Abstract
Direct skin feeding experiments are sensitive assays to determine human infectiousness to mosquitoes but are rarely used in malaria epidemiological surveys. We determined the infectiousness of inhabitants of a malaria hypoendemic area in Senegal. Gametocyte prevalence by microscopy was 13.5% (26 of 192). Of all individuals who were gametocyte positive, 44.4% (11 of 25) infected ≥ 1 Anopheles arabiensis mosquito and 10.8% (54 of 500) of mosquitoes became infected. Of all individuals who were gametocyte negative by microscopy, 4.3% (7 of 162) infected ≥ 1 mosquito and 0.4% (12 of 3240) of mosquitoes became infected. The 18.2% (12 of 66) of all mosquito infections was a result of submicroscopic gametocyte carriage and two individuals without asexual parasites or gametocytes by microscopy were infectious to mosquitoes. When infectivity and local demography was taken into account, children 5–14 years of age contributed 50.8% of the human infectious reservoir for malaria. Adults and submicroscopic gametocyte carriers may contribute considerably to onward malaria transmission in our setting.
Introduction
Malaria is one of the most deadly infections in the world with ~600,000 deaths annually, predominantly in young children in sub-Saharan Africa.1–3 In Senegal, malaria transmission has declined recently,4 although Plasmodium falciparum remains endemic throughout the country.5 Malaria transmission occurs from mosquito to man and from man to mosquito. Transmission from man to mosquitoes is poorly quantified6 and a focus often lies on entomological parameters like aggressiveness of anophelines to humans, the entomological inoculation rate, vectorial capacity,7,8 and indicators of parasite prevalence in the human population.9 However, these parameters fail to directly assess human infectivity that is most relevant in describing malaria transmission from mosquito to man.10 Only a handful of surveys on the natural process of transmission of Plasmodium from man to mosquito have been taken. Some indices have been defined, including the proportion of humans with malaria transmission stages, gametocytes in their peripheral blood, the proportion of infectious individuals, and the proportion of mosquitoes that have become infected after feeding.11–15 Often, these studies were restricted to microscopically detected gametocyte carriers, even though it has been repeatedly shown that submicroscopic gametocyte densities may result in mosquito infection.16,17 Although microscopically detectable gametocyte densities are more likely to result in mosquito infections compared with submicroscopic gametocyte densities, the contribution of submicroscopic gametocyte carriers to the total infectious reservoir may be considerable18 and can only be determined in xenodiagnostic surveys where individuals are recruited for feeding experiments, regardless of their parasitemic status. A recent meta-analysis further suggests that direct skin feeding assays may be the most sensitive approach to determine human infectiousness to mosquitoes.16
In this study, we determine infectivity of the human population to mosquitoes in a hypoendemic area of Senegal using direct skin feeding mosquito assays and observe a considerable contribution of adults and submicroscopic gametocyte carriers to malaria transmission.
Materials and methods
This study was carried out in 2010 in the Thies region 90 Km north of Dakar. Malaria transmission was described by Robert and others19 in the bordering zone of Niakhar. Anopheles arabiensis is the main vector. Malaria transmission occurs mainly during the rainy season from July to October with about 400–500 mm of annual rainfall and a long dry season from November to June. The level of malaria transmission in this area is hypo-endemic; older estimates of the sporozoite rate in field-caught mosquitoes were 1.6% to 1.8%.19 The study procedures were previously described by Bonnet and others.20 Families were invited to participate in the study during community sensitization meetings. From all participating families, family members ≥ 5 years of age were invited for mosquito feeding assays. Children < 5 years of age were not included because of ethical concerns.
Ethical approval was granted by the National Ethical committees of Senegal for this study. The study protocol and objectives were carefully explained to the local administrative, community leaders, and the assembled village population. Participants were offered free treatment of malaria, intestinal helminthes infections and anemia, and received during the project an insecticide impregnated bed net. Individual written informed consent was obtained from all participants and all caretakers of participants < 18 years of age.
Measurements.
Thick blood smears were prepared from each individual and slides were stained with 10% giemsa for 30 minutes and microscopically examined. Two-hundred fields were read for asexual parasites and gametocytes. Assuming 10–20 white blood cells per high power field and 8,000 white blood cells per μL, this gives an estimated sensitivity of microscopy of 2–4 parasites/μL. Sulfadoxine-pyrimethamine (Fansidar, Laboratoire Roche, France) was provided for individuals who were infected with > 1,000 parasites/μL.
Mosquito feeding assays.
Study participants > 5 years of age were included in an evaluation of infectiousness by feeding laboratory-bred mosquitoes directly on their skin.11,16 A local strain of An. arabiensis that has been reared in IRD (Institut de Recherche pour le Developpement) insectary in Dakar was used. Batches containing 25 3-day-old mosquitoes, female anophelines, were starved for 12 hours before being applied to the participant arm. Each individual receives one batch of mosquitoes. Mosquitoes were allowed to feed 10–15 minutes and after feeding, disinfection with alcohol and anti-histamine ointment was put on the skin. Participants were instructed to avoid movement and tolerated the blood feeding without any side effects. After feeding, mosquitoes were transferred from the cups to the cages 12 × 12 × 12 cm and labeled (number of the gametocyte carrier, date of infection). With a mouth aspirator, unfed mosquitoes were removed. Sugar solution was provided for 7 days during which mosquitoes were kept at 25°C, 70–80% humidity. Only cages with at least 20 mosquitoes surviving until Day 7 were included in the analysis. The midguts of surviving females were dissected in a drop of physiological saline (PBS) with 1% mercurochrome to facilitate observation of oocysts detection at the microscope. The infectivity of each patient participant was assessed by determining the percentage of mosquito midguts infected with at least one oocyst.
Statistical analysis.
Data were analyzed in Epi Info version 7 (Centers for Disease Control and Prevention, Atlanta, GA) and Stata version 12 (StataCorp, College Station, TX). Proportions were compared by the χ2 test or Fisher exact; mean densities by the Mann-Whitney test or the Kruskall Wallis test. The contribution of different age groups to malaria transmission (Ci) was estimated using the proportion (Pi) of the total population in each age group and the age group-specific probability of mosquito infection (Ti), which corresponds to the proportion of mosquitoes infected in feeding assays involving individuals from each age group, regardless of their parasite status:
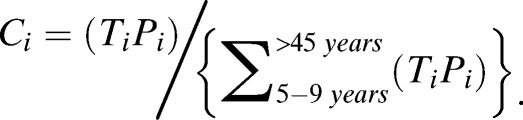 |
Results
We enrolled a total of 192 individuals 5–65 years of age. Parasite prevalence was 49.0% (94 of 192) and negatively associated with age as a categorical variable (Table 1, P = 0.05). Gametocyte prevalence was 13.5% (26/192) and negatively associated with age as a categorical variable (Table 1, P = 0.005). Geometric mean gametocyte density was 53.7 gametocytes/μL (interquartile range [IQR] 4–226) and there were too few microscopy positive gametocyte observations to allow a robust statistical analysis of gametocyte density in relation to age.
Table 1.
Characteristics of the study population
| Ages groups | |||||
|---|---|---|---|---|---|
| 5–9 | 10–14 | 15–24 | 25–44 | ≥ 45 | |
| N | 45 | 44 | 31 | 38 | 34 |
| Asexual parasite prevalence, % (n/N)* | 53.3 (24/45) | 63.6 (28/44) | 45.2 (14/31) | 34.2 (13/38) | 44.1 (15/34) |
| Asexual parasite density, GM (IQR) | 418.4 (90.5–2192.5) | 500.3 (94.5–5052.0) | 176.7 (76.0–198.0) | 83.8 (65.0–119.0) | 129.2 (56.0–195.0) |
| Gametocyte prevalence, % (**n/N) | 28.9 (13/45) | 11.4 (5/44) | 9. 7 (3/31) | 5.3 (2/38) | 8.8 (3/34) |
N = proportion of each age group in the sample population.
n = number of individuals with asexual parasite.
n = number of gametocyte carriers in each age group.
IQR = interquartile range; GM = Geometric mean.
Mosquito feeding experiments.
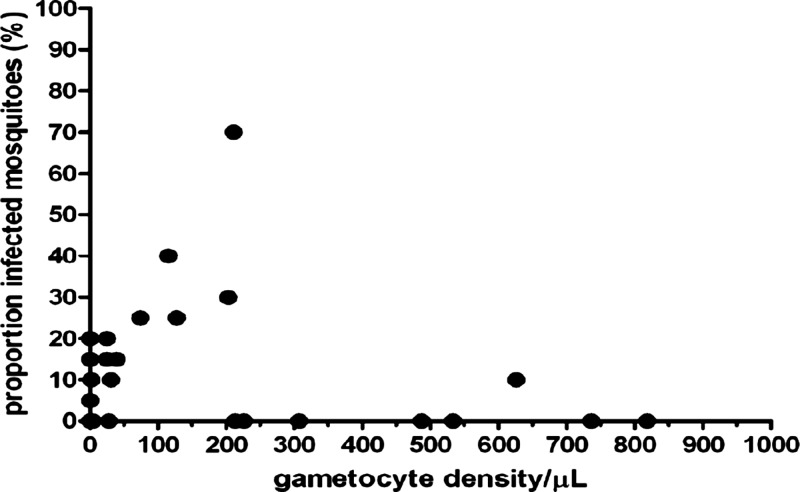
Direct skin feeding experiments were successful for 187 individuals (97.4%, 187 of 192); four experiments failed because fewer than 20 mosquitoes survived until Day 7 post feeding (5, 7, 9, and 11 mosquito observations) and one subject was found to be Plasmodium malariae infected; this individual did not infect mosquitoes and was excluded from the analyses. Exactly 20 mosquitoes were dissected and examined for the remaining 187 experiments, giving 3,740 mosquito observations. Eighteen experiments (9.4%, 18 of 187) resulted in at least one infected mosquito; 66 mosquitoes (1.8%, 66 of 3740) became infected. The results of the feeding experiment for each age group and gametocyte-positive and -negative individuals are reported in Table 2 and shows that gametocyte-positive individuals were considerably more likely to infect mosquitoes (P < 0.001) and infected more mosquitoes (P < 0.001). Of all individuals who were gametocyte positive by microscopy, 44.4% (11 of 25) infected at least one mosquito and 10.8% (54 of 500) of mosquitoes became infected. Of all individuals who were gametocyte negative by microscopy, 4.3% (7 of 162) infected at least one mosquito and 0.4% (12 of 3,240) of mosquitoes became infected. In children and adults some individuals who were gametocyte negative by microscopy infected mosquitoes. In our study setting 18.2% (12 of 66) of all mosquito infections was a result of submicroscopic gametocyte carriage. Among the seven individuals who infected mosquitoes despite being gametocyte negative, two were completely slide negative (asexual and sexual parasite slide negative); the remaining five had asexual parasites detected by microscopy but no gametocytes. Although there was a positive correlation between gametocyte density and mosquito infection rates (Figure 1; r = 0.47, P < 0.0001), the association appeared non-linear and there were a number of individuals with high gametocyte densities that did not infect any mosquitoes.
Table 2.
Infectiousness in relation to age and parasite status
| Gametocyte status | Number of experiments | Proportion of individuals infecting ≥ 1 mosquito, % (n/N) | Proportion of infected mosquitoes, % (n/N) | |
|---|---|---|---|---|
| 5–9 | Positive | 12 | 25.0 (3/12) | 5.0 (12/240) |
| Negative | 31 | 6.5 (2/31) | 0.3 (2/680) | |
| 10–14 | Positive | 5 | 80.0 (4/5) | 15.0 (15/100) |
| Negative | 39 | 5.1 (2/39) | 0.6 (5/780) | |
| 15–24 | Positive | 3 | 66.7 (2/3) | 36.7 (22/60) |
| Negative | 28 | 0.0 (0/28) | 0 (0/500) | |
| 25–44 | Positive | 2 | 50.0 (1/2) | 7.5 (3/40) |
| Negative | 35 | 0.0 (0/35) | 0 (0/700) | |
| ≥ 45 | Positive | 3 | 33.3 (1/3) | 3.3 (2/60) |
| Negative | 29 | 10.3 (3/29) | 0.9 (5/580) | |
| Total | 187 | 9.6 (18/187) | 1.8 (66/3740) |
Figure 1.
The association between microscopy gametocyte density and the proportion of infected mosquitoes. Each dot represents a single experiment from a single individual.
The human infectious reservoir for malaria.
The contribution of different age groups to the human infectious reservoir for malaria was estimated by including demographic data from a recent demographic survey in the study area (Table 3).
Table 3.
The contribution of different age groups to the human infectious reservoir in the population
| Proportion in the current study (n) | Proportion of population in a census (%) = (P) | Proportion of individuals infecting ≥ 1 mosquito, % (n/N) | Proportion of infected mosquitoes, % (n/N) = (T) | Relative contribution to the infectious reservoir of individuals ≥ 5 years* | |
|---|---|---|---|---|---|
| < 5 years | ND | 19.3 | ND | ND | ND |
| 5–9 | 23.0 (43) | 17.0 | 11.6 (5/43) | 1.6 (14/860) | 19.7% |
| 10–14 | 23.5 (44) | 12.3 | 13.6 (6/44) | 2.3 (20/880) | 19.9% |
| 15–24 | 16.6 (31) | 17.3 | 6.5 (2/31) | 3.5 (22/620) | 43.7% |
| 25–44 | 19.8 (37) | 20.3 | 2.7 (1/36) | 0.4 (3/740) | 5.9% |
| ≥ 45 | 17.1 (32) | 13.8 | 12.5 (4/32) | 1.1 (7/640) | 10.8% |
| Total | (187) | 100.0 | 9.6 (18/187) | 1.8 (66/3740) | 100% |
ND = non-determined; contribution to the infectious reservoir estimated by the formula.
The validity of the estimation of malaria transmission from man to mosquito depends on the representativeness of our sample to the total population. We compared the proportions of each age group between this sample and the national census in the study area. There was a significant difference between the demographic profile of the population on which mosquitoes fed and the demographic profile of the total population in this area. The under-representation of 5–14 age groups and the over 44 age-groups was a result of the absence of some schoolboys far from the village, and some adults working outside the study area and thereby not available for the surveys. When we adjust our estimates of infectiousness to mosquitoes for the contribution of different age groups to the total population, we found that ~51.5% of all mosquito infections that resulted from blood sampling on individuals ≥ 5 years of age was a result of children 5–14 years of age. Young adults, 15–24 years of age comprised 17.3% of the village population according to census data and contributed to 33.3% of all mosquito infections. The remaining infected mosquitoes 15.15% (10 of 66) were contributed by those over 24 years of age.
Discussion
In this study, we determined the human infectious reservoir in an area of hypoendemic malaria transmission in Senegal. We observed that ~44% of the microscopically detectable gametocyte carriers were capable of infecting An. arabiensis mosquitoes. Although we did not directly determine gametocyte carriage by molecular methods, we found evidence for subpatent gametocyte carriage: 4.3% of gametocyte-negative individuals were capable of infecting mosquitoes, although with low mosquito infection rates. Adults comprised a considerable proportion of the human infectious reservoir, more than half of all mosquito infections occurred from individuals > 15 years of age.
We performed a xenodiagnostic evaluation of transmission from man to mosquito selecting individuals irrespective of gametocytaemia or parasitaemia.13,17,21 Direct feeding of laboratory-bred An. arabiensis was performed by using a convenience sampling approach in which families were invited to participate during community meetings and all family members 5 years of age and above were invited to participate in the study. Although there is variation in the manner in which skin feeding is performed, it appears to be a more efficient tool to measure Plasmodium falciparum transmission from man to mosquito than the more widely used membrane feeding assays.16,22,23 We chose a minimum number of fed mosquitoes per batch to maximize the informativeness of individual feeding experiments.24 Our findings confirm previous findings on a higher likelihood of mosquito infection from microscopically detectable gametocyte densities16,24 and a relevant contribution of submicroscopic gametocyte carriers to onward malaria transmission.18 Because of the methodology we used, direct skin feeding, we could not directly determine gametocyte density in the mosquito blood meal. There are indications that gametocyte densities may be higher in the subdermal vasculature compared with venous blood16 and potentially that there are higher gametocyte densities in the subdermal vasculature compared with finger prick blood samples.25 One may therefore hypothesize that mosquitoes sample gametocytes more efficiently from the subdermal vasculature than microscopy can detect gametocytes in finger prick blood samples that are taken from slightly deeper capillaries. The phenomenon of relatively efficient mosquito transmission from low gametocyte densities has also been reported for membrane feeding assays where gametocytes were detected in the venous blood sample that was offered to mosquitoes18 and suggests that clustering of gametocytes in the mosquito blood meal26 may contribute to the efficient transmission of malaria at gametocyte densities below the threshold density, which allows gametocyte detection by microscopy.6 Specific studies must be carried out to elucidate this long-standing question on gametocyte densities and infectiousness in different blood compartments that may have fundamental consequences for the interpretation of malariometric indices from venous blood or finger prick blood samples.
Shortcomings of our study include the fact that we did not perform a formal random selection of individuals but approached families and recruited all family members who were willing to participate. The most important shortcoming may be that we excluded children < 5 years of age for ethical reasons.27 In our study setting, it was considered unacceptable to include this young age group. Membrane feeding assays may be more acceptable but have a lower sensitivity and form a less natural assessment of infectivity where loss of gametocyte infectiousness caused by handling of venous blood samples cannot be excluded.16 Our choice for direct skin feeding precluded any statements on the infectiousness of the youngest age group who may have the highest gametocyte densities6 and may be particularly infectiousness to mosquitoes and form an important part of the human infectious reservoir. Despite this important limitation, our findings illustrate that adults, in particular young adults 15–24 years of age, contribute considerably to onward malaria transmission. This age group is currently not directly targeted in malaria control interventions in Senegal, and this may limit the impact of current interventions on malaria transmission intensity because a considerable proportion of the human infectious reservoir is neglected. Another shortcoming is our dependence on microscopy. We did not use molecular methods to detect gametocytes that are known to substantially increase the proportion of gametocyte carriers that are detected in a population.6 By reading 200 microscopic fields, double the number of fields routinely examined, we have attempted to maximize the sensitivity of microscopy detection. Despite this extensive slide reading, not all infectious individuals were detected. Many authors report that individuals with submicroscopic gametocytes can successfully infect mosquitoes,18,20,21,28,29 and we show that reading 200 microscopic fields does not overcome the limited sensitivity of microscopy for gametocyte detection. Although the limited sensitivity of microscopy to detect infectious individuals is widely accepted, this is not the same as evidence for a contribution of submicroscopic infections to malaria transmission.30 In our setting, the majority of individuals who were infectious to mosquitoes but gametocyte negative by microscopy had asexual parasites detected by microscopy and where therefore not truly subpatent infections. This is important to put our findings in perspective because these submicroscopic gametocyte carriers would have been detected by conventional diagnostics that detect (asexual) malaria parasites. We observed that two individuals without asexual parasites or gametocytes by microscopy were infectious to mosquitoes, comprising 11% of the total number of infectious individuals. One of these two individuals infected 20% (4 of 20) of the feeding mosquitoes, the other 5% (1 of 20). These individuals will have remained undetected by conventional diagnostics. If such subpatent infections are also found to be infectious in low endemic areas, where subpatent infections are particularly prevalent,31 they may form a stumbling block for successful elimination initiatives and may need to be included in malaria interventions to maximize their effect.
In conclusion, our study showed that ~10% of all individuals ≥ 5 years of age were infectious to An. arabiensis mosquitoes in the peak transmission season in an area of intense malaria transmission. Infectiousness to mosquitoes was not restricted to any age group or to individuals with gametocytes detected by microscopy. We observed successful mosquito infections from individuals without microscopically detectable asexual parasites or gametocytes. If the infectiousness of such subpatent infections is confirmed in other settings, notably low endemic settings that aim for malaria elimination, they may fulfill an important role in maintaining malaria transmission and may require inclusion in malaria control and elimination efforts.
ACKNOWLEDGMENTS
We thank the inhabitants of Anene, Keur Mamour, and Keur Madaro (Thies rural area) for their appreciated collaboration. We are grateful to Moussa Gueye and Maty for their technical assistance. We also thank Kevin Opondo for his assistance in the data analysis.
Footnotes
Financial support: This work was supported by the Institute of Research for Development (IRD).
Authors' addresses: Abdoulaye Gaye and Musa Jawara, Medical Research Council Unit, Banjul, Fajara, The Gambia, E-mails: abgaye@mrc.gm/layegaye@gmail.com and jawaramusa@mrc.gm. Teun Bousema, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Immunology and Infection, London School of Hygiene and Tropical Medicine, London, UK, E-mail: Teun.Bousema@lshtm.ac.uk. Gadiaga Libasse, Programme Nationale de Lutte contre le Paludisme, Dakar-Fann, Sénégal, E-mail: lgadius@gmail.com. Mamadou O. Ndiath, Institut Pasteur de Bangui, République Centrafricaine, E-mail: ousmane.ndiath@gmail.com. Lassana Konaté and Ousmane Faye, Universite Cheikh Anta Diop de Dakar, Departement de Biologie Animale, Dakar, Senegal, E-mails: konatela@yahoo.fr and jogomaye@yahoo.fr. Cheikh Sokhna, Unite de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, UMR 198, Campus UCAD – IRD, Dakar, Senegal, E-mail: cheikh.sokhna@ird.fr.
References
- 1.RTS,S Clinical Trials Partnership A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e100029. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray JC, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, Dieye-Ba F, Roucher C, Bouganali C, Badiane A, Sarr FD, Mazenot C, Touré-Baldé A, Raoult D, Druilhe P, Mercereau-Puijalon O, Rogier C, Sokhna C. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bed nets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 5.Thiam S, Thior M, Faye B, Ndiop M, Diouf ML, Diouf MB, Diallo I, Fall FB, Ndiaye JL, Albertini A, Lee E, Jorgensen P, Gaye O, Bell D. Major reduction in anti-malarial drug consumption in Senegal after nation-wide introduction of malaria rapid diagnostic tests. PLoS ONE. 2011;6:e18419. doi: 10.1371/journal.pone.0018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousema T, Drakeley C. Vivax gametocytes in relation to malaria Plasmodium falciparum and Plasmodium epidemiology and infectivity of control and elimination. Clin Microbiol Rev. 2011;24:377. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald G. The Epidemiology and Control of Malaria. London: Oxford University Press; 1957. [Google Scholar]
- 8.Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae. Bull World Health Organ. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 9.Gething PW, Patil AP, Smith DL, Guerra1 CA, Elyazar IR, Johnston GL, Tatem AJ, Hay SI. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malaria Journal. 2011 doi: 10.1186/1475-2875-10-378. http://www.malariajournal.com/content/10/1/378 doi:10.1186/1475-2875. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muirhead-Thomson R. The malaria infectivity of an African village population to mosquitoes (A. gambiae): a random xenodiagnostic survey. Am J Trop Med Hyg. 1957;6:971–979. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- 12.Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. Measurement of malarial infectivity of human populations to mosquitoes in the Madang Area, Papua, New Guinea. Parasitology. 1988;96((Pt 2)):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- 13.Githeko AK, Brandling-Bennett AD, Beier MS, Atieli F, Owaga M, Collins FH. The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg. 1992;86:355–358. doi: 10.1016/0035-9203(92)90216-y. [DOI] [PubMed] [Google Scholar]
- 14.Boudin C, Bonnet S, Tchuinkam T, Gouagna LC, Gounoue R, Manga L. Levels of malaria transmission: methods and parameters. Med Trop (Mars) 1998;58:69–75. (French) [PubMed] [Google Scholar]
- 15.Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA. Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans R Soc Trop Med Hyg. 2000;94:472–476. doi: 10.1016/s0035-9203(00)90056-7. [DOI] [PubMed] [Google Scholar]
- 16.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg. 1993;48:700–706. doi: 10.4269/ajtmh.1993.48.700. [DOI] [PubMed] [Google Scholar]
- 18.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Lin Ouédraogo A, Basáñez M-G. Predicting Mosquito Infection from Plasmodium falciparum Gametocyte Density and Estimating the Reservoir of Infection. 2013. http://dx.doi.org/10.7554/eLife.00626 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert V, Dieng H, Lochouarn L, Traoré SF, Trape J-F, Simondon F, Fontenille D. La transmission du paludisme dans la zone de Niakhar, Sénégal. Trop Med Int Health. 1998;3:667–677. doi: 10.1046/j.1365-3156.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet S, Gouagna LC, Paul RE, Safeukui I, Meunier J-Y, Boudin C. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: evaluation and comparison of several indices. Trans R Soc Trop Med Hyg. 2003;97:53–59. doi: 10.1016/s0035-9203(03)90022-8. [DOI] [PubMed] [Google Scholar]
- 21.Muirhead-Thomson RC. Factors determining the true reservoir of infection of Plasmodium falciparum and Wuchereria bancrofti in a West African village. Trans R Soc Trop Med Hyg. 1954;48:208–225. doi: 10.1016/0035-9203(54)90067-x. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet S, Gouagna C, Safeukui I, Meunier J-Y, Boudin C. Comparison of artificial membrane feeding with direct skin feeding to estimate infectiousness of Plasmodium falciparum gametocyte carriers to mosquitoes. Trans R Soc Trop Med Hyg. 2000;94:103–106. doi: 10.1016/s0035-9203(00)90456-5. [DOI] [PubMed] [Google Scholar]
- 23.Diallo M, Touré AM, Traoré SF, Niaré O, Kassambara1 L, Konaré A, Coulibaly M, Bagayogo M, Beier JC, Sakai RK, Touré YT, Doumbo OK. Evaluation and optimization of membrane feeding compared to direct feeding as an assay for infectivity. Malar J. 2008;7:248. doi: 10.1186/1475-2875-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, Biswas S, Da DF, Cohuet A, Sinden RE. Measuring the blockade of malaria transmission: an analysis of the Standard Membrane Feeding Assay. Int J Parasitol. 2012;42:1037–1044. doi: 10.1016/j.ijpara.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Chardome, Janssen PJ. Inquiry on malarial incidence by the dermal method in the region of Lubilash, Belgian Congo. Annales de la Société belge de médecine tropicale. 1952;32:209–211. [PubMed] [Google Scholar]
- 26.Pichon G, Awono-ambene HP, Robert V. High heterogeneity in the number of Plasmodium falciparum gametocytes in the blood meal of mosquitoes fed on the same host. Parasitology. 2000;121:115–120. doi: 10.1017/s0031182099006277. [DOI] [PubMed] [Google Scholar]
- 27.Bousema T, Churcher TS, Morlais I, Dinglasan RR. Can field-based mosquito feeding assays be used for evaluating transmission-blocking interventions? Trends Parasitol. 2013;29:53–59. doi: 10.1016/j.pt.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Pethleart A, Prajakwong S, Suwonkerd W, Corthong B, Webber R, Curtis C. Infectious reservoir of Plasmodium infection in Mae Hong Son Province, north-west Thailand. Malar J. 2004;3:34. doi: 10.1186/1475-2875-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, Sauerwein RW. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–474. [PubMed] [Google Scholar]
- 30.Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30:183–190. doi: 10.1016/j.pt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]