Abstract
Leptospirosis is a common disease in tropical countries, and the kidney is one of the main target organs. Membrane proteins of Leptospira are capable of causing endothelial damage in vitro, but there have been no studies in humans evaluating endothelial glycocalyx damage and its correlation with acute kidney injury (AKI). We performed a cohort study in an outbreak of leptospirosis among military personnel. AKI was diagnosed in 14 of 46 (30.4%) patients. Leptospirosis was associated with higher levels of intercellular adhesion molecule-1 (ICAM-1; 483.1 ± 31.7 versus 234.9 ± 24.4 mg/L, P < 0.001) and syndecan-1 (73.7 ± 15.9 versus 21.2 ± 7.9 ng/mL, P < 0.001) compared with exposed controls. Patients with leptospirosis-associated AKI had increased level of syndecan-1 (112.1 ± 45.4 versus 41.5 ± 11.7 ng/mL, P = 0.021) and ICAM-1 (576.9 ± 70.4 versus 434.9 ± 35.3, P = 0.034) compared with leptospirosis patients with no AKI. Association was verified between syndecan-1 and ICAM-1 with serum creatinine elevation and neutrophil gelatinase-associated lipocalin (NGAL) levels. This association remained even after multivariate analysis including other AKI-associated characteristics. Endothelial injury biomarkers are associated with leptospirosis-associated renal damage.
Introduction
Leptospirosis is caused by a microorganism of the genus Leptospira. In total, nine pathogenic species are known, including L. interrogans.1 The annual incidence is estimated at 10–100 cases per 100,000 population in tropical countries.2
The early phase of leptospirosis manifestations lasts 3–7 days and includes fever, headaches, myalgia (especially in the calves), nausea, vomiting, malaise, and conjunctival hyperemia. Only 10% progress to the second phase: the Weil syndrome.3 This phase lasts from 4 to 30 days, and more severe symptoms, such as jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (AKI), can occur.4
The kidney is one of the main target organs of Leptospira, and AKI can occur in 20–85% of patients in the second phase of the disease.5,6 Interstitial nephritis is the main pathological finding in patients with leptospirosis, even in those without AKI.7,8 Leptospira or its fragments can be found in renal tubules and interstitium.9 Observational studies have indicated that Leptospira spread through the hematogenous route to the kidney, circulate to peritubular capillaries, migrate to the interstitium and renal tubule, and finally, remain in the proximal tubular lumen. In the kidney, proteins present in the outer membrane of Leptospira induce an inflammatory response. This response seems to be, at least in part, mediated by toll-like receptors.10
The outer membrane proteins (OMPs) of Leptospira are capable of promoting up-regulation of cell adhesion molecules (for instance, intercellular adhesion molecule-1 [ICAM-1]) expressed mainly in endothelial cells.11,12 Although these studies were performed in vitro with umbilical vein endothelial cells, they suggested a role of endothelial activation in leptospiral pathogenesis.11,12 Also, experimental and autopsy studies have shown endothelial necrosis and capillary thrombosis in lung tissue.13,14
Another important component of the endothelium is the glycocalyx, a 1-μm carbohydrate-rich structure located at the top of the endothelium. It has antiadhesive and anticoagulant properties that protect the endothelium and maintain the vascular barrier function. Glycocalyx damage is associated with pathophysiological sequelae, such as capillary leakage and edema formation, accelerated inflammation, platelet activation and hypercoagulability, and loss of vascular responsiveness.15,16 It is unknown whether glycocalyx lesions have any role in leptospirosis-related AKI pathophysiology.
In this study, we evaluated young patients from a leptospirosis outbreak among military personnel. Our objective was to evaluate the association between the presence and severity of renal lesions with biomarkers representative of glycocalyx and endothelial injury.
Methods
Subjects.
In this study, we analyzed subjects involved in an outbreak of leptospirosis among military personnel. Three military physicians were responsible for the patients' medical care. They made all decisions regarding need for hospitalization, frequency of diagnostic procedures, and antibiotic therapy. Immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA; PanBio Kit, Alere, Orlando, FL) and microagglutination (MAT) with 19 different serovars were carried out in all patients. The Ceará State Department of Health collected blood samples from all subjects 3 weeks after convalescence. IgM-ELISA and MAT were performed by the Central Laboratory of Public Health (LACEN), a government-sponsored reference laboratory. Positive cases were defined according to the Centers for Disease Control and Prevention (CDC) laboratory criteria for supportive (Leptospira agglutination titer ≥ 200 but < 800 by MAT in one serum specimen or detection of IgM antibodies against Leptospira in one acute-phase serum specimen) or confirmed (fourfold or greater increase in Leptospira agglutination titer between acute- and convalescent-phase serum specimens studied at the same laboratory or Leptospira agglutination titer ≥ 800 by MAT in one serum specimen) diagnosis. MAT confirmation criteria included seroconversion or a fourfold rise in titers between acute- and convalescent-phase sera obtained on the day of admission and after 14–30 days of convalescence or a titer ≥ 1:800 in one or more samples. This definition has been used in other studies.17 Other infectious diseases (hepatitis A, dengue, typhoid fever, and others) were excluded.
Symptoms, physical findings, and routine laboratory tests were retrospectively retrieved from medical records. Serum samples from the first blood drawn from each in-hospital patient were processed and stored at −70°C for posterior analyses (see below). Patients with less than three serum creatinine (SCr) measurements during hospitalization were excluded. Anemia was defined as hemoglobin less than 12 g/dL, and low platelet count was defined as a value < 150 × 103/μL. Another 31 soldiers exposed to the disease but with no laboratory diagnosis of leptospirosis (neither supportive nor confirmed) were selected as the exposed control group. The institutional ethical committee approved the study, and participants signed informed consent.
AKI definition and classification.
AKI was defined according to the Kidney Disease Improving Global Outcome (KDIGO) definition. Briefly, changes in SCr during hospitalization were calculated, and AKI was diagnosed by an increase in SCr ≥ 50% or ≥ 0.3 mg/dL compared with baseline SCr values and after 48 hours. AKI severity was classified as stage 1 (SCr increase by 50–100% or ≥ 0.3 mg/dL), stage 2 (SCr increase by 101–200%), or stage 3 (SCr increase by > 200%). Baseline SCr was considered as the lowest value during hospitalization, and estimated glomerular filtration rate (eGFR) was calculated using CKD-EPI formula. Also, the difference between the highest and lowest SCr values (ΔSCr) was considered as a marker of Acute Kidney Injury Network (AKI) severity.
Markers of inflammation, oxidative stress, renal lesion, glycocalyx, and endothelium injury.
Inflammatory status was evaluated using high-sensitivity C-reactive protein (hsCRP) by the nephelometry method. Oxidative stress was assessed through malondialdehyde (MDA) concentration based on thiobarbituric acid (TBA) reactivity. Other than SCr, we measured neutrophil gelatinase-associated lipocalin (NGAL), a marker of renal proximal tubule damage, using a commercial ELISA kit (Boster Biological Technology, Fremont, CA). Syndecan-1 was measured as a biomarker of endothelial glycocalyx injury (Abcam, Cambridge, MA). Finally, ICAM-1 was also measured using a commercial ELISA kit (Life Technologies Brasil, São Paulo, Brazil) as a marker of endothelial injury. All tests were performed in serum samples.
Statistical analysis.
Analysis was performed using SPSS 19.0 for Windows. Variables were tested for normal distribution. Continuous variables are reported as medians and interquartile ranges. Continuous data were compared using Mann–Whitney tests with Tukey post-tests. Categorical data are reported as proportions and compared using Fisher's exact tests. Univariate analysis is shown as Pearson's coefficient when indicated. Two multiple linear regression analyses were performed to identify the independent factors associated with AKI markers (ΔSCr and NGAL). A P value < 0.05 was considered statistically significant for all comparisons.
Results
Subjects' characteristics.
During a 4-day period, 83 subjects performed military training activities in an open-air field in July of 2012 in Ceará in the northeast of Brazil. For personal reasons, 3 military individuals withdrew before any exposure to the field, with 80 military soldiers remaining in the training. Among the military activities, those that entailed the highest exposure of skin or mucous membranes to water or soil contaminated by Leptospira were performed on the second and third days of training. On these days, transposition of watercourses and crawling exercises with water immersion were performed. At that place, there was stagnant water mixed with feces from urban and wild animals found in the area. During training, the soldiers crawled close to the ground, leaning on forearms and legs, which resulted in immersion in mud.
In total, 80 patients were exposed to Leptospira infection, and 49 had leptospirosis confirmed. Of these, 3 patients were excluded, because they had fewer than three SCr measurements available. In the end, 46 patients were included in the final analysis. All patients were male and between 18 and 24 years old, with a median age of 21 (19–23) years. No patient had any comorbidity, and all patients had a basal eGFR higher than 80 mL/min. The index case reported symptom onset 5 days after exposure, and the last case reported symptoms 21 days after exposure. The most common symptoms were headache (98%), myalgia, fever and prostration (96.1%), vomiting, diarrhea, calf pain (30%), and abdominal pain (17.1%). Laboratory data are disclosed in Table 1. Overall, 17 (36.9%) patients had anemia, and 16 (34.8%) patients had low platelet count. AKI was diagnosed in 14 (30.4%) patients, and all patients except for 1 had the milder form of AKI (AKIN stage 1). No patient had pulmonary hemorrhage. All patients were treated with ceftriaxone.
Table 1.
Clinical and laboratory data in patients according AKI diagnosis
| Exposed control group (N = 31) | All patients (N = 46) | P value* | No AKI patients (N = 32) | AKI patients (N = 14) | P value† | |
|---|---|---|---|---|---|---|
| Age (years) | 21.3 ± 1.7 | 21.3 ± 1.7 | 0.645 | 21.5 ± 1.3 | 20.8 ± 1.9 | 0.911 |
| Baseline GFR (mL/min per 1.73 m2) | 104.1 ± 10.2 | 102.6 ± 12.5 | 0.896 | 104.1 ± 13.1 | 99.2 ± 9.8 | 0.769 |
| Hemoglobin (g/dL) | 13.1 ± 1.7 | 11.7 ± 2.4 | 0.011 | 11.5 ± 3.4 | 12.0 ± 0.9 | 0.596 |
| White blood cells (cells/mm3) | 6.3 ± 2.0 | 11.9 ± 2.9 | < 0.001 | 12.2 ± 3.4 | 11.6 ± 2.5 | 0.784 |
| Platelet count (/μL) | 212.6 ± 26.4 | 155.4 ± 39.1 | < 0.001 | 159.3 ± 39.9 | 150.1 ± 40.8 | 0.573 |
| Alanine transaminase (U/L) | 26.2 ± 9.4 | 106.9 ± 50.7 | < 0.001 | 113.9 ± 49.8 | 95.8 ± 55.0 | 0.369 |
| Creatine kinase (U/L) | 72.9 ± 28.1 | 171.4 ± 57.2 | < 0.001 | 104.6 ± 73.9 | 263.6 ± 81.5 | 0.062 |
| hsCRP (mg/L) | 3.9 ± 3.4 | 110.0 ± 30.9 | < 0.001 | 109.0 ± 66.1 | 111.1 ± 58.3 | 0.940 |
| Malondialdehyde | 3.5 ± 1.8 | 9.5 ± 2.7 | < 0.001 | 8.9 ± 2.6 | 10.6 ± 2.6 | 0.081 |
| ICAM-1 (mg/L) | 234.9 ± 24.4 | 483.1 ± 31.7 | < 0.001 | 434.9 ± 35.3 | 576.9 ± 70.4 | 0.034 |
| Syndecan-1 (ng/mL) | 21.2 ± 7.9 | 73.7 ± 15.9 | < 0.001 | 41.5 ± 11.7 | 112.1 ± 45.4 | 0.021 |
| NGAL (ng/mL) | 32.3 ± 9.7 | 139.4 ± 22.2 | < 0.001 | 139.3 ± 22.6 | 139.8 ± 22.0 | 0.952 |
Exposed control group versus all patients.
No AKI patients versus AKI patients.
Additionally, we selected 31 exposed soldiers without leptospirosis diagnosis, and their data are show in Table 1. As expected, these subjects had lower levels of inflammatory, oxidative stress, and endothelial biomarkers. None of subjects in the exposed control group had low platelet level or altered renal function.
Endothelial biomarkers in leptospirosis.
Patients with leptospirosis had elevated serum levels of ICAM-1 (483.1 ± 31.7 versus 234.9 ± 24.4 mg/L, P < 0.001) and syndecan-1 (73.7 ± 15.9 versus 21.2 ± 7.9 ng/mL, P < 0.001) compared with patients in the exposed control group. There was a significant correlation between syndecan-1 and ICAM-1 levels (r = 0.629, P < 0.0001). No significant correlation was observed between syndecan-1 or ICAM-1 and hsCRP, MDA, hemoglobin level, creatine phosphokinase (CPK) or platelet count (Table 2).
Table 2.
Correlation between endothelial and renal damage biomarkers and other laboratory parameters
| ICAM-1 | Syndecan-1 | |||
|---|---|---|---|---|
| Pearson correlation coefficient | P value | Pearson correlation coefficient | P value | |
| Hemoglobin | 0.086 | 0.676 | −0.087 | 0.673 |
| White blood cells | 0.005 | 0.979 | 0.167 | 0.414 |
| Platelet count | 0.035 | 0.864 | −0.082 | 0.689 |
| Creatine kinase | −0.002 | 0.994 | −0.026 | 0.867 |
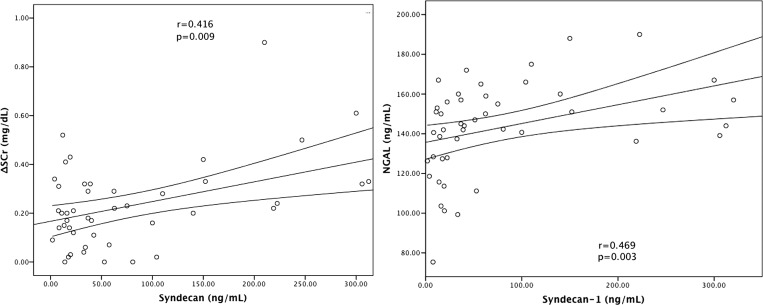
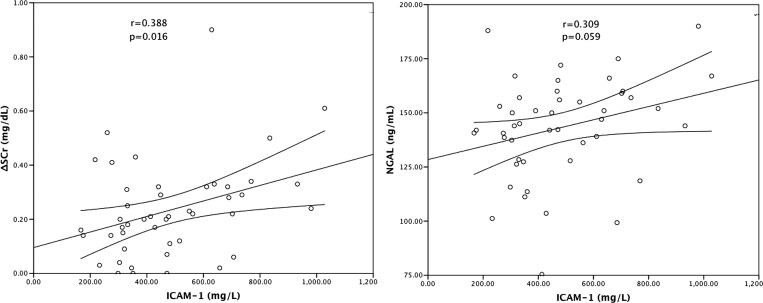
| ΔSCr | 0.388 | 0.016 | 0.416 | 0.009 |
| NGAL | 0.309 | 0.059 | 0.469 | 0.003 |
| hsCRP | 0.128 | 0.444 | 0.184 | 0.269 |
| Malondialdehyde | 0.169 | 0.309 | 0.094 | 0.573 |
Endothelial damage is associated with leptospirosis-associated AKI.
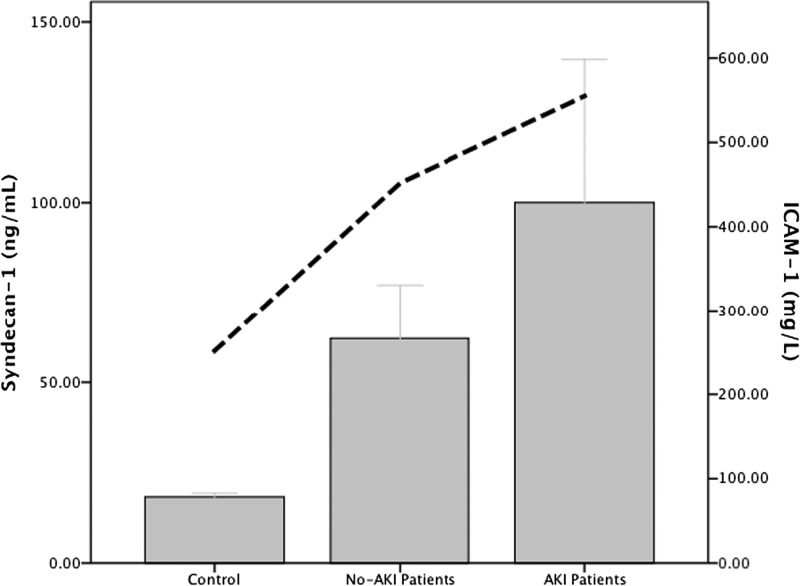
Patients with leptospirosis-associated AKI had increased levels of syndecan-1 (112.1 ± 45.4 versus 41.5 ± 11.7 ng/mL, P = 0.021) and ICAM-1 (576.9 ± 70.4 versus 434.9 ± 35.3, P = 0.034) compared with leptospirosis patients with no AKI (Figure 1 ). Also, a significant association was verified between syndecan-1 and ICAM-1 levels with ΔSCr in both absolute and relative values in relation to basal SCr (Figures 2 and 3 and Table 2).
Figure 1.
Mean values of syndecan-1 (bars) and ICAM-1 (dotted lines) according to groups. There is significance difference between all groups (in the text).
Figure 2.
Correlations between renal biomarkers and syndecan. Pearson coefficient correlation.
Figure 3.
Correlations between renal biomarkers and ICAM-1. Pearson coefficient correlation.
NGAL was similar in leptospirosis patients with or without AKI (139.8 ± 22.0 versus 139.3 ± 22.6 ng/mL, P = 0.952), and there was no significant association between NGAL and ΔSCr. Although there was no correlation between serum NGAL and the presence of AKI, there was a positive correlation between syndecan-1 and serum NGAL levels (r = 0.469, P = 0.003) (Figure 2 and Table 2). A trend was observed in the positive correlation between ICAM-1 and serum NGAL levels (r = 0.309, P = 0.059) (Figure 3).
To further explore the association between syndecan/ICAM-1 and renal damage markers, models of linear multivariate analysis were performed to evaluate independent association between endothelium markers with ΔSCr and NGAL. In these models, we forced all variables potentially associated with leptospirosis AKI: inflammatory status (white blood cells and hsCRP), oxidative stress (MDA), rhabdomyolysis (creatine kinase), serum liver enzymes, and platelet count. Even after forcing all variables into the model, syndecan and ICAM-1 remained independently associated with ΔSCr and NGAL (Table 3).
Table 3.
Multivariate analysis, including all laboratory parameters, shown to verify the independent association between syndecan-1 and renal damage markers
| Variable | Standardized β-coefficient | P value |
|---|---|---|
| Dependent variable: ΔSCr (mg/dL) | ||
| Hemoglobin (g/dL) | 0.077 | 0.793 |
| White blood cells (×103/mm3) | −0.224 | 0.479 |
| Platelet count (×103/mm3) | −0.329 | 0.484 |
| Alanine transaminase (U/L) | −0.450 | 0.160 |
| Creatine kinase (U/L) | 0.295 | 0.305 |
| hsCRP (mg/L) | 0.145 | 0.613 |
| Malondialdehyde | −0.043 | 0.893 |
| Syndecan-1 (ng/mL) | 0.682 | 0.021 |
| ICAM-1 (mg/L) | 0.560 | 0.032 |
| Dependent variable: NGAL (ng/mL) | ||
| Hemoglobin (g/dL) | −0.102 | 0.738 |
| White blood cells (×103/mm3) | 0.145 | 0.683 |
| Platelet count (×103/mm3) | 0.234 | 0.444 |
| Alanine transaminase (U/L) | −0.097 | 0.730 |
| Creatine kinase (U/L) | 0.432 | 0.108 |
| hsCRP (mg/L) | −0.101 | 0.712 |
| Malondialdehyde | 0.149 | 0.629 |
| Syndecan-1 (ng/mL) | 0.600 | 0.034 |
| ICAM-1 (mg/L) | 0.480 | 0.047 |
Discussion
In this study, for the first time, endothelial lesion biomarkers in leptospirosis were studied in humans. Also, we disclosed an important association between endothelial lesion (mainly glycocalyx) and the presence and severity of renal lesion. There was a correlation with both reductions in GFR and markers of renal proximal tubular damage (ΔSCr and NGAL, respectively).
The patients were extracted from an outbreak that occurred among military personnel in 2011 in northeastern Brazil. This fact provides support to our study mainly regarding two points: all patients had similar characteristics (same gender, similar age, and absence of comorbidities), and because all exposed individuals were submitted to medical assessment and laboratory tests, there was no selection bias regarding disease severity. Although the subjects were submitted to the same exposure, Leptospira burden was not necessarily the same, because possible injuries and cuts on the skin can influence the observed attack rate.
Overall, the patients included in the study had mild forms of leptospirosis. According to stringent definitions of severe leptospirosis (presence of at least one of the following criteria: AKI requiring dialysis, shock treated with vasoactive drugs, alveolar hemorrhage, bleeding requiring blood transfusion, respiratory failure needing mechanical ventilation, or death during hospitalization),18 no patient in this study had the severe form. Although 30% of included patients developed AKI, AKIN criteria was used, which is more sensitive than the previous AKI definition. This AKI definition can significantly overestimate AKI incidence; however, in other leptospirosis cohorts, it has been associated with increasing mortality.19
Although it is known that leptospirosis can induce vessel inflammation, only few studies have evaluated the role of endothelium in the pathophysiology of leptospirosis. In an ancillary study, De Brito and others14 described swollen endothelium and microvascular thrombosis in guinea pigs infected with Leptospira. Recently, a group of investigators described that the OMP of Leptospira can induce endothelial cell damage in vitro.12 To the best of our knowledge, this study is the first to disclose endothelial glycocalyx injury in human leptospirosis. Interestingly, these alterations could be seen, even in milder forms of leptospirosis.
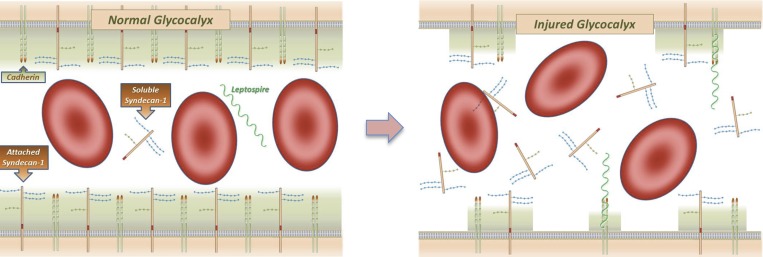
Migration of Leptospira to tissues involves the spirochetes adhering to and escaping from vasculature. Recently, it has been described that Leptospira adheres to vascular endothelium cadherin,20 which plays an important role in maintaining the vasculature barrier properties. Attachment of Leptospira to the vasculature through cadherin may result in vascular damage, facilitating the escape of the pathogen from the bloodstream into different tissues. One can speculate that glycocalyx and endothelial lesions occur during this translocation and that endothelial biomarkers are a marker of disease severity in not only the kidney but also, other organs (Figure 4). Alternatively, one can suppose that glycocalyx and endothelium lesions occur first, favoring spirochete adhesion and translocation. These hypotheses cannot be further explored in our findings because of the study design.
Figure 4.
Endothelial lesion, a suggested mechanism. Leptospira attaches to endothelium through cadherin followed by syndecan-1 release into circulation.
The most severely affected organs in leptospirosis are the kidney, liver, and lungs. In our population, no patients had pulmonary manifestations or liver failure, but almost all patients had mild to moderate liver enzyme elevation, and nearly 30% had AKI. Although AKI was milder in all except for one patient, there was a positive correlation between syndecan-1 and ICAM-1 levels and ΔSCr. This finding supports a role of endothelium activation/damage in the pathophysiology of leptospirosis-associated AKI. As stated in the Introduction, Leptospira affects mainly the proximal tubular cells after transmigration through the peritubular capillary network. To further study this association, we have measured a renal biomarker that is more specific for proximal tubular injury (NGAL), and it showed a positive correlation with both syndecan-1 and ICAM-1.
Even after controlling for several laboratory findings, syndecan-1, but not ICAM-1, remained associated with AKI severity. This highlights the potential and unexplored role of endothelial glycocalyx in leptospirosis-associated AKI. There have been only two studies evaluating syndecan-1 levels in patients with renal disease.21 In one study, patients undergoing maintenance hemodialysis had higher levels of syndecan-1 than controls. The other study excluded any accumulation effect of syndecan-1 into the circulation because of reduced renal clearance,22 and thus, it is unlikely that only the reduced GFR may be responsible for the increase in syndecan levels.
Although not the main focus of the study, one point that also deserves consideration is the lack of correlation between NGAL and AKI, contrary to what has been shown by many other studies. Also, AKI in leptospirosis has been associated with low platelet count,23 rhabdomyolysis,24 and other clinical parameters. In this study, we were unable to show these associations. The lack of associations can also be explained by the milder AKI developed by patients.
Our study has some limitations, but two deserve consideration. First, although we measured endothelial and glycocalyx biomarkers during the first medical consultation, some patients already had some kidney damage and GFR decrease, making it difficult to ascertain a causal association between endothelial damage and AKI. Second, all except for one patient had milder AKI. It is highly probable the associations disclosed in this study become stronger with increasing AKI severity, but that can only be speculated at this point.
In conclusion, we studied a homogeneous group of young male patients from the military community exposed to a leptospirosis outbreak and observed that endothelial and mainly, glycocalyx injury biomarkers are related with renal damage, even in patients with milder AKI.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: A.B.L., A.M.C.M. and E.d.F.D. are recipients of a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Authors' addresses: Alexandre Braga Libório and Elizabeth de Francesco Daher, Department of Clinical Medicine, Faculty of Medicine, Universidade Federal do Ceará, Fortaleza, Ceara, Brazil, E-mails: alexandreliborio@yahoo.com.br and ef.daher@uol.com.br. Marcelo Boecker Munoz Braz, Fernanda Macedo de Oliveira Neves, and Danielle Carvalho Pedrosa, Medical Sciences Post-Graduate Program, Department of Clinical Medicine, Universidade Federal do Ceará, Fortaleza, Ceara, Brazil, E-mails: boecker@oi.com.br, fepinhal@ig.com.br, and dannycpmed@hotmail.com. Antonio Carlos Seguro, Laboratório de Investigação Médica (LIM 12) do Hospital das Clínicas da Faculdade de Medicina USP, São Paulo, SP, Brazil, E-mail: trulu@usp.br. Gdayllon C. Meneses and Alice Maria Costa Martins, Department of Clinical and Toxicological Analysis, Faculty of Pharmacy, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mails: gdayllon@yahoo.com.br and martinsalice@gmail.com. Luciano Pamplona de Góes Cavalcanti, Departamento de Saúde Comunitária, Universidade Federal do Ceará, Fortaleza, Ceara, Brazil, E-mail: pamplona.luciano@gmail.com.
References
- 1.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008;12:351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade L, de Francesco Daher E, Seguro AC. Leptospiral nephropathy. Semin Nephrol. 2008;28:383–394. doi: 10.1016/j.semnephrol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Daher EF, Silva GB, Jr, Lima RS, Mota RM, Rocha HA, de Abreu KL, Barreto AG, Pereira ED, Araújo SM, Libório AB. Different patterns in a cohort of patients with severe leptospirosis (Weil syndrome): effects of an educational program in an endemic area. Am J Trop Med Hyg. 2011;85:479–484. doi: 10.4269/ajtmh.2011.11-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daher EF, Silva GB, Jr, Karbage NN, Carvalho PC, Jr, Kataoka RS, Silva EC, Magalhães MM, Mota RM, Araújo SM, Gutiérrez-Adrianzén OA, Libório AB. Predictors of oliguric acute kidney injury in leptospirosis. A retrospective study on 196 consecutive patients. Nephron Clin Pract. 2009;112:c25–c30. doi: 10.1159/000210571. [DOI] [PubMed] [Google Scholar]
- 7.Sitprija V, Pipatanagul V, Mertowidjojo K, Boonpucknavig V, Boonpucknavig S. Pathogenesis of renal disease in leptospirosis: clinical and experimental studies. Kidney Int. 1980;17:827–836. doi: 10.1038/ki.1980.95. [DOI] [PubMed] [Google Scholar]
- 8.Penna D, De Brito T, Pupo AA, Machado MM, Galvão PAA, de Almeida SS. Kidney biopsy in human leptospirosis. Am J Trop Med Hyg. 1963;12:896–901. doi: 10.4269/ajtmh.1963.12.896. [DOI] [PubMed] [Google Scholar]
- 9.De Brito T, Menezes LF, Lima DMC, Lourenço S, Silva AMG, Alves VAF. Immunohistochemical and in situ hybridization studies of the liver and kidney in human leptospirosis. Virchows Arch. 2006;448:576–583. doi: 10.1007/s00428-006-0163-z. [DOI] [PubMed] [Google Scholar]
- 10.Yang C-W. Leptospirosis renal disease: understanding the initiation by Toll-like receptors. Kidney Int. 2007;72:918–925. doi: 10.1038/sj.ki.5002393. [DOI] [PubMed] [Google Scholar]
- 11.Gómez RM, Vieira ML, Schattner M, Malaver E, Watanabe MM, Barbosa AS, Abreu PA, de Morais ZM, Cifuente JO, Atzingen MV, Oliveira TR, Vasconcellos SA, Nascimento AL. Putative outer membrane proteins of Leptospira interrogans stimulate human umbilical vein endothelial cells (HUVECS) and express during infection. Microb Pathog. 2008;45:315–322. doi: 10.1016/j.micpath.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Vieira ML, D'Atri LP, Schattner M, Habarta AM, Barbosa AS, de Morais ZM, Vasconcellos SA, Abreu PA, Gómez RM, Nascimento AL. A novel leptospiral protein increases ICAM-1 and E-selectin expression in human umbilical vein endothelial cells. FEMS Microbiol Lett. 2007;276:172–180. doi: 10.1111/j.1574-6968.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 13.De Brito T, Aiello VD, da Silva LF, Gonçalves da Silva AM, Ferreira da Silva WL, Castelli JB, Seguro AC. Human hemorrhagic pulmonary leptospirosis: pathological findings and pathophysiological correlations. PLoS ONE. 2013;8:e71743. doi: 10.1371/journal.pone.0071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Brito T, Böhm GM, Yasuda PH. Vascular damage in acute experimental leptospirosis of the guinea-pig. J Pathol. 1979;128:177–182. doi: 10.1002/path.1711280403. [DOI] [PubMed] [Google Scholar]
- 15.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 16.Palaiologou M, Delladetsima I, Tiniakos D. CD138 (syndecan-1) expression in health and disease. Histol Histopathol. 2014;29:177–189. doi: 10.14670/HH-29.177. [DOI] [PubMed] [Google Scholar]
- 17.Reis EA, Hagan JE, Ribeiro GS, Teixeira-Carvalho A, Martins-Filho OA, Montgomery RR, Shaw AC, Ko AI, Reis MG. Cytokine response signatures in disease progression and development of severe clinical outcomes for leptospirosis. PLoS Negl Trop Dis. 2013;7:e2457. doi: 10.1371/journal.pntd.0002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubiana S, Mikulski M, Becam J, Lacassin F, Lefèvre P, Gourinat AC, Goarant C, D'Ortenzio E. Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl Trop Dis. 2013;7:e1991. doi: 10.1371/journal.pntd.0001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva Júnior GB, Abreu KL, Mota RM, Barreto AG, Araújo SM, Rocha HA, Libório AB, Daher EF. RIFLE and Acute Kidney Injury Network classifications predict mortality in leptospirosis-associated acute kidney injury. Nephrology (Carlton) 2011;16:269–276. doi: 10.1111/j.1440-1797.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista K, Franco R, Schwab A, Coburn J. Leptospira interrogans binds to cadherins. PLoS Negl Trop Dis. 2014;8:e2672. doi: 10.1371/journal.pntd.0002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900–1908. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, Lukasz A, Oberleithner H, Pavenstädt H, Brand M, Kümpers P. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234:335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Raoult D, Jeandel P, Mailloux M, Rougier Y. Thrombocytopenia and renal failure in leptospirosis. Am J Trop Med Hyg. 1983;32:1464. doi: 10.4269/ajtmh.1983.32.1464. [DOI] [PubMed] [Google Scholar]
- 24.Coursin DB, Updike SJ, Maki DG. Massive rhabdomyolysis and multiple organ dysfunction syndrome caused by leptospirosis. Intensive Care Med. 2000;26:808–812. doi: 10.1007/s001340051252. [DOI] [PMC free article] [PubMed] [Google Scholar]