Abstract
In the context of malaria elimination, novel strategies for detecting very low malaria parasite densities in asymptomatic individuals are needed. One of the major limitations of the malaria parasite detection methods is the volume of blood samples being analyzed. The objective of the study was to compare the diagnostic accuracy of a malaria polymerase chain reaction assay, from dried blood spots (DBS, 5 μL) and different volumes of venous blood (50 μL, 200 μL, and 1 mL). The limit of detection of the polymerase chain reaction assay, using calibrated Plasmodium falciparum blood dilutions, showed that venous blood samples (50 μL, 200 μL, 1 mL) combined with Qiagen extraction methods gave a similar threshold of 100 parasites/mL, ∼100-fold lower than 5 μL DBS/Instagene method. On a set of 521 field samples, collected in two different transmission areas in northern Cambodia, no significant difference in the proportion of parasite carriers, regardless of the methods used was found. The 5 μL DBS method missed 27% of the samples detected by the 1 mL venous blood method, but most of the missed parasites carriers were infected by Plasmodium vivax (84%). The remaining missed P. falciparum parasite carriers (N = 3) were only detected in high-transmission areas.
Background
For several years, the number of malaria cases in Southeast Asia has been significantly reduced as a result of the implementation of effective strategies.1,2 However, to achieve the goal of elimination, and especially the elimination of artemisinin-resistant Plasmodium falciparum parasites, which are emerging in this region,3–12 additional efforts are needed. Indeed, the detection of the reservoir of malaria parasites in asymptomatic populations is challenging and needs new actions, in particular, a change from passive case detection to active case detection, and new tools capable to detect very low parasitemia in field settings.13
For two decades, the sensitivity of malaria diagnostic tests have considerably been improved with the introduction of molecular assays.14 One major limitation is the volume of blood samples collected and analyzed, obviously limited in mass screening studies (usually 5–30 μL). Indeed, higher volumes of blood may allow the detection of very low parasite density, and could reveal a higher than expected proportion of malaria parasite carriers, including mixed infections. To date, no studies have been published to assess the prevalence of asymptomatic parasite carriers in the population by comparing different sampling volumes of blood (from 5 μL to 1 mL).
Here, the detection limit of malaria polymerase chain reaction (PCR) assays, and then the prevalence of parasite carriers in a set of field samples, were evaluated by using different volumes of blood and DNA extraction methods, by comparing the following conditions: 1) DNA extracted from dried blood spots (DBS) (5 μL) with a fast and inexpensive extraction method, commonly performed in large-scale epidemiological studies in malaria-endemic countries15; 2 and 3) DNA extracted from 50 μL and 200 μL venous blood using the Qiagen mini kit based extraction method (Courtaboeuf, France), commonly used in research studies; 4) DNA extracted from 1 mL venous blood using the Qiagen midi kit based extraction method, representing here the gold standard. To this end, 521 samples were collected in Preah Vihear province, a low malaria transmission area (north eastern Cambodia) and aliquoted into four volumes: 5 μL capillary blood spot onto filter paper, 50 μL, 200 μL, and 1 mL venous blood. Extracted DNAs were screened for the presence of Plasmodium using a qualitative real-time PCR assay targeting the cytochrome b gene. Samples identified as positives were then analyzed for Plasmodium species using four real-time PCR assays specifically amplifying P. falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae.15,16
The aim of this study is to gain a better knowledge of the unseen malaria reservoir in Cambodia, and to answer a critical question: How many malaria parasite carriers are missed by applying the conventional sampling such as small volume DBS? Resulting data should provide guidance on the best protocol to be used for mass screening studies to adapt our strategy aiming to clear the reservoir.
Materials and Methods
Participants, blood collection, and processing.
A total of 521 venous blood samples were collected in October 2013 in Preah Vihear province (located in north eastern Cambodia, along the Thailand and the Lao People's Democratic Republic borders) as a sub-study of the cross-sectional population-based study described by Bosman and others.17 Two villages were selected, one with an expected high levels and another with an expected low levels of malaria transmission, based on the number of P. falciparum malaria cases confirmed by both microscopy and rapid diagnostic tests reported through the Cambodian Malaria Information System in 2012. In both villages, 75 households were selected by systematic random sampling. A volume of 2 mL venous blood was collected in an EDTA tube from each participant aged at least 2 years who did not report anti-malarial drugs intake in the preceding month and who would freely consent to participate. For each participant, the age, sex and history of fever in the last 24 hours were recorded.
Blood samples were stored at 4°C in cool boxes, sent to the Institut Pasteur in Cambodia (IPC) within 24 h after blood collection, and aliquoted into four volumes: 5 μL aliquoted in 96 well-plates containing a 4 mm 3MM Whatman filter paper, 50 μL, 200 μL, and 1 mL, aliquoted in individual tubes. Additionally, twice 30 μL were aliquoted on 3MM Whatman filter paper as back-up. Plasma and buffy coat were removed after centrifugation on the 1 mL aliquots, to prevent PCR inhibition caused by the high amount of human DNA.
DNA extractions.
The 5 μL DBS samples were lysed overnight with hepes buffered saline (HBS) 1×/Saponin 0.5% and DNAs were extracted using the Bio-Rad Instagene matrix (Bio-Rad, Singapore) as previously described.15 The DNAs from the 50 and 200 μL aliquots were extracted using the QiaAmp DNA blood mini kit (Qiagen, Courtaboeuf, France) and 1 mL aliquots were extracted using the QiaAmp DNA blood midi kit (Qiagen) following manufacturer's recommendations. All DNA samples were eluted with 200 μL buffer AE. After optimization experiences, DNA extracts from 200 μL and 1 mL volumes were not used pure but diluted by one-half and one-fifth, respectively, before the PCR assay, to avoid inhibition of the PCR reactions.
Real-time PCR assays.
Samples were screened for the presence of Plasmodium DNA using a qualitative real-time PCR assay targeting the cytochrome b gene. Positive samples were then analyzed for Plasmodium species using four real-time PCR assays targeting the same gene and specifically amplifying P. falciparum, P. vivax, P. ovale, and P. malariae. All real-time PCR assays were performed using SYBR green ready-to-use PCR mix (Solis Biodyne, Tartu, Estonia) on a 5 μL DNA template on the BioRad CFX96 real-time PCR system (BioRad). Details on the PCR program, mix composition, and primer sequences have been described in Canier and others.15 Real-time PCR assays were followed by a melt curve analysis.
Assay performance assessment.
The detection threshold of the malaria screening assay was assessed by using the four volumes of blood: 5 μL (dried blood spot), 50 μL, 200 μL, and 1 mL of blood. To this end, cultured 3D7 ring-stage parasites were adjusted to 1% parasitemia and serially diluted 10-fold using uninfected blood from the blood bank, to concentrations ranging from 1,000 to 0.01 parasites/μL. Each concentration was aliquoted into the four studied volumes and was screened for malaria parasites following the same protocols as study samples. In addition, the specificity of each four volumes of assay was assessed by analyzing blood from 29 malaria negative volunteers.
Statistical analysis.
All data were recorded and analyzed using Excel software (Microsoft, Redmond, WA) and MedCalc (MedCalc Software, Mariakerke, Belgium). The Fisher's exact test or χ2 test were used to compare the proportions of positive PCR results using the different blood sample methods, according to the transmission areas and the Plasmodium species. A P value < 0.05 was considered to indicate statistical significance.
Ethical consideration.
The study protocol was submitted for approval to the Ethics Review Board of Médecins Sans Frontières and to the Cambodian National Ethics Committee on Health Research (0094 NECHR, 24 June 2013). Written informed consent was obtained from each participant or legal representative for children < 18 years of age before participation. Participants with a positive by PCR for Plasmodium were offered free treatment according to national guidelines: dihydroartemisinin/piperaquine (DHA-PIP) for 3 days or quinine for 7 days for women in the first trimester of pregnancy.
Results
Blood volume and assays performance (using quality control samples).
The limit of detection of the PCR screening assay for each studied volume was evaluated on P. falciparum blood serial dilutions. Results are presented in Table 1. Using 5 μL DBS samples, the assay was able to detect 100% of infections at 10 parasites/μL, and about 50% of infections at 1 parasites/μL.
Table 1.
Proportion of positive cases per dilution, for each studied volume, Preah Vihear, Cambodia 2013*
| Parasite concentration | 5 μL DBS | 50 μL blood | 200 μL blood | 1 mL blood | |||||
|---|---|---|---|---|---|---|---|---|---|
| p/μL | p/mL | No. of positive | % | No. of positive | % | No. of positive | % | No. of positive | % |
| 1,000 | 1,000,000 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 |
| 100 | 100,000 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 |
| 10 | 10,000 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 |
| 1 | 1,000 | 11/21 | 52 | 21/21 | 100 | 21/21 | 100 | 21/21 | 100 |
| 0.1 | 100 | 1/21 | 5 | 19/21 | 90 | 20/21 | 95 | 20/21 | 95 |
| 0.01 | 10 | N/A | 0/21 | 0 | 7/21 | 33 | 2/21 | 10 | |
| 0 | 0 | 0/21 | 0 | 0/21 | 0 | 0/21 | 0 | 0/21 | 0 |
Each dilution was tested in triplicate in seven polymerases chain reaction (PCR) assays (N = 21).
DBS = dried blood spots.
Using different volumes of venous blood (50 μL, 200 μL, 1 mL), the PCR detection rate was 90–95% for samples at 0.1 parasite/μL (or 100 parasites/mL). Less than 50% of samples with 0.01 parasite/μL (or 10 parasites/mL) were detected using the 200 μL and 1 mL volumes of venous blood. The use of venous blood volumes (50 μL, 200 μL, and 1 mL) combined with Qiagen extraction methods, resulted in a similar detection threshold of 100 parasites/mL, ~100-fold lower than the protocol using 5 μL DBS/Instagene extraction (proportion of positive PCR at 100 parasites/mL, P < 10−4). Surprisingly, samples at 10 parasites/ml gave a higher proportion of positive PCR by using 200 μL of venous blood (7/21) compared with 1 mL of venous blood (2/21) but this difference was not significant (P = 0.09). All negative samples (malaria negative volunteers, N = 29) were found negative with the four volumes tested.
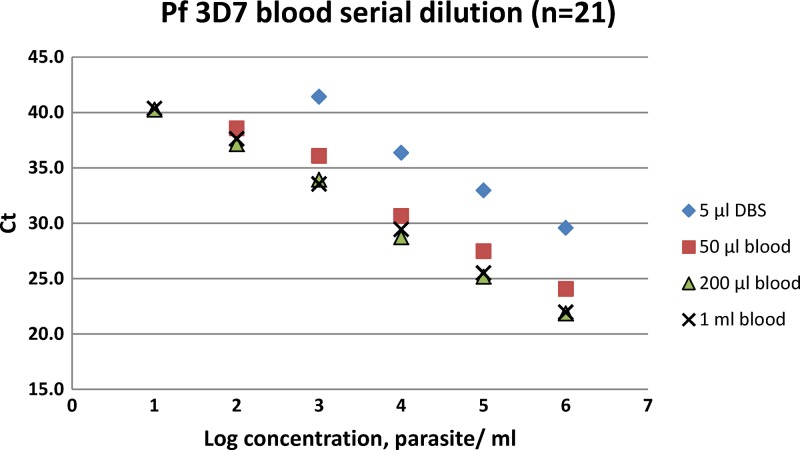
The screening assay showed a reproducible linearity with R2 > 0.9 for each volume analyzed, as shown by the standard curve presented in Figure 1.
Figure 1.
Standard curve of the malaria screening assay for each volume (triplicates), according to parasite concentrations (per mL) and cycle threshold (Ct) values.
Blood volume and prevalence of Plasmodium carriage (using field samples).
Malaria screening.
A total of 521 blood samples were analyzed using the four different volumes of blood. Samples were collected in two villages: 246 samples in a “low malaria transmission” village, 59% of the participants being female and 9% < 5 years of age; and 275 samples in a “high malaria transmission” village, 56% of the participants being female and 8% < 5 years of age.
In the low transmission village, 6/246, 6/246, 7/246, and 7/246 Plasmodium carriers were detected using the 5 μL, 50 μL, 200 μL, and 1 mL methods, respectively. The global prevalence of Plasmodium carriage rose from 2.4% to 2.8% when increasing the analyzed volume from 5 μL to 1 mL. Only one Plasmodium vivax infection was detected by using higher volumes of blood (200 μL and 1 mL) (Table 2).
Table 2.
Prevalence of Plasmodium spp. carriage and number of carriers detected per village, per tested volume, and per Plasmodium species, Preah Vihear, Cambodia 2013
| Village | N | Blood volumes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 μL | 50 μL | 200 μL | 1 mL | |||||||
| No. of positive | % of positive | No. of positive | % of positive | No. of positive | % of positive | No. of positive | % of positive | |||
| Low | 246 | Total | 6 | 2,44 | 6 | 2,44 | 7 | 2,85 | 7 | 2,85 |
| Pf | 1* | 0,41 | 1* | 0,41 | 1* | 0,41 | 1* | 0,41 | ||
| Pv | 5 | 2,03 | 5 | 2,03 | 6 | 2,44 | 6 | 2,44 | ||
| High | 275 | Total | 43 | 15,64 | 55 | 20,00 | 57 | 20,73 | 60 | 21,82 |
| Pf | 7 | 2,55 | 10† | 3,64 | 10† | 3,64 | 10† | 3,64 | ||
| Pv | 36 | 13,09 | 46† | 16,73 | 48† | 17,45 | 51† | 18,55 | ||
| Total | 521 | 49 | 9,40 | 61 | 11,71 | 64 | 12,28 | 67 | 12.9 | |
| Pf | 8 | 1,54 | 11† | 2,11 | 11† | 2,11 | 11† | 2,11 | ||
| Pv | 41 | 7,87 | 51† | 9,79 | 54† | 10,36 | 57† | 10,94 | ||
Including one Pf/Pm mixed infection.
One Pf/Pv mixed infection.
In the “high transmission” village; 43/275, 55/275, 57/275 and 60/275 Plasmodium carriers were detected using the 5 μL, 50 μL, 200 μL and 1 mL methods, respectively, raising the global prevalence of Plasmodium carriage from 15.6% (5 μL) to 21.8% (1 mL) (Table 2). However, the difference of prevalence observed between the different volumes was not significant (P = 0.09). All positive samples with the 5 μL DBS methods were also positive with the three other blood volumes methods. According to the detected Plasmodium species, the prevalence of P. falciparum, which increased from 2.5% (5 μL) to 3.6% (1 mL) were not significantly different (P = 0.4); most of the individuals that failed to be detected by using 5 μL DBS were infected by P. vivax (84%). However, the prevalence of P. vivax increasing from 7.9% (5 μL) to 10.9% (1 mL) were not significantly different (P = 0.10).
The 5 μL DBS method was capable of detecting > 73% of the samples detected positive using the 1 mL method (73% and 72% for P. falciparum and P. vivax, respectively), in both the “low” (86%) and “high” transmission villages (72%). The estimation of the Plasmodium carriage prevalence increased by 1.4-fold with the 1 mL method, compared with the 5 μL DBS method (1.2-fold in the “low setting”; 1.4-fold in the “high setting”). It is worth noting that the same prevalence were found for P. falciparum infections in low setting areas, regardless of the volume of blood used (0.41%), although in a high-transmission area, the prevalence was 1.4-fold higher by using 1 mL method compared with the 5 μL DBS method.
Methods using 50 μL, 200 μL, and 1 mL of venous blood were equally effective in detecting P. falciparum parasite carriers in the same set of blood samples, regardless of the transmission areas. However, in both areas, P. vivax parasite carriers were more frequently detected by using higher volumes of blood (1.3- to 1.4-fold).
Malaria species.
Malaria species identification was performed on 5 μL and 1 mL DNA samples. Among the 521 tested samples, P. vivax was the most prevalent species, representing more than 80% of the positive samples, followed by P. falciparum present in < 20% of the samples. Only one carrier of P. malariae and no P. ovale infection were identified. Almost all infections were single infections, one P. falciparum/P. malariae mixed infection was detected with the 5 μL method, and two mixed infections were detected with the 1 mL method (the same P. falciparum/P. malariae, and a P. falciparum/P. vivax) (Table 3).
Table 3.
Malaria species identification, for 5 μL and 1 mL of tested volume, Preah Vihear, Cambodia 2013
| Blood volumes | Increased malaria PCR diagnostic accuracy (CI 95%) | ||
|---|---|---|---|
| 5 μL | 1 mL | ||
| Total positives | 49 | 67 | 1.36 (1.06–1.73) |
| P. falciparum | 8 | 9 | 1.12 (0.51–2.13) |
| P. vivax | 40 | 56 | 1.40 (1.06–1.82) |
| P. malariae | 0 | 0 | |
| P. ovale | 0 | 0 | |
| P. falciparum/P. vivax | 0 | 1 | |
| P. falciparum/P. malariae | 1 | 1 | |
| % of each species among positives | |||
| P. falciparum | 18.4% | 16.4% | |
| P. vivax | 81.6% | 85.1% | |
| P. malariae | 2.0% | 1.5% | |
| P. ovale | 0.0% | 0.0% | |
PCR = polymerase chain reaction; CI = confidence interval.
Species results obtained from the 5 μL were 100% concordant with results obtained using 1 mL DNA extracts (N = 49). Additional infections detected with the 1 mL method (N = 18) consisted of 16 P. vivax, 1 P. falciparum, 1 P. falciparum/P. vivax (Table 3).
Discussion
The use of different volumes of calibrated venous blood, i.e., 50 μL, 200 μL, and 1 mL, combined with Qiagen extraction methods resulted in a similar detection threshold of 100 parasites/mL, which is more sensitive than the protocol using 5 μL DBS/Instagene extraction, whose detection threshold is usually around 1,000–10,000 parasites/mL.18 However, when analyzing field samples collected in two different transmission areas, there was no evidence of a difference in the parasite carrier detection rate of the methods using 5 μL DBS, 50 μL, 200 μL, and 1 mL of venous blood (P = 0.78 and P = 0.09, respectively). On a set of 521 samples from Preah Vihear province, the 5 μL DBS method missed 27% of the parasite carriers detected using the 1 mL method. Most of the parasite carriers missed by the 5 μL DBS method were infected by P. vivax (84%). The remaining P. falciparum parasite carriers (N = 3) missed by the 5 μL DBS were detected among samples from high-transmission areas.
Similar results performed in symptomatic patients were also observed in different malaria transmission areas. In Tanzania, a high-transmission area, Strøm and others18 found almost 2-fold more positives when using a 200 μL venous blood protocol in febrile children: positivity of PCR was 24.5% (78 of 319) and 11.2% (52 of 442) using 200 μL whole blood and DBS, respectively. Although in Iran, which is considered as a low-transmission area, Ataei and others19 observed a malaria prevalence of 42.7% and 46.7% (1.1-fold increase) when using DBS and 200 μL of venous blood, respectively, among malaria suspected patients.
It was observed that the PCR assay using pure DNA extracts failed to amplify a large part of 1 mL samples compared with the 1/5 dilution of DNA extracts, probably because of the presence of inhibitors. A similar effect was also occasionally observed with the 200 μL DNA extracts. Repetition of the serial dilution of samples detected as negative with pure DNA and positive with diluted DNA confirmed the assumption of an inhibition effect. Obviously, the addition of a dilution step solved this inhibition issue, but likely reduced the initial sensitivity potential of the screening assay combined with 200 μL and 1 mL venous blood volumes.
Although the use of DNA extracted from 5 μL DBS missed detecting parasite carriers, blood collection on filter paper remains a practical method with multiple advantages: 1) sample blood collection from finger prick, which is less invasive; 2) storage and transport of the samples at ambient temperature; 3) quick training for sample collection; and 4) minor biohazard risks. Indeed, venipuncture sampling for detecting malaria parasites using PCR assays implies a number of limitations such as the reluctance of patients, some difficulties in transportation of blood samples and freezing them for a long time 19. Therefore, the 5 μL DBS remains one of the methods of choice for large-scale studies on prevalence of malaria carriage (especially P. falciparum infections in low-transmission areas) and/or studies into resistance. However, for studies in elimination contexts, where detection of every single carrier of Plasmodium is paramount, the 5 μL DBS approach may be suboptimal.
Combined with Instagene DNA extraction (or chelex), the 5 μL DBS approach allows a quick, easy, and inexpensive preparation of samples for PCR assays. From our experience, malaria screening using Instagene DNA extraction from 5 μL DBS, Qiagen mini kit (50–200 μL blood), Qiagen midi kit (1 mL blood) cost US$2.5, US$6, and US$12, respectively, per sample and allow to proceed 192, 72, and 32 samples per day per technician. Therefore, the replacement of the 5 μL DBS/Instagene by the “venous blood”/Qiagen is challenging in resource-poor settings.
Conclusions
The study presented here, clearly shows that malaria parasite detection rates are not significantly increased by using venous blood samples/Qiagen method compared with 5 μL DBS/Instagene, especially for P. falciparum infections in low-transmission areas. Most of the samples missed by the 5 μL DBS/Instagene method were P. vivax infections, which are often found at very low parasite density in asymptomatic individuals. In addition, finger prick blood spots collection presents numerous advantages, including cost, and remains a more practical method for large-scale epidemiological studies, especially in low transmission malaria-endemic settings, where active case detection strategies need to be implemented.
Finally, in the context of malaria elimination, the main challenge is to detect all falciparum malaria parasite transmitters (i.e., individuals with a sufficient number of sexual parasites to be transmitted by mosquito bites). Further studies are needed to assess the association between PCR detection data (and especially gametocytes detection) and mosquito feeding assays, which is used as a gold standard approach, to determine the infectiousness of P. falciparum gametocyte carriers. This would help defining the required sensitivity of a diagnostic tool to be able to detect parasite carriers that are infectious.
ACKNOWLEDGMENTS
We thank everyone in Preah Vihear province for participating in the study. We are grateful to people involved in the MSF project, especially health workers and staff of the Ministry of Health of Cambodia in Preah Vihear province, the National Center for Parasitology, Entomology and Malaria Control, and the Institut Pasteur in Cambodia (IPC) for their collaboration. We are thankful to Pascale Chaillet, Amine Dahmane, Mark Debackere, and Rafael Van den Bergh for their support and relevant advices. We also thank the study teams, MSF field team, and the villagers who took part in this study.
Footnotes
Financial support: This study was supported by Médecins Sans Frontières, Belgium. Didier Ménard was supported by the French Ministry of Foreign Affairs.
Authors' addresses: Lydie Canier, Nimol Khim, Saorin Kim, Rotha Eam, Chanra Khean, Kaknika Loch, Malen Ken, and Didier Ménard, Institut Pasteur du Cambodge, Malaria Molecular Epidemiology Unit, Phnom Penh, Cambodia, E-mails: lcanier@pasteur-kh.org, knimol@pasteur-kh.org, ksaorin@pasteur-kh.org, rotha@pasteur-kh.org, kchanra@pasteur-kh.org, lkaknika@pasteur-kh.org, kmalen@pasteur-kh.org, and dmenard@pasteur-kh.org. Pieter Pannus, Philippe Bosman, Jorgen Stassijns, William Etienne, Martin De Smet, and Jean-Marie Kindermans, Médecins Sans Frontières, Médecins Sans Frontières, Brussels, Belgium, E-mails: pieter.pannus@Brussels.msf.org, bosmanphil@yahoo.com, Jorgen.Stassijns@Brussels.msf.org, William.Etienne@Brussels.msf.org, Martin.De.Smet@Brussels.msf.org, and Jean-Marie.Kindermans@Brussels.msf.org. Fabienne Nackers, Médecins Sans Frontières, Médecins Sans Frontières, Paris, France, E-mail: Fabienne.Nackers@Brussels.msf.org. SweetC Alipon, Médecins Sans Frontières, Médecins Sans Frontières, Phnom Penh, Cambodia, E-mail: sweetc.alipon@gmail.com. Meng Chuor Char and Nguon Chea, Ministry of Health, National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia, E-mails: mengchuor@gmail.com and cheanguon@yahoo.com.
References
- 1.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Malaria Report 2013. Geneva: WHO publications; 2013. [Google Scholar]
- 3.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57:818–826. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 7.Amaratunga C, Witkowski B, Khim N, Menard D, Fairhurst RM. Artemisinin resistance in Plasmodium falciparum. Lancet Infect Dis. 2014;14:449–450. doi: 10.1016/S1473-3099(14)70777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Aye MM, Lindegardh N, Tarning J, Imwong M, Jacob CG, Rasmussen C, Perin J, Ringwald P, Nyunt MM. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS ONE. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al- Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TH, Nguyen TT, Nguyen HP, Boni MF, Ngo VT, Nguyen TN, Le HT, Cao QT, Pham VT, Phung DT, Le TL, Le TD, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J. 2013;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WR, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MalERA Consultative Group on Diagnoses and Diagnostics A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 2011;8:e1000396. doi: 10.1371/journal.pmed.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 15.Canier L, Khim N, Kim S, Sluydts V, Heng S, Dourng D, Eam R, Chy S, Khean C, Loch K, Ken M, Lim H, Siv S, Tho S, Masse-Navette P, Gryseels C, Uk S, Van Roey K, Grietens KP, Sokny M, Thavrin B, Chuor CM, Deubel V, Durnez L, Coosemans M, Menard D. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J. 2013;12:405. doi: 10.1186/1475-2875-12-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyer S, Nguon S, Kim S, Habib N, Khim N, Sum S, Christophel EM, Bjorge S, Thomson A, Kheng S, Chea N, Yok S, Top S, Ros S, Sophal U, Thompson MM, Mellor S, Ariey F, Witkowski B, Yeang C, Yeung S, Duong S, Newman RD, Menard D. Focused Screening and Treatment (FSAT): a PCR-based strategy to detect malaria parasite carriers and contain drug resistant P. falciparum, Pailin, Cambodia. PLoS ONE. 2012;7:e45797. doi: 10.1371/journal.pone.0045797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosman P, Stassijns J, Nackers F, Canier L, Kim N, Khim S, Alipon SC, Chuor Char M, Chea N, Dysoley L, Van den Bergh R, Etienne W, De Smet M, Menard D, Kindermans JM. Plasmodium prevalence and artemisinin-resistant falciparum malaria in Preah Vihear Province, Cambodia: a cross-sectional population-based study. Malar J. 2014;13:394. doi: 10.1186/1475-2875-13-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strøm GE, Moyo S, Fataki M, Langeland N, Blomberg B. PCR targeting Plasmodium mitochondrial genome of DNA extracted from dried blood on filter paper compared to whole blood. Malar J. 2014;13:137. doi: 10.1186/1475-2875-13-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ataei S, Nateghpour M, Hajjaran H, Edrissian GH, Foroushani AR. High specificity of semi-nested multiplex PCR using dried blood spots on DNA Banking Card in comparison with frozen liquid blood for detection of Plasmodium falciparum and Plasmodium vivax. J Clin Lab Anal. 2011;25:185–190. doi: 10.1002/jcla.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]