Abstract
In Tunisia, malaria transmission has been interrupted since 1980. However, the growing number of imported cases and the persistence of putative vectors stress the need for additional studies to assess the risk of malaria resurgence in the country. In this context, our aim was to update entomological data concerning Anopheles mosquitoes in Tunisia. From May to October of 2012, mosquito larval specimens were captured in 60 breeding sites throughout the country and identified at the species level using morphological keys. Environmental parameters of the larval habitats were recorded. Specimens belonging to the An. maculipennis complex were further identified to sibling species by the ribosomal deoxyribonucleic acid (rDNA)–internal transcribed spacer 2 (ITS2) polymerase chain reaction (PCR) technique. In total, 647 Anopheles larvae were collected from 25 habitats. Four species, including An. labranchiae, An. multicolor, An. sergentii, and An. algeriensis, were morphologically identified. rDNA-ITS2 PCR confirmed that An. labranchiae is the sole member of the An. maculipennis complex in Tunisia. An. labranchiae was collected throughout northern and central Tunisia, and it was highly associated with rural habitat, clear water, and sunlight areas. Larvae of An. multicolor and An. sergentii existed separately or together and were collected in southern Tunisia in similar types of breeding places.
Introduction
Until its elimination in 1980, malaria was endemic in Tunisia, with an annual mean incidence of 10,000 cases.1 Anopheles (An.) labranchiae Falleroni, 1926 and An. (Cellia) sergentii Theobald, 1907 were reported as the main incriminated vectors of the disease in the northern and southern parts of the country, respectively (Wernsdorfer W and Iyengar MO, unpublished data). Since 1903 and mainly after the World War II, numerous control campaigns combining environmental interventions, vector control and screening, and treatment of infected people have led to successful interruption of autochthonous malaria transmission.2,3 Currently, only imported cases (mainly from sub-Saharan African countries and caused by Plasmodium falciparum) and some post-transfusion cases are observed.4,5 However, the increase of the annual incidence of imported cases of malaria associated with the persistence of Anopheles mosquitoes highlights the risk of a resumption of the disease transmission in Tunisia.1,3,5,6
The first map of Anopheles distribution in Tunisia was based on data collected between 1968 and 1974 during the malaria eradication campaign. A literature review by Brunhes and others7 compiled 12 species in 1999: An. algeriensis, An. cinereus, An. claviger, An. dthali, An. labranchiae, An. marteri, An. multicolor, An. petragnani, An. plumbeus, An. sergentii, An. superpictus, and An. ziemanni. The most recent investigation dating back to the 1990s detected only six species, including those suspected as malaria vectors in Tunisia.8–10
Despite the public health importance of An. labranchiae, An. sergentii, and An. multicolor, their larval biology has not, to our knowledge, been explored. A good understanding of larval habitat diversity and selection can provide relevant information about areas that are at higher risk of malaria transmission and could improve vector control implementation through targeted strategies.
Most of the important malaria vectors are members of species complexes or species groups, which are often difficult to distinguish morphologically from one another. Members of the Maculipennis complex have different ecologies, biological attributes, and vectorial capacities, and hence, correct species identification is necessary for a better understanding of their potential roles in malaria transmission in areas where they are known to occur. Species that are endemic to Europe, Asia, and North Africa and considered to belong to this group are An. labranchiae, An. atroparvus, An. messeae, An. sacharovi, An. maculipennis, and An. melanoon. According to most studies, with the exception of An. sacharovi, these species are impossible to distinguish morphologically at the adult as well as larval stages,11–14 even if some reports suggest possible diagnostic characters for some members of the complex at the larval stage.15,16 Recent studies based on the analysis of DNA sequences have leveraged part of the problem by providing a straight-forward and reliable polymerase chain reaction (PCR)-based molecular identification tool that allows species discrimination within the Maculipennis group in many countries, including Italy, Romania, Iran, England, Algeria, Morocco, and Greece.11,13,14,17–28 These studies have retained An. labranchiae as the only member of the Maculipennis complex found in Morocco and Algeria,22 whereas species composition within the Maculipennis complex is still pending for Tunisia.
This study aimed to update available data on the endemic Anopheles species present in Tunisia and their geographical distribution and determine their larval habitat preference.
Materials and Methods
Larval sampling and habitat characterization.
In total, 60 localities in nine governorates from northern, central, and southern Tunisia were visited during the 2012 summer season (May to October) (Figure 1 and Table 1). The selection of the region of interest was based on bibliographic research and data provided by the Regional Directories of Public Health. Anopheline mosquito larvae were collected from each larval development site using the standard dipping technique (350-mL dipper).29 Arbitrarily, sites with over 200 larvae after 15 dips were considered as hosting high larval densities. Sites with less than 100 larvae were considered as low-density habitats, and sites with no anopheline larvae after 15 dips were recorded as negative. The Anopheles larvae were separated from the culicine larvae and classified as early- (I and II) or late-instar (III and IV) stage. The late instars were preserved in 70% ethanol and transported to the laboratory for morphological identification.
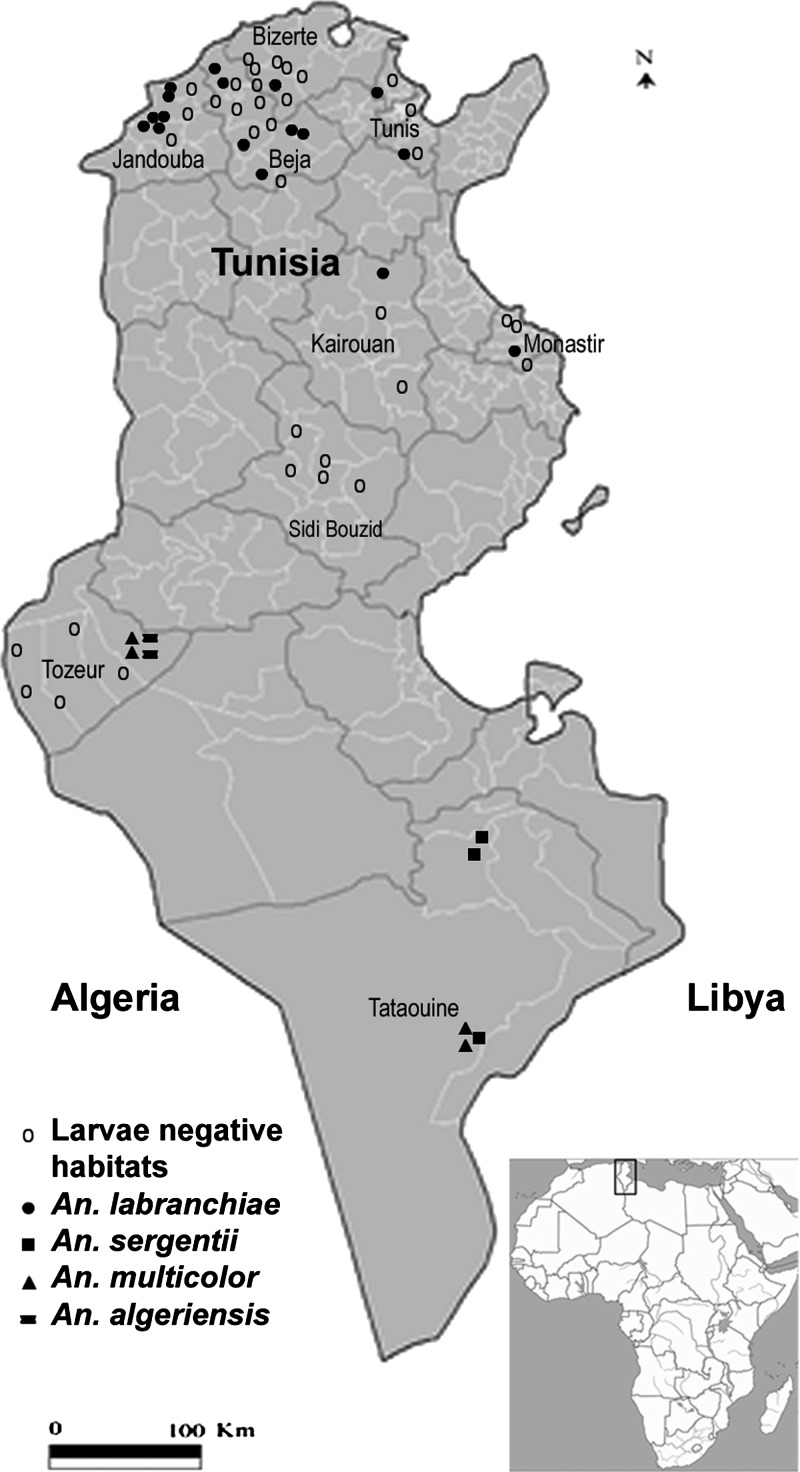
Figure 1.
Distribution of Anopheles mosquitoes in Tunisia.
Table 1.
Larval habitats characterization and composition species of Anopheles mosquitoes in Tunisia
| Governorate | GPS (north/east) | Locality | Chemical characteristics | Physical characteristics | Anopheline species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | pH | Dissolved oxygen (mg/L) | Salinity (mg/L) | Habitat type | Altitude (m) | Sunlight exposure | Water turbidity | Substrate | Vegetation | Culicine larvae | Fish | ||||
| Biezrte | 36°55′/9°22′ | Bagrat | 32.2 | 8.8 | 12.1 | 506 | Suburban | 251.7 | Sunny | Clear | Muddy | No vegetation | + | − | An. labranchiae |
| Ariana | 36°54′/10°03′ | Chorfesh | 28.6 | 8.9 | 11.8 | 1,480 | Rural | 54.1 | Sunny | Clear | Sandy | No vegetation | + | − | An. labranchiae |
| Ben arous | 36°36′/10°09′ | Siguel | 37.2 | 8.7 | 18.5 | 952.1 | Rural | 130.7 | Sunny | Clear | Sandy | P. communis/algae | + | − | An. labranchiae |
| Beja | 36°58′/9°00′ | Wechtat1 | 32.8 | 2.6 | 14.5 | 330 | Rural | 76.9 | Sunny | Clear | Sandy | T. angustifolia | + | − | An. labranchiae |
| Beja | 36°57′/8°59′ | Wechtat2 | 28.1 | 7.7 | 25.4 | 168 | Rural | 103.7 | Sunny | Clear | Rocky | No vegetation | + | − | An. labranchiae |
| Beja | 36°30′/9°14′ | Bir touta | 33.4 | 8.8 | 20.2 | 841 | Rural | 411.3 | Sunny | Clear | Muddy | P. communis | + | − | An. labranchiae |
| Beja | 36°42′/9°27′ | Babouche | 31.5 | 8.4 | 21 | 1,751 | Rural | 261.8 | Sunny | Clear | Muddy | Algae | + | + | An. labranchiae |
| Beja | 36°41′/9°27′ | Deroua | 28.5 | 8.3 | 10 | 3,030 | Rural | 256.2 | Sunny | Clear | Muddy | P. communis/algae | + | + | An. labranchiae |
| Beja | 36°35′/9°11′ | Elhamri1 | 25.6 | 7.6 | 14.2 | 333 | Rural | 213.76 | Sunny | Clear | Muddy | Algae | + | − | An. labranchiae |
| Jandouba | 36°55′/8°45′ | Kaffaf | 27.8 | 7.3 | 9.4 | 235 | Rural | 65.6 | Sunny | Clear | Sandy | No vegetation | + | − | An. labranchiae |
| Jandouba | 36°52′/8°43′ | Ktayreyia | 28.6 | 7.6 | 18.3 | 252 | Rural | 93.1 | Sunny | Clear | Muddy | P. communis | + | − | An. labranchiae |
| Jandouba | 36°45′/8°32′ | Rouii | 26.7 | 7.2 | 5.3 | 372 | Rural | 196.9 | Sunny | Clear | Muddy | T. angustifolia | + | − | An. labranchiae |
| Jandouba | 36°43′/8°35′ | Ncham1 | 33.2 | 7.1 | 12.3 | 392 | Rural | 672.8 | Sunny | Clear | Sandy | Algae | + | − | An. labranchiae |
| Jandouba | 36°44′/8°35′ | Ncham2 | 31.6 | 7.6 | 10.1 | 178.1 | Rural | 646.3 | Sunny | Clear | Sandy | No vegetation | + | − | An. labranchiae |
| Jandouba | 36°38′/8°39′ | Damous | 29.5 | 7.8 | 11.1 | 162.5 | Rural | 337.5 | Sunny | Clear | Muddy | No vegetation | + | − | An. labranchiae |
| Kairouan | 35°56′/10°01′ | Sbikha | 28.9 | 8.9 | 18.4 | 1,139 | Suburban | 136.2 | Sunny | Clear | Sandy | Algae | + | − | An. labranchiae |
| Monastir | 35°30′/10°46′ | Jnaiha | 34.5 | 8.6 | 15.1 | 941 | Rural | 122.1 | Shaded | Clear | Muddy | P. communis/T. angustifolia | + | − | An. labranchiae |
| Tozeur | 33°57′/8°13′ | Gite2 | 24.4 | 6.5 | 7.5 | 9,010 | Rural | 49.2 | Sunny | Clear | Sandy | P. communis/Juncus sp. | + | − | An. algeriensis |
| Tozeur | 33°58′/8°14′ | Gite9 | 28.6 | 6.6 | 10.6 | 13,660 | Rural | 57 | Shaded | Clear | Sandy | P. communis | + | − | An. multicolor |
| Tozeur | 33°59′/8°14′ | Mejed | 36.4 | 6 | 2.1 | 23,600 | Suburban | 75.3 | Sunny | Turbid | Sandy | Sarcocornia sp. | + | − | An. multicolor |
| Tozeur | 34°00′/8°09′ | Chnichina | 29.8 | 6.5 | 2.7 | 9,970 | Suburban | 75.1 | Shaded | Clear | Sandy | P. communis | + | − | An. algeriensis |
| Tataouine | 32°58′/10°22′ | Tlalet | 21.4 | 8.4 | 17.1 | 6,440 | Suburban | 282.2 | Shaded | Clear | Sandy | P. communis/algae | + | + | An. sergentii |
| Tataouine | 32°22′/10°23′ | Lachouch | 24.5 | 7.7 | 17.9 | 36,800 | Suburban | 315.6 | Sunny | Clear | Sandy | No vegetation | + | − | An. multicolor/An. sergentii |
| Tataouine | 32°19′/10°23′ | Ettboul | 24.7 | 8.5 | 13.2 | 4,520 | Rural | 332.1 | Shaded | Clear | Sandy | P. communis | + | − | An. multicolor |
| Tataouine | 33°03′/10°20′ | Ennasr | 19.4 | 8.7 | 11.3 | 2,820 | Urban | 305.9 | Sunny | Clear | Sandy | No vegetation | + | − | An. sergentii |
GPS = global positioning system..
Environmental variables, including chemical and physical characteristics of larval development sites, were recorded during larval collections. Chemical characteristics, including dissolved oxygen, salinity, and pH, were measured using a digital multimeter (CP1000/Wagtech WTD, Palintest Ltd, Gateshead, UK). Water temperature at the time of collection was also recorded. Physical characteristics of the mosquito larval habitats included altitude, habitat type (i.e., rural, suburban, or urban), sunlight exposure (i.e., sunny versus shaded), water turbidity (clear versus turbid), and substrate (i.e., muddy, sandy, or rocky). Presence/absence of vegetation (Phragmites communis, Typha angustifolia, Juncus sp., Sarcocornia sp., and algae), predators (Gambusia affinis), and competitors (e.g., culicinae larvae) was noted.
Anopheles larvae identification.
Third- and fourth-instar larvae were morphologically identified in the field using the standardized key for the mosquitoes of Mediterranean Africa.7 Twenty larvae of the Maculipennis complex were then randomly selected from positive larval collections sites to be further identified into sibling species by the ribosomal deoxyribonucleic acid (rDNA)–internal transcribed spacer 2 (ITS2) PCR technique.30
Genomic DNA was extracted from individual whole-larvae specimens as previously described.31 Amplification targeted the conserved ITS2 region of the rDNA cluster. The amplification was done with the conditions described previously.30
Statistical analysis.
The associations of the environmental variables with the occurrence of Anopheles mosquito larvae were tested using SPSS software (IBM SPSS statistics 20). A descriptive analysis of the data was carried out. The quantitative variables were described by means ± SEMs, and for the categorical variables, the percentages were calculated. The χ2 or Fisher's exact test was used to compare qualitative variables (percentages), whereas t test or analysis of variance (ANOVA) and the corresponding non-parametric tests (Mann–Whitney and Kruskal–Wallis tests, respectively) were used to compare the quantitative variables (means).
Results
Morphological identification of anopheline mosquito larvae.
In total, 25 of 60 water collections prospected revealed the presence of anopheline mosquito larvae (41.7%) (Figure 1). They produced a total of 647 anopheline mosquito larvae. According to morphological identification, four species belonging to two subgenera were recorded, including members of the Maculipennis complex: most probably An. (An.) labranchiae (N = 252; 38.9%), An. (Ce.) multicolor (N = 233; 36%), An. (Ce.) sergentii (N = 150; 23.2%), and An. (An.) algeriensis (N = 12; 1.9%).
Molecular identification.
The rDNA-PCR technique performed on 20 specimens collected from 11 larval habitats amplified a single 374-base pair (bp) -long fragment, which was expected from An. labranchiae, therefore confirming morphological identification and suggesting the presence of a single member of the Maculipennis complex in Tunisia.
Habitat characterization of anopheline mosquito larvae.
Six hundred forty-seven larvae of Anopheles, including An. labranchiae, An. multicolor, An. sergentii, and An. Algeriensis, were collected from 25 habitats located in northern, central, and southern Tunisia (Figure 1); 4 of 10 studied variables were significantly associated with species distribution: water temperature (P = 0.031), salinity (P = 0.001), bottom surface (P = 0.022), and habitat type (P = 0.017) (Tables 2 and 3). These characteristics were found to be the key factors that are associated with species occurrence.
Table 2.
Statistical analysis results: distribution of species (percentage) according to qualitative environmental conditions
| Sunlight situation | Fauna | Water transparency | Bottom surface | Habitat type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shaded (N = 5) | Sunlight (N = 20) | Absence (N = 22) | Presence (N = 3) | Clear (N = 24) | Turbid (N = 1) | Muddy/rocky (N = 9) | Sandy (N = 16) | Rural (N = 18) | Suburban/urban (N = 7) | |
| An. labranchiae (%) | 33.3 | 78.9 | 68.2 | 66.7 | 70.8 | 0 | 100 | 50 | 83.3 | 16.7 |
| Others (%) | 66.7 | 21.1 | 31.8 | 33.3 | 29.2 | 100 | 0 | 50 | 28.6 | 71.4 |
| P value* | 0.059 | NS | NS | 0.022 | 0.017 | |||||
NS = not significant.
Fisher's exact test.
Table 3.
Statistical analysis results: comparison of quantitative environmental conditions by species
| Code species | Altitude (m) | Salinity (mg/L) | Dissolved oxygen (mg/L) | pH | Water temperature (°C) |
|---|---|---|---|---|---|
| An. labranchiae | |||||
| Mean | 237.0924 | 20,160.747 | 14.5776 | 7.7494 | 30.5118 |
| N | 17 | 17 | 17 | 17 | 17 |
| SD | 187.55885 | 79,847.2568 | 5.12223 | 1.46602 | 3.09493 |
| Median | 196.9400 | 506.000 | 14.2000 | 7.7800 | 29.5000 |
| Others | |||||
| Mean | 186.5488 | 13,352.500 | 9.9013 | 7.3588 | 26.1500 |
| N | 8 | 8 | 8 | 8 | 8 |
| SD | 131.84250 | 11,476.1800 | 5.45234 | 1.08320 | 5.35057 |
| Median | 178.7600 | 9,490.000 | 10.9300 | 7.1000 | 24.6000 |
| Total | |||||
| Mean | 220.9184 | 17,982.108 | 13.0812 | 7.6244 | 29.1160 |
| N | 25 | 25 | 25 | 25 | 25 |
| SD | 170.59059 | 65,569.1193 | 5.57845 | 1.34522 | 4.36441 |
| Median | 196.9400 | 1,139.000 | 12.3300 | 7.7000 | 28.6000 |
| Test statistics | |||||
| Mann–Whitney U | 57.000 | 9.000 | 39.000 | 45.000 | 31.000 |
| Asymptomatic significance (two-tailed) | 0.522 | 0.001 | 0.091 | 0.180 | 0.031 |
The mean water temperature was significantly higher among An. labranchiae (30.5°C ± 3.09°C) than others species (26.15°C ± 5.35°C). Likewise, analysis of the association between chemical characteristics of habitats and species revealed that salinity mean was significantly higher among An. labranchiae (Tables 2 and 3).
An. labranchiae is significantly less frequent in sandy bottom surface (50%) than other types of bottom surface (rocky and muddy; 100%; P = 0.022), significantly more common in rural (83.3%) than urban and suburban (28.6%; P = 0.017) areas, and more frequent in sunlight areas (78.9%) versus shaded one (33.3%), with a tendency to significance (P = 0.059).
In this study, the other measured parameters, such as dissolved oxygen (P = 0.091), altitude (P = 0.522), pH (P = 0.18), fauna (P = 1), and water transparency (P = 0.32), differed between species but were not significantly associated with species distribution (Tables 2 and 3).
Anopheline larvae and Gambusia fish only coexisted in three habitats where the predators were recently introduced (Table 1).
The 252 An. labranchiae larvae were collected in 17 habitats located in northern and central Tunisia. It was the only species encountered at these sites (Figure 1), and it always occurred at low density in the breeding sites. The most common habitats for An. labranchiae larvae in Tunisia were rural (88.2%) with clear water (100%), no larvivorous fishes (88.2%), and sunny areas (94.1%) (Table 1).
An. multicolor and An. sergentii, the suspected vector species in central and southern Tunisia during the endemic period, were found separately in 83.3% of the positive breeding places of both species, where they frequently occurred at low density. They were collected together in only 16.7% of the positive breeding places of both species, corresponding often to high-density larval habitats (Figure 1 and Table 1). The most common habitats for both species are characterized by clear water (83.3%), no larvivorous fishes (83.3%), and sand substrate (100%) (Table 1). An. algeriensis, reported as a non-vector species during the endemic period in Tunisia, was collected at low density from only 8% of larval habitats.
Discussion
Only four species of Anopheles were found in this study, despite the sampling effort and the appropriate season of the captures corresponding to the Anopheles activity period in Tunisia: An. labranchiae, An. multicolor, An. sergentii, and An. algeriensis. Between 1968 and 1974 (i.e., during the malaria eradication campaign), 12 species had been reported.7 None of these species are specific to Tunisia. More recent investigations found six species, including An. cinereus and An. claviger.8,9 An. labranchiae was the predominant species in northern Tunisia, whereas An. sergentii and An. multicolor were prevalent in southern Tunisia. These results are similar to those of previous studies.8,9
As reported by Krida and others,10 anopheline larvae were found in rural, suburban, and urban habitats. An. labranchiae was the only widely distributed species throughout northern and central Tunisia in subhumid and semiarid climate, respectively (Figure 1). Our results showed that habitats sustaining the development of An. multicolor, An. sergentii, and An. algeriensis were not significantly different in relation to the environmental variables measured (Tables 2 and 3). The three species were captured in localities with arid climate located in southern Tunisia. Only water temperature, salinity, habitat type, and bottom surface were associated with species distribution. As reported in other studies,32,33 the existence and abundance of Anopheles immature stages were not correlated with water temperature, dissolved oxygen, pH, and bottom surface. However, a significant role of temperature and light exposure on Anopheles distribution was supported by Christophe and others.33 Surprisingly, exceptional tolerance to low pH of An. labranchiae larvae was observed (pH 2.6). However, no study showed comparative results for Anopheles mosquitoes, and additional investigations are required on the larvae biology of this species.
The species belonging to Maculipennis complex are difficult to identify because of the morphological overlap that exists within the groups.34 The molecular identification of species revealed the presence of a single member of the Maculipennis complex in Tunisia, namely An. labranchiae. The proportion of An. labranchiae (identified according to morphological characters) might have been correctly reported in previous entomological surveys in the area.10,35 The distribution of An. labranchiae is somewhat unusual, in that it is believed to be the only African member of the Maculipennis complex. It is highly abundant and widespread in the Maghreb countries: Morocco,22,36–40 Algeria,22,41,42 and Tunisia.10,35 It is assumed that An. labranchiae was the principal malaria vector in a large part of the country, particularly in the northern governorates. However, data are quite confusing because of the scanty and old infectivity tests conducted.43–48 Laboratory studies performed with An. labranchiae revealed that this species can transmit P. ovale,45 whereas populations collected in Italy were refractory to African strains of P. falciparum.44,45 Nevertheless, recent research with populations from Corsica (France) and Principina (Grosseto, Italy) have indicated that the P. falciparum cycle can be successfully completed in An. labranchiae.49,50 Moreover, An. labranchiae has also been involved in autochthonous transmission of P. vivax in Corsica, Greece, and Italy.46–48 P. vivax malaria has been reported from different regions of Tunisia, and the number of imported cases is on the rise,51 highlighting a risk for the re-emergence of local foci in Tunisia. Furthermore, An. labranchiae has been involved in the epidemic transmission of P. falciparum, P. malariae, and P. vivax during recent epidemics in Morocco.52 It will be necessary to assess the vector competence of local An. labranchiae populations from Tunisia for sub-Saharan strains of African malaria parasites to more accurately assess the risk for re-emergence of malaria transmission in the highly populated northern parts of the country.
An. sergentii has been incriminated in malaria transmission in the southern part of Tunisia (Wernsdorfer W and Iyengar MO). The role of An. multicolor where it exists with An. sergentii or alone in the oases remains unknown. An. multicolor has not been incriminated in nature, but it is suspected to be a vector on epidemiological grounds, because it has been found alone in some oases where malaria is transmitted.53 In Egypt, An. multicolor and An. sergentii have been found infected with P. vivax and P. falciparum in natural conditions.54 An. algeriensis is only considered a potential or secondary malaria vector in endemic regions without proof of natural transmission. Because of its scarcity in Tunisia,9 catholic feeding preferences, and exophilic behavior, the species does not presently and did not historically pose a risk.55–57
The presence of putative malaria vector species together with high numbers of imported malaria cases each year in Tunisia highlight a risk for re-emergence of autochthonous transmission in the country. Additional investigations are required on the ecology, bionomics, and vector competence of local Anopheles populations to implement tailored vector surveillance and control programs and prevent re-emergence of the disease.
ACKNOWLEDGMENTS
We are grateful to the Regional Directories of Public Health that participated in the study. A special thanks to Dr. S. Fessi, Dr. A. Houerbi, Dr. L. Sakhri, Dr. T. Barhoumi, Dr. A. Hedfi, Dr. M. Raouane, Mrs. S. Kilani, H. Hajlaoui, A. Mribai, H. Dellaii, H. Aloui, A. Rezeigui, N. Mejri, S. Ben Bdira, N. Shili, E. Trilla, A. Khlifi, H. Ghribi, and S. Traoui for their help. We also thank Dr. Thierry Baldet for his advice and Dr. I. Ben Abda, Dr. E. Siala, and Dr. M. Ben Abid for their assistance.
Footnotes
Financial support: This study was supported by the Tunisian Ministry of High Education and Research in the frame of the Research Laboratory LR 11-IPT-06.
Authors' addresses: Ahmed Tabbabi, Adel Rhim, Aïda Bouratbine, and Karim Aoun, Laboratoire de Parasitologie Médicale, Biotechnologies et Biomolécules and Laboratoire de Parasitologie-Mycologie, Institut Pasteur de Tunis, Tunis, Tunisia, E-mails: tabbabiahmed@gmail.com, adel.rhaim@pasteur.rns.tn, aida.bouratbine@pasteur.rns.tn, and karim.aoun@pasteur.rns.tn. Philippe Boussès, Cécile Brengues, Didier Fontenille, and Frédéric Simard, Maladies Infectieuses et Vecteurs: Écologie, Génétique, Évolution et Contrôle (MIVEGEC; UMR IRD 224, CNRS 5290, UM1, UM2), Centre IRD France-Sud, Montpellier, France, E-mails: philippe.bousses@ird.fr, Cecile.Brengue@ird.fr, didier.fontenille@ird.fr, and frederic.simard@ird.fr. Jabeur Daaboub, Direction de l'Hygiène du Milieu et de la Protection de l'Environnement, Ministère de la santé publique, Tunis, Tunisia, E-mail: jadaaboub@yahoo.fr. Nissaf Ben-Alaya-Bouafif, Observatoire National des Maladies Nouvelles et Émergentes, Tunis, Tunisia, E-mail: nissaf.bouafif@rns.tn.
References
- 1.Chadli A, Kennou MF, Kooli J. Le paludisme en Tunisie: historique et état actuel. Bull Soc Pathol Exot. 1985;78:844–851. [PubMed] [Google Scholar]
- 2.Chadli A, Kennoun MF, Kooli J. Les campagnes d'éradication du paludisme en Tunisie: historique et état actuel. Arch Inst Pasteur Tunis. 1986;63:35–50. [PubMed] [Google Scholar]
- 3.Ben Rachid MS, Ben Ammar R, Redissi T, Ben Said M, Hellel H, Bach-Hamba D, el Harabi M, Nacef T. Géographie des parasitoses majeures en Tunisie. Arch Inst Pasteur Tunis. 1984;61:17–41. [PubMed] [Google Scholar]
- 4.Anonymous . Bulletins épidémiologiques de la Direction de Soins de Santé de Base-Ministère de la Santé publique, Tunis, Tunisia. 1980–2013. [Google Scholar]
- 5.Gmara D. Situation actuelle du paludisme dans le monde et en Tunisie (DSSB) Cairo, Egypt: Réunion OMS; 2006. [Google Scholar]
- 6.Bouratbine A, Chahed MK, Aoun K, Krida G, Ayari S, Ben Ismail R. Le paludisme d'importation en Tunisie. Bull Soc Pathol Exot. 1998;91:203–207. [PubMed] [Google Scholar]
- 7.Brunhes J, Rhaim A, Geoffroy B, Angel G, Hervy JP. Les moustiques de l'Afrique méditerranéenne CD-ROM d'identification et d'enseignement, Edition IRD, Montpellier, France. 1999. [Google Scholar]
- 8.Bouchité B, Kennou MF, Chauvet G. Ethologie et capacité vectorielle des anophèles de Tunisie dans la région de Sidi Bouzi, multigrade. ORSTOM/IPT; 1991. [Google Scholar]
- 9.Bouattour A, Rhaim A, Bach Hamba D. Etude de la capacité vectorielle d'Anopheles labranchiae dans la région de Nefza. Tunis, Tunisia: 1993. Document multigrade disponible IPT et ANPE. [Google Scholar]
- 10.Krida G, Bouattour A, Rhaim A, el Kebir A, Jlidi R. Preliminary investigation of four Anopheles larvae samples susceptibility to chlorpyrifos in Tunisia. Arch Inst Pasteur Tunis. 1998;75:199–203. [PubMed] [Google Scholar]
- 11.Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterization and recognition of a new species. Bull Entomol Res. 2003a;93:527–535. doi: 10.1079/ber2003272. [DOI] [PubMed] [Google Scholar]
- 12.Nicolescu G, Linton YM, Vladimirescu A, Howard TM, Harbach RE. Mosquitoes of the Anopheles maculipennis group (Diptera: Culicidae) in Romania, with the discovery and formal recognition of a new species based on molecular and morphological evidence. Bull Entomol Res. 2004;94:525–535. doi: 10.1079/ber2004330. [DOI] [PubMed] [Google Scholar]
- 13.Linton YM, Smith L, Harbach RE. Observations on the taxonomic status of Anopheles subalpinus Hackett and Lewis and An. melanoon Hackett. Eur Mosq Bull. 2002c;13:8–16. [Google Scholar]
- 14.Patsoula E, Samanidou-Voyadjoglou A, Spanakos G, Kremastinou J, Nasioulas G, Vakalis NC. Molecular characterization of Anopheles maculipennis complex during surveillance for the 2004 Olympic Games in Athens. Med Vet Entomol. 2007;21:36–43. doi: 10.1111/j.1365-2915.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 15.Doosti S, Azari-Hamidian S, Vatandoost H, Oshaghi MA, Hosseini M. Taxonomic differentiation of Anopheles sacharovi and An. maculipennis s.l. (Diptera: Culicidae) larvae by seta 2 (antepalmate hair) Acta Med Iran. 2006;44:21–27. [Google Scholar]
- 16.Doosti S, Vatandoost H, Oshaghi MA, Hosseini M, Sedaghat MM. Applying morphometric variation of seta 2 (antepalmate hair) among the larvae of the members of the Maculipennis subgroup (Diptera: Culicidae) in Iran. Iran J Arthropod Borne Dis. 2007;1:28–37. [Google Scholar]
- 17.Linton YM, Samanidou-Voyadjoglou A, Harbach RE. Ribosomal ITS2 sequence data for Anopheles maculipennis and An. messeae in northern Greece, with a critical assessment of previously published sequences. Insect Mol Biol. 2002a;11:379–383. doi: 10.1046/j.1365-2583.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 18.Boccolini D, Di Luca M, Marinucci M, Romi R. Further molecular and morphological support for the formal synonymy of Anopheles subalpinus Hackett and Lewis with An. melanoon Hackett. Eur Mosq Bull. 2003;16:1–5. [Google Scholar]
- 19.Di Luca M, Boccolini D, Marinucci M, Romi R. Intrapopulation polymorphism in Anopheles messeae (Anopheles maculipennis complex) inferred by molecular analysis. J Med Entomol. 2004;41:582–586. doi: 10.1603/0022-2585-41.4.582. [DOI] [PubMed] [Google Scholar]
- 20.Djadid ND, Gholizadeh S, Tafsiri E, Romi R, Gordeev M, Zakeri S. Molecular identification of Palearctic members of Anopheles maculipennis in northern Iran. Malar J. 2007;6:6. doi: 10.1186/1475-2875-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kampen H. Integration of Anopheles beklemishevi (Diptera: Culicidae) in a PCR assay diagnostic for Palaearctic Anopheles maculipennis sibling species. Parasitol Res. 2005;97:113–117. doi: 10.1007/s00436-005-1392-9. [DOI] [PubMed] [Google Scholar]
- 22.Laboudi M, Faraj C, Sadak A, Harrat Z, Boubidi SC, Harbach RE, El Aouad R, Linton YM. DNA barcodes confirm the presence of a single member of the Anopheles maculipennis group in Morocco and Algeria: An. sicaulti is conspecific with An. labranchiae. Acta Trop. 2011;118:6–13. doi: 10.1016/j.actatropica.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Linton YM, Samanidou-Voyadjoglou A, Smith L, Harbach RE. New occurrence records for Anopheles maculipennis and An. messeae in northern Greece based on DNA sequence data. Eur Mosq Bull. 2001;11:31–36. doi: 10.1046/j.1365-2583.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 24.Linton YM, Smith L, Harbach RE. Molecular confirmation of sympatric populations of Anopheles messeae and Anopheles atroparvus overwintering in Kent, southeast England. Eur Mosq Bull. 2002;13:8–16. [Google Scholar]
- 25.Linton YM, Smith L, Koliopoulos G, Samanidou-Voyadjoglou A, Zounos AK, Harbach RE. Morphological and molecular characterization of Anopheles (Anopheles) maculipennis Meigen, type species of the genus and nominotypical member of the Maculipennis Complex. Syst Entomol. 2003;28:39–55. [Google Scholar]
- 26.Linton YM, Lee AS, Curtis C. Discovery of third member of Maculipennis group in SW England. Eur Mosq Bull. 2005;19:5–9. [Google Scholar]
- 27.Linton YM, Smith L, Koliopoulos G, Zounos AK, Samanidou- Voyadjoglou A, Harbach RE. The Anopheles (Anopheles) maculipennis complex (Diptera: Culicidae) in Greece. J Nat Hist. 2007;41:2683–2699. [Google Scholar]
- 28.Marinucci M, Romi R, Mancini P, Di Luca M, Severini C. Phylogenetic relationships of seven Palaearctic members of the Maculipennis Complex inferred from ITS2 sequence data. Insect Mol Biol. 1999;8:469–480. doi: 10.1046/j.1365-2583.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- 29.Service M. Mosquito Ecology: Field Sampling Methods. 2nd Ed. London, UK: Elsevier Applied Science; 1993. [Google Scholar]
- 30.Proft J, Maier W, Kampen H. Identification of six sibling species of the Anopheles maculipennis complex (Diptera: Culicidae) by polymerase chain reaction assay. Parasitol Res. 1999;85:837–843. doi: 10.1007/s004360050642. [DOI] [PubMed] [Google Scholar]
- 31.Morlais I, Ponçon N, Simard F, Cohuet A, Fontenille D. Intraspecific nucleotide variation in Anopheles gambiae: new insights into the biology of malaria vectors. Am J Trop Med Hyg. 2004;71:795–802. [PubMed] [Google Scholar]
- 32.Herrel N, Amerasinghe FP, Ensink J, Mukhtar M, van der Hoek W, Konradsen F. Breeding of Anopheles mosquitoes in irrigated areas of South Punjab, Pakistan. Med Vet Entomol. 2001;15:236–248. doi: 10.1046/j.0269-283x.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 33.Christophe AN, Cyrille N, Carlo C, Parfait AA, Didier F, Frédéric S. Distribution and larval habitat characterization of Anopheles moucheti, Anopheles nili, and other malaria vectors in river networks of southern Cameroon. Acta Trop. 2009;112:270–276. doi: 10.1016/j.actatropica.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Romi R, Boccolini D, Di Luca M, La Rosa G, Marinucci M. Identification of the sibling species of the Anopheles maculipennis complex by heteroduplex analysis. Insect Mol Biol. 2000;9:509–513. doi: 10.1046/j.1365-2583.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 35.Chahed MK, Bouratbine A, Krida G, Ben Hamida A. Receptivity of Tunisia to malaria after its eradication: analysis of the situation for adequacy of the surveillance. Bull Soc Pathol Exot. 2001;94:271–276. [PubMed] [Google Scholar]
- 36.Gaud J. Notes biogéographiques sur les culicidés au Maroc. Arch Inst Pasteur Maroc. 1953;4:443–490. [Google Scholar]
- 37.Guy Y. Les Anopheles du Maroc. Mem Soc Sci Nat Phys Zool. 1959;7:235. [Google Scholar]
- 38.Guy Y. Bilan épidémiologique du paludisme au Maroc (données recueillies entre 1960, 1961 et 1962) Ann Parasitol Hum Comp. 1963;38:823–857. [PubMed] [Google Scholar]
- 39.Benmansour N, Laaziri M, Mouki B. Note sur la faune anophélienne du Maroc. Bull Inst Hyg (Maroc) 1972;52:103–112. [Google Scholar]
- 40.Faraj C, Adlaoui E, Saaf N, Romi R, Boccolini D, Di Luca M, Lyagoubi M. Note sur le complexe Anopheles maculipennis au Maroc. Bull Soc Pathol Exot. 2004;97:293–294. [PubMed] [Google Scholar]
- 41.Senevet G, Andarelli L. Les Anopheles d'Afrique du Nord et du Bassin Méditérranéen. Encycl Entomol. 1956;33:1–280. [Google Scholar]
- 42.Senevet G, Andarelli L. Contribution à l'étude de la biologie des Anopheles algériens. Arch Inst Pasteur Alger. 1961;39:393–400. [PubMed] [Google Scholar]
- 43.Constantinescu P, Negulici E. The experimental transmission of Plasmodium malariae to Anopheles labranchiae atroparvus. Trans R Soc Trop Med Hyg. 1967;61:182–188. doi: 10.1016/0035-9203(67)90155-1. [DOI] [PubMed] [Google Scholar]
- 44.Ramsdale CD, Coluzzi M. Studies on the infectivity of tropical African strains of Plasmodium falciparum to some southern European vectors of malaria. Parassitologia. 1975;17:39–48. [PubMed] [Google Scholar]
- 45.de Zulueta J, Ramsdale CD, Coluzzi M. Receptivity to malaria in Europe. Bull World Health Organ. 1975;52:109–111. [PMC free article] [PubMed] [Google Scholar]
- 46.Sautet J, Quilici R. A propos de quelques cas de paludisme autochtone contractés en France pendant l'été. Presse Med. 1971;79:524. [Google Scholar]
- 47.Zahar AR. Vector Bionomics in the Epidemiology and Control of Malaria. Part II: The WHO European Region and the WHO Eastern Mediterranean Region. Geneva: World Health Organization; 1987. [Google Scholar]
- 48.Baldari M, Tamburro A, Sabatinelli G, Romi R, Severini C, Cuccagna P, Fiorilli G, Allegri MP, Buriani C, Toti M. Introduced malaria in Maremma, Italy, decades after eradication. Lancet. 1998;351:1246–1248. doi: 10.1016/S0140-6736(97)10312-9. [DOI] [PubMed] [Google Scholar]
- 49.Toty C, Barré H, Le Goff G, Larget-Thiéry I, Rahola N, Couret D, Fontenille D. Malaria risk in Corsica, former hot spot of malaria in France. Malar J. 2010;9:231. doi: 10.1186/1475-2875-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romi R, Boccolini D, Vallorani R, Severini F, Toma L, Cocchi M, Tamburro A, Messeri G, Crisci A, Angeli L, Costantini R, Raffaelli I, Pontuale G, Thiéry I, Landier A, Le Goff G, Fausto AN, Di Luca M. Assessment of the risk of malaria re-introduction in the Maremma plain (Central Italy) using a multi-factorial approach. Malar J. 2012;11:98. doi: 10.1186/1475-2875-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aoun K, Siala E, Tchibkere D, Ben Abdallah R, Zallagua N, Chahed MK, Bouratbine A. Paludisme d'importation en Tunisie: conséquences sur le risque de réintroduction de la maladie. Med Trop. 2010;70:33–37. [PubMed] [Google Scholar]
- 52.Houel G, Donadille F. Vingt ans de lutte antipaludique au Maroc. Bull Inst Hyg (Maroc) . 1953;13:1–51. [Google Scholar]
- 53.Zahar AR. Review of the ecology of malaria vectors in the WHO Eastern Mediterranean Region. Bull World Health Org. 1974;50:427–440. [PMC free article] [PubMed] [Google Scholar]
- 54.Kenawy MA, Beier JC, Asiago CM, el Said SE, Roberts CR. Interpretation of low-level Plasmodium infection rates determined by ELISA for anophelines (Diptera: Culicidae) from Egyptian oases. J Med Entomol. 1990;27:681–685. doi: 10.1093/jmedent/27.4.681. [DOI] [PubMed] [Google Scholar]
- 55.Peus F. Die Fiebermücken des Mittelmeergebietes. Leipzig, Germany: Verlag Dr. Paul Schöps; 1942. [Google Scholar]
- 56.Russell PF, Rozeboom LE, Stone A. Keys to the Anopheline Mosquitoes of the World. Philadelphia, PA: The American Entomological Society and The Academy of Natural Sciences; 1943. [Google Scholar]
- 57.Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A. Mosquitoes and Their Control. New York, NY: Springer; 2010. [Google Scholar]