. Our base case analysis showed that AL and DP were equally effective in reducing malaria prevalence (Figures 2). However, DP was more effective than AL in reducing the incidence of clinical malaria. The additional incidence of clinical episodes averted by DP was greater in areas with high transmission (Figures 2 ). At an EIR of 50 ibpy, DP prevented an additional episode of clinical malaria per 100 persons annually compared with AL. At 100 ibpy, DP averted an additional 4 clinical episodes per 100 persons annually compared with AL, and at 200 ibpy, DP averted an additional 12 clinical episodes per 100 persons per year compared with AL (Figure 3 ). For EIR lower than 20 ibpy AL and DP where equally effective in reducing incidence of clinical episodes (Figure 3).
Figure 2.
Differential effectiveness of artemether-lumefantrine (AL) and dihydroartemisinin-piperaquine (DP) on malaria transmission. Results were computed using based values of Supplemental Table 1
Figure 3.
Percentage reduction of malaria transmission from using dihydroartemisinin-piperaquine (DP) compared with artemether-lumefantrine (AL). The difference in effectiveness between DP and AL was measured in terms of percentage reduction in malaria prevalence and prevented episodes of clinical malaria per 100 people annually. (A and B) The sensitivity of the differential impact on the infectiousness of treated individuals under DP and AL treatment was computed by varying the probability of mosquito infection upon biting a treated human (CT) by ±50%. (C and D) The sensitivity of the differential impact to the duration of the infectiousness period of individuals treated with DP and AL, respectively, was computed by varying the infectiousness period of treated individuals (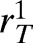 ) between its maximum and minimum values.
) between its maximum and minimum values.
To evaluate the sensitivity of our base case analysis to the probability of mosquito infection upon biting a treated individual (CT) and the duration of infectiousness for treated individuals (1/rT) under AL and DP, we performed a one-way sensitivity analysis. We found that the base case analysis was relatively robust to variation of infectiousness (CT) and infectiousness duration (1/rT) of treated individuals (Figure 3).
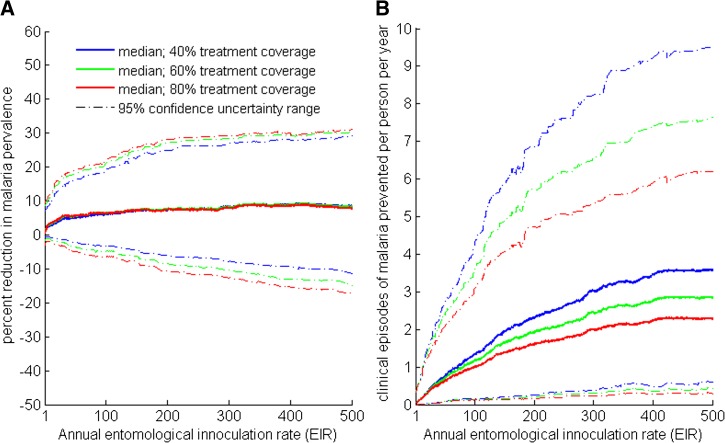
In addition to the one-way sensitivity analysis, we also conducted a probabilistic uncertainty analyses to evaluate the effect of empirical uncertainty in epidemiological parameters on the predictions of our model. Uncertainty analysis confirms that DP is more effective than AL in reducing the incidence of clinical episodes of malaria (Figure 4B), and that on average DP is more effective than AL in reducing malaria prevalence (Figure 4A ). The impact of parameter uncertainty on the relative effectiveness of DP for reducing malaria prevalence and incidence of clinical episodes increases with transmission intensity (Figure 4). The relative effectiveness of DP for reducing incidence of clinical episodes decreases with expanding treatment coverage (Figure 4B). Global sensitivity analysis using partial rank correlation coefficient showed that variation in the percent reduction of malaria prevalence under DP treatment relative to AL was primarily driven by duration of the prophylactic periods for DP and AL treatments, and the biting rate on humans by mosquitoes, which here is the main determinant for malaria transmission intensity (Figure 5 ).
Figure 4.
Uncertainty analysis of percentage reduction of malaria transmission from using dihydroartemisinin-piperaquine (DP) compared with artemether-lumefantrine (AL). Probabilistic uncertainty analysis was conducted for a different level of treatment coverage: 40%, 60%, and 80% of clinically infected individuals seek treatment. Individuals are all treated with AL or DP. The difference in effectiveness between DP and AL was measured in terms of (A) percentage reduction in malaria prevalence and (B) prevented episodes of clinical malaria per person annually.
Figure 5.
Partial rank correlation coefficients (PRCCs) of model parameters. A parameter was considered to be important in affecting the relative effectiveness of dihydroartemisinin-piperaquine (DP) versus artemether-lumefantrine (AL) for reducing malaria prevalence if ∣PRCC∣ > 0.3. The PRCCs with absolute value > 0.2 were statistically significant (P value < 0.05). The dashed lines show cutoff of significance.
Discussion
To evaluate the differential effectiveness between AL and DP as first-line antimalarial treatments in sub-Saharan Africa, we developed a malaria transmission model that we parameterized using clinical and epidemiological findings from studies on the efficacy of AL and DP in sub-Saharan Africa. We showed that DP may more effectively reduce incidence of clinical episodes compared with AL. In areas of low transmission, DP may be equally effectively as AL in reducing malaria prevalence, whereas in areas of high transmission, DP may be more effective than AL in reducing malaria prevalence. These results suggest that health policymakers should consider using DP rather than AL as first-line antimalarial treatment in P. falciparum highly endemic areas.
Previous modeling studies have shown that ACTs have a greater potential for reducing malaria transmission in low-transmission areas than in high-transmission areas,18,20 and that ACT partner-drugs with longer prophylactic periods, such as piperaquine, could confer more health benefits than partner drugs with shorter prophylactic periods, such as lumefantrine, in higher-transmission settings.20 However, these studies did not consider the differential effectiveness between ACTs in reducing malaria prevalence and clinical episodes of malaria.18,20 Our model extends previous modeling studies by providing a framework for quantifying and comparing the effectiveness between ACTs in different P. falciparum malaria transmission settings. Understanding the differential effectiveness between ACTs is paramount for optimizing malaria control policies in sub-Saharan Africa.
Malaria transmission is very heterogeneous with some individuals at higher risk than others, particularly between ages.21–23 To account for this heterogeneity in the risk of disease transmission within the human population, we considered differential biting rates, susceptibility, and infectiousness between children and adults in our model. Future studies could further stratify age-specific heterogeneity in disease transmission and the efficacy of ACT regimens to facilitate the design of age-specific malaria treatment.22,24
A new combination of DP with a single dose of primaquine is currently being evaluated in many countries to reduce malaria transmission.25 This new drug combination may make DP more efficacious than AL in terms of reducing the transmission potential of treated individuals and thus may shift the balance of the trade-off between DP and AL. As clinical trials are conducted and clinical parameters regarding efficacy are collected, modeling studies could be used to evaluate the effectiveness and relative benefits of this new drug combination. Although evaluating the effectiveness of other malaria intervention strategies such as intermittent preventative treatment, indoor residual spraying campaigns, and insecticide-treated bed nets is beyond the scope of this study, ACT is generally implemented in combination with these interventions and complements their effectiveness in reducing malaria transmission.3–6,18 Future studies may consider comparing the effectiveness of different ACTs in the presence of existing interventions and those under development against malaria. A limitation on the effectiveness of ACTs that remains to be addressed is the emergence and spread of P. falciparum resistance to artemisinin derivatives, partner drugs, or both.26,27 Resistance may be of particular concern with regimens containing longer-acting partner drugs present at low levels for extended periods, such as is the case with piperaquine, and reports of reduced efficacy of DP are now emerging.16,28 If resistance to ACTs spreads into sub-Saharan Africa, this will certainly alter the relative benefits of each regimen. Additional epidemiological and clinical data on the efficacy of different combination therapies and the risk for emergence of drug resistance are needed to fully evaluate and optimize the effectiveness of malaria treatment policies. Our model assumed that the difference between AL and DP to be only based on the infectious period, prophylactic period, and infectiousness of treatment individuals. However, other mechanism such as heterogeneity in drug failure, for example failing 5% of the time versus 95% successful on everyone, may also contribute to the difference in efficacy between AL and DP. Further studies should investigate the impact of these potential mechanisms on the effectiveness of AL and DP, ands epidemiological, entomological, and clinical criteria under which AL may perform better than DP in reducing malaria prevalence.
In addition to reducing malaria transmission, clinical studies have shown that artemisinin-based therapy may reduce schistosomiasis health burden in sub-Saharan Africa.29 Given the interactive pathology between malaria and schistosomiasis and their co-endemicity in many sub-Saharan African communities,30–32 mass screening and treatment of malaria using ACT could contribute to reducing both malaria transmission and the schistosomiaisis burden in sub-Saharan Africa. Therefore, future studies should investigate the complementary effects of ACTs and mass praziquantel administration for reducing both malaria and schistosomiais transmission in co-endemic communities.
Current protocols recommend AL as the first-line treatment of uncomplicated P. falciparum malaria in many sub-Saharan Africa countries. Our results show that, in highly endemic communities, DP should be considered as a replacement for first-line treatment. To optimize the effectiveness of malaria combination therapies for individuals and wider at-risk communities in sub-Saharan Africa, our results suggest that it is necessary to design treatment policies that are tailored to the transmission intensity of different settings.
Supplementary Material
Disclaimer: The sponsors of the study had no role in the study design, analysis, interpretation of results, writing of the report, or the decision to submit for publication. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication. We report no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Financial support: This work was supported by the National Institute of General Medical Sciences [MIDAS grant 2U01GM087719-06] and the National Institute of Child Health and Human Development under award number R01HD068174.
Authors' addresses: Martial L. Ndeffo Mbah, Yale University, School of Public Health, New Haven, CT, E-mail: martial.ndeffo-mbah@yale.edu. Sunil Parikh and Alison P. Galvani, Yale School of Public Health, Epidemiology of Microbial Diseases, New Haven, CT, E-mails: sunil.parikh@yale.edu and alison.galvani@yale.edu.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 4.Nyarango PM, Gebremeskel T, Mebrahtu G, Mufunda J, Abdulmumini U, Ogbamariam A, Kosia A, Gebremichael A, Gunawardena D, Ghebrat Y, Okbaldet Y. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bed net coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370:1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes KI, Chanda P, Ab Barnabas G. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8((Suppl 1)):S8. doi: 10.1186/1475-2875-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beeson JG, editor. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8:e1001119. doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thwing J, Eisele TP, Steketee RW. Protective efficacy of malaria case management and intermittent preventive treatment for preventing malaria mortality in children: a systematic review for the Lives Saved Tool. BMC Public Health. 2011;11((Suppl 3)):S14. doi: 10.1186/1471-2458-11-S3-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 11.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, Manjurano A, Kavishe RA, Beshir KB, Yussuf RU, Omar SA, Hermsen CC, Okell L, Schallig HD, Sauerwein RW, Hallett RL, Bousema T. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis. 2013;207:1637–1645. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 12.Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, Kamya MR, Vora N, Greenhouse B, Rosenthal PJ, Tappero J, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–1637. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, McMorrow M, Onyango P, Otieno K, Odero C, Williamson J, Kariuki S, Kachur SP, Slutsker L, Desai M. A randomized trial of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated malaria among children in western Kenya. Malar J. 2013;12:254. doi: 10.1186/1475-2875-12-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanzira H, Kakuru A, Arinaitwe E, Bigira V, Muhindo MK, Conrad M, Rosenthal PJ, Kamya MR, Tappero JW, Dorsey G. Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis. 2014;59:509–516. doi: 10.1093/cid/ciu353. [DOI] [PubMed] [Google Scholar]
- 15.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menéndez C, Nambozi M, Valéa I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A, D'Alessandro U. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomized, non-inferiority trial. PLoS ONE. 2009;4:e7871. doi: 10.1371/journal.pone.0007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creek DJ, Bigira V, McCormack S, Arinaitwe E, Wanzira H, Kakuru A, Tappero JW, Sandison TG, Lindegardh N, Nosten F, Aweeka FT, Parikh S. Pharmacokinetic predictors for recurrent malaria after dihydroartemisinin-piperaquine treatment of uncomplicated malaria in Ugandan infants. J Infect Dis. 2013;207:1646–1654. doi: 10.1093/infdis/jit078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abay S. Blocking malaria transmission to Anopheles mosquitoes using artemisinin derivatives and primaquine: a systematic review and meta-analysis. Parasit Vectors. 2013;6:278. doi: 10.1186/1756-3305-6-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basáñez M-G, Ghani AC. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:17. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254:178–196. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okell LC, Drakeley CJ, Bousema T, Whitty CJM, Ghani AC. Modeling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008;5:e226. doi: 10.1371/journal.pmed.0050226. discussion e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, Otieno S, Carneiro I, Cox J, Msuya E, Kleinschmidt I, Maxwell C, Greenwood B, Riley E, Sauerwein R, Chandramohan D, Gosling R. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 24.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Syafruddin D, Kusriastuti R, Hawley WA, Lobo NF, Ter Kuile FO. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in south Sumatra, western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis. 2013;56:685–693. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- 26.Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci USA. 2008;105:14216–14221. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders DL, Vanachayangkul P, Lon C. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med. 2014;371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 29.Utzinger J, N'Goran EK, N’Dri A, Lengeler C, Shuhua X, Tanner M. Oral artemether for prevention of Schistosoma mansoni infection: randomized controlled trial. Lancet. 2000;355:1320–1325. doi: 10.1016/s0140-6736(00)02114-0. [DOI] [PubMed] [Google Scholar]
- 30.Sokhna C, Le Hesran J-Y, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium falciparum malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trop Dis. 2010;4:e882. doi: 10.1371/journal.pntd.0000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.