Abstract
Conducting research in areas with diverse cultures requires attention to community sensitization and involvement. The process of community engagement is described for a large community-based, cluster-randomized, controlled trial comparing daily 4% chlorhexidine umbilical cord wash to dry cord care for neonatal mortality prevention in Southern Province, Zambia. Study preparations required baseline formative ethnographic research, substantial community sensitization, and engagement with three levels of stakeholders, each necessitating different strategies. Cluster-specific birth notification systems developed with traditional leadership and community members using community-selected data collectors resulted in a post-natal home visit within 48 hours of birth in 96% of births. Of 39,679 pregnant women enrolled (93% of the target of 42,570), only 3.7% were lost to follow-up or withdrew antenatally; 0.2% live-born neonates were lost by day 28 of follow-up. Conducting this trial in close collaboration with traditional, administrative, political, and community stakeholders facilitated excellent study participation, despite structural and sociocultural challenges.
Introduction
Until recently, most global health interventions to reduce child mortality have focused on the post-neonatal period, leaving neonates vulnerable and stalling progress toward achievement of Millennium Development Goal (MDG) 4. Annually, an estimated 3.1–3.6 million neonatal deaths occur worldwide, predominantly in low- and middle-income countries.1–3 Simple, effective, low-cost, community-based interventions that will reduce neonatal mortality are critically needed. Research at the community level, where the greatest burden of morbidity and mortality exists, is required to discover and test these interventions.
Infections are responsible for approximately 36% of neonatal deaths in resource-limited countries.4 The umbilical cord is a potential portal or source of serious infections that may lead to sepsis and death. The use of 4% chlorhexidine as a topical umbilical antiseptic has been recently shown to reduce omphalitis and all-cause neonatal mortality in southern Nepal, Bangladesh, and Pakistan.5–8 The choice of 4% chlorhexidine was based on the safety of this concentration and its efficacy in reducing cord colonization.9,10 At the time of our study initiation in 2010, only one of these studies (Nepal) had been completed. South Asia and sub-Saharan Africa have different labor/delivery and care-seeking practices, cord care practices, population densities, mortality rates, and cultural practices.11–15 Evidence is, thus, needed from sub-Saharan Africa on the efficacy and effectiveness of chlorhexidine to inform umbilical cord care global policy; an effectiveness trial is being conducted in Zambia, and an efficacy trial is being conducted in Pemba Island, Tanzania.
Conducting a large cluster-randomized, controlled trial at the community level in an African country with poor road infrastructure, human resource shortages, and widely dispersed rural populations presents significant logistical barriers that must be addressed in study design and implementation. Community belief systems and the need to obtain informed consent from stakeholders ranging from traditional leaders to individual pregnant women and guardians/husbands of pregnant minors also present challenges to the conduct of clinical trials.16,17
We developed our approach to the implementation of the Zambia Chlorhexidine Application Trial (ZamCAT) with these issues in mind. The primary goal of this manuscript is to describe the process of community engagement and how this influenced the quality of study implementation. We believe that the strategies used for the design and implementation of this trial should prove useful for other investigators planning large-scale, community-based research in sub-Saharan Africa.
Materials and Methods
Study site.
At study initiation, Southern Province consisted of 11 districts with a total population of 1.59 million,18 which is populated mainly by the Tonga ethnic group. The total land area of Southern Province is 85,283 km2; average household size is 5.4 persons, and population density is 18.8 people/km2. There is one paved road through the center of the province that connects the administrative capital of Choma with Livingstone and Lusaka. Nearly all side roads are unpaved; thus, short distances can take many hours to cover. According to the 2007 Demographic Health Survey, the neonatal mortality rate in Southern Province was 37 deaths/1,000 live births, and adult human immunodeficiency virus (HIV) prevalence is 14.5%. More than 90% of all pregnant women have at least one interaction with a health facility for antenatal care (ANC) and were, thus, eligible for participation in ZamCAT.
Study design and sample size.
ZamCAT was a cluster-randomized, controlled trial that compared daily cord cleansing with 4% chlorhexidine (chlorhexidine arm) with the Ministry of Health (MoH) -recommended practice (dry cord care arm) in Zambia's Southern Province. Because the study was designed as an effectiveness trial, mothers or other care providers were trained to perform chlorhexidine cord washes. In the case of facility-based deliveries, a member of the healthcare team performed the initial application, whereas the infant's mother or caregiver did all subsequent applications. Similarly, at home deliveries, either a trained traditional birth attendant (TBA) or the mother performed the initial application, and then, the mother or caregiver was responsible for subsequent applications. Clusters consisted of primary healthcare centers and their respective catchment areas as defined by the MoH. In general, each rural health center is situated at the center of a catchment area with an approximate 25-km radius around the health center; urban health centers have a geographically smaller catchment area, because they serve more densely populated areas.
Primary outcomes were all-cause neonatal mortality at 28 days postpartum and all-cause neonatal mortality among newborns that survived the first day of life. To detect a 17% decrease in all-cause neonatal mortality from a neonatal mortality rate of 29 deaths/1,000 live births6,19 with 90% power, α = 0.05, and k = 0.08, the target sample size was 42,570 live births (90 clusters × 473 women per cluster), including a 10% increase to account for loss to follow-up or withdrawal. Because of financial limitations toward the end of the trial, we stopped recruitment when 39,679 pregnant women had been enrolled (93% of the target of 42,570).
Site assessment and cluster selection.
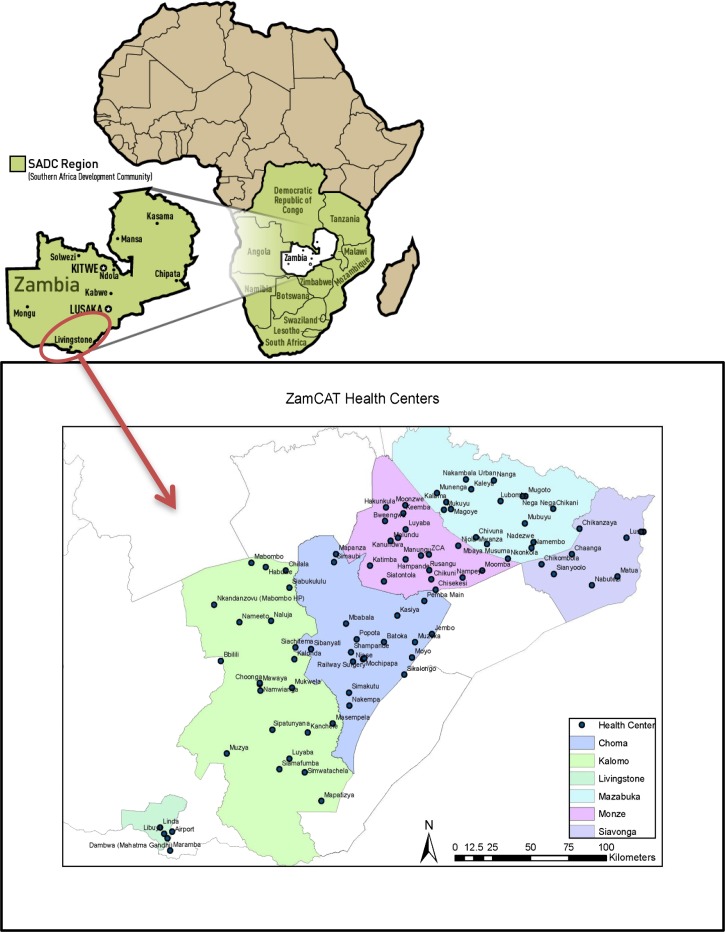
To select the study's 90 clusters, we conducted a site assessment of 128 facilities in six districts (Mazabuka, Siavonga, Monze, Choma, Kalomo, and Livingstone). In consultation with the MoH and Southern Province Medical Office (SPMO), we developed eligibility criteria, including (1) health facilities offering routine ANC services, (2) more than 160 births per annum in the catchment area regardless of birth location (home or facility), and (3) willingness of the facility management to participate. The birth data were based on actual health facility statistics, including their estimates of the number of home deliveries in their catchment areas. To conduct stratified restricted randomization, the final list of 90 sites was stratified based on three levels of distance from the main tarred road—health centers in town along the road (12 sites), sites within 40 km of the road (56 sites), and sites more than 40 km from the road (22 sites) (Figure 1). Within each stratum, clusters were randomized with restriction to achieve balance among five factors: health center catchment population, total births, distance to referral facility, total facility staff, and number of associated community-based TBAs. Cluster characteristics were well-balanced across the study arms post-randomization.
Figure 1.
Map of Zambia and ZamCAT clusters in Southern Province, Zambia.
Study implementation.
Community sensitization about study aims and procedures promoted understanding of the study and delineated expected roles of community leaders, members, and potential study participants. We first performed social mapping of the selected districts to understand the political and sociocultural processes between various levels of leadership and the community.20 The social mapping results identified three levels of stakeholders, with each requiring different approaches for communication and community sensitization (Table 1). The first level consisted of the traditional chiefdom administration, community leaders, community-based healthcare providers, and the community. The second level included the district health and political administration. The third level comprised the MoH and its supporting ministries, such as the Ministries of Local Government, Agriculture and Community Development, and Mother and Child Health. Meetings were held with each level to explain the study and determine the best methods of study implementation.
Table 1.
Community sensitization
| Level/target group | Number of participants |
|---|---|
| District | |
| Political administrator | 14 |
| Health administrators | 34 |
| Coordinating committees | 100 |
| Traditional leadership | |
| Chiefs and royal secretaries | 95 |
| Village headmen | 600 |
| Cluster (study sites) | |
| Departmental heads | 600 |
| Religious leaders | 500 |
| Traditional practitioners | 100 |
| Community-based providers | 400 |
| Health facility workers | 200 |
| Community health workers | 300 |
| Women age > 15 years old | 7,000 |
| Men age > 15 years old | 2,000 |
In addition to extensive rounds of community sensitization (September to December of 2010) before participant recruitment, we integrated ongoing sensitization into routine ZamCAT activities. During the trial (February of 2011 to September of 2013), quarterly meetings were held with health center staff and traditional leadership in each cluster. Over the course of the study, sensitization meetings were held with more than 11,500 individuals. Per community leaderships' requests, enrolled numbers and any challenges that occurred were shared so that they could help resolve barriers (Table 2). The influence of religious beliefs on health practices presented a major challenge. For example, one sect believed in prayer rather than ANC or healthcare-seeking in the government-sponsored health sector. We engaged church leadership to try to increase local understanding of the study and the importance of ANC for a mother's health. After our discussions, we witnessed an increased uptake in enrollment in this cluster. In certain facilities where health workers emphasized male involvement, pregnant women would not be booked for ANC visits without their spouses, adversely affecting ANC attendance and enrollment. To resolve this challenge, we involved the District Medical Office (DMO) and community leaders to ensure that ANC booking could be done without spouses present. In locations where male involvement was required, a local religious group opened ANC registration at church and school premises.
Table 2.
Quotations from chiefs at community meetings
| Quotations |
|---|
| “Welcome my children. I fully understand the importance of research in development and urge you all to feel free to ask for my support at any time during the implementation period of this study.” Chief Moyo, Choma District |
| “Here in Chief Singani's area, our preferred method of notifying the study team on the delivery with 24 hours is by sending the family member. Also, our traditional birth attendants can also be useful, and most of them among us are willing to physically travel to the Centre to notify the study team on a delivery in the community. We are willing to share our cell phones wherever we have the network.” Chief Chipepo, Women's Meeting, Siavonga District |
| “Work that is intended to improve the health of our children and the mothers is exactly what we want, and I will personally ensure that this is given the support necessary in my chiefdom.” Chief Chikanta, Kalomo District |
| “I welcome this research in my chiefdom. Let me acknowledge the new developments in the health sector and especially the ones aimed at reducing maternal and child illnesses and deaths; and further state that the research is welcome. I pray that it will bring about further reduction of these illnesses and deaths in our women and new babies. My palace is open to you all anytime.” Chief Hamaundu, Choma District |
| “I am very happy to receive the good news of such a big study in my district. Let me assure you of my support. Any program or project, whether research or service delivery, that aims to help improve our children and women wellbeing is greatly appreciated by the people of Kalomo District. Yours is even special in that you are here for the rarely considered area of health, NEWBORN UMBILICAL CORD CARE!” Kalomo District Commissioner, Kalomo District |
| “I would like to thank the ZamCAT team for the good work aimed at trying to find an answer to neonatal mortality in our district of Mazabuka. The area of umbilical cord care is a very important subject matter if we are serious about improving neonatal health in Zambia. I am challenging the ZamCAT team to ensure quality research work as outlined in the protocol is replicated in the field if we are to meaningfully contribute towards the attainment of MDG #4 in reducing infant mortality. I pledge my support and that of my district staff to the ZamCAT team.” District Community Medical Officer, Mazabuka District |
| “I am the custodian of healthcare for my chiefdom, and therefore, I am welcoming this progressive project, ZamCAT, in my chiefdom. I will make reference in my remarks to all the good works that the Boston University team is doing in my chiefdom, especially preventing babies from getting infected with HIV and now babies' umbilical cord care. I, however, challenge you to ensure you adhere to all ethical procedures in your work with my subjects. You should ensure all ethical requirements are followed in order to protect my subjects from research abuse.” Chief Sianjalika, Mazabuka District |
| “I am very happy to have such a big study that includes my district of Siavonga. Pregnant women and their newborns from the valley of Siavonga rarely receive such support and care. For your team to include the valley as part of the study area is appreciated greatly. Let me emphasize the importance of effective community sensitization considering that many studies that have involved people in the valley in the past have not explained the benefits of participation to community members. This has contributed to reluctance in participation on research studies. Please ensure you effectively share the study aims and procedures. Also, manage any expectations from participants before beginning the implementation. You are welcome to Siavonga.” District Commissioner, Siavonga District, December of 2010 |
Understanding community norms through formative research.
From 2009 to 2010, we conducted formative research using qualitative methods to inform study implementation. Focus group discussions (FGDs; N = 36 FGDs with 339 respondents) and in-depth interviews (IDIs; N = 42 respondents) were designed to provide an understanding of newborn care-seeking behaviors, labor/delivery and cord care cultural practices, sociocultural norms regarding visitation in the early neonatal period, and acceptance of various chlorhexidine packaging methods through a trial of improved practices (TIPS) with 96 recently delivered women and their newborns. Full details are described elsewhere.21
The formative research provided important contextual and sociocultural information needed for study implementation by yielding a deeper insight into cultural norms and acceptability. For example, we learned about (1) characteristics of field monitors (FMs; data collectors) that would make them acceptable to local communities, (2) potential substances typically applied to the umbilical cord, (3) acceptability of timing of visits in the early neonatal period, (4) maternal preference for chlorhexidine bottle size, (5) culturally appropriate questions for our quantitative survey, (6) perceptions of newborn illness, and (7) care-seeking practices for newborns.
Formative research to determine the FM characteristics.
FGD participants provided rich insight into the cultural context of early infant life. Many participants shared information about the types of visitors not welcome in the home of a newborn, especially in the first days of life. These include other pregnant women, women who are menstruating, men who have recently had sex, individuals having extramarital affairs, and prostitutes; visits by these persons were believed to cause newborn illnesses. Families of newborns might place a warning sign at the entrance to the home to ward off these types of visitors or encourage them to self-identify. Because ZamCAT FMs needed to visit infants' homes on days 1, 4, 10, and 28 of life for an anticipated 170,280 post-natal visits, it was imperative to select staff who would be culturally acceptable to study participants and not be accused of causing illness in the newborn. As a result, we limited our recruitment of FMs to women, ideally post-menopausal, who were well-respected in their communities and known to have extensive newborn care experience (as a result of past community-based health volunteer work).
FM selection, training, and supervision.
Community leaders and neighborhood health committees in the 90 clusters identified appropriate FM candidates. The local health facility staff assisted by ranking lists prepared by the community according to the selection criteria, including the characteristics mentioned above, at least a grade 9 level of education, ability to read and write in English and Tonga, and a reputation for being honest and reliable.
The FMs underwent an interview process conducted by the health centers in charge, traditional leadership representatives, and ZamCAT district-based staff. We conducted intensive initial training, multiple refresher trainings, and supportive supervision throughout the study. On recruitment, FMs participated in a 5-day training on study procedures, ethics, and data collection instruments followed by a written and practical examination. A second round of cluster-level refresher training was conducted when enrollment was initiated. On-site refresher trainings were also conducted quarterly throughout the study. A 1-day training was also held for health clinic staff and associated community-based agents (e.g., TBAs, community health workers, family planning agents, etc.) at study initiation. After study initiation, FMs were expected to enroll pregnant women and then schedule and conduct all home visits, with a focus on visiting the newborn within 24 hours of birth. At the peak of the study, FMs had to ride their bicycles 15–30 km a day to conduct follow-up visits.
District managers or field supervisors oversaw the FMs during weekly visits to the cluster. During these visits, the supervisor reviewed study procedures, reviewed and collected completed forms, answered questions that had recently arisen, met with study stakeholders in conjunction with FMs, and visited a sample of households to confirm the FM visitations. Additionally, study supplies were replenished and FM concerns were addressed.
Study personnel and retention.
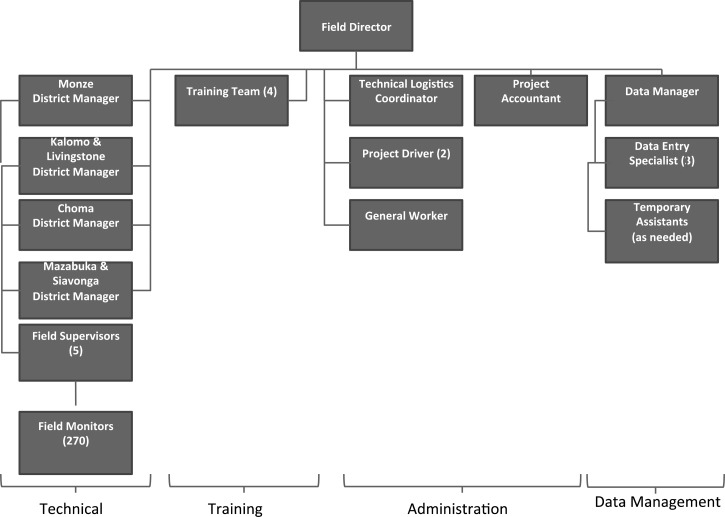
At study initiation, the total number of study personnel included 270 FMs (3 per cluster), 4 district managers, 5 field supervisors, 3 district-level logistics officers, and a central office consisting of the field director, 5 administrative staff (including 2 project drivers), a training team of 4 retired midwives, and a 4-person data management team (Figure 2). By the time of study completion, only 13 people had left the group (seven resignations and six dismissals). This high level of retention was made possible because of recruitment consistent with community input, motivated staff with adequate salary, daily supervisory support, monthly information sharing meetings across the districts, and an approachable, collaborative management style.
Figure 2.
ZamCAT organizational chart.
Supply chain.
Over the course of the study, a significant proportion of time and personnel focus was placed on supply chain management and ensuring that chlorhexidine, clean delivery kits (CDK), and paper and toner for printing of data collection forms were made available. At the field level, we purchased items in bulk, always had a buffer stock of the supply on hand, had a locked stock room, contracted local supplies whenever possible, and conducted monthly stock management. To supply the sites, which involved long distances, poor roads, and seasonal flooding, we provided a buffer stock at each site with all study supplies stored in two locked metal trunks at the health center. The district managers and field supervisors conducted weekly inventories at the cluster level. FMs contacted their field supervisor in case of low supplies, who in turn, would contact the logistics officer at the central office for an order to be placed and sent out to the cluster.
Birth notification.
Before enrollment, we worked with community leaders and members to develop cluster-specific birth notification systems of deliveries by ZamCAT mothers to ensure that a FM visited the newborn infant within 48 hours of delivery (ideally within the first 24 hours). The most feasible and effective birth notification methods identified were (1) cell phones (applicable only in communities with a functional cell phone network), (2) a facility-based ZamCAT cell phone, (3) sending family members by foot or bicycle to notify FMs, and (4) orienting local healthcare providers to birth notification strategies and study procedures.
In each cluster, an incentive system augmented the above-mentioned birth notification strategies. These incentives included reimbursement of cell phone talk time to mothers or their families or use of a community cell phone to facilitate calling the FMs. For accountability, the district manager and field supervisor monitored monthly phone usage. In addition, each district team documented each birth notification by community-based healthcare providers, family members, and health center committees. The team quarterly reviewed the documented notifications made by each community-based provider. If a given provider made at least 10 notifications, the provider received an incentive package containing two of the following products: 1 kg sugar, 750 mL cooking oil, 500 g washing powder, a 250-g soap bar, and 500 g salt. Providers who made more than 20 notifications received all four products for that quarter.
Participant enrollment and follow-up.
We initiated enrollment in stepwise fashion: starting in February of 2011 with 15 clusters and scaling up until all 90 clusters were covered by mid-May of 2011. Pregnant women in their second or third trimester ages ≥ 15 years old who planned to stay in the health facility catchment area for delivery and 1 month postpartum, were willing to provide informed consent, and were willing to perform cord care as per the protocol of their cluster were eligible for study participation. Eligible pregnant women were enrolled during ANC visits at health centers and in the community during ANC outreach activities conducted by health center personnel. Study team members assisted with outreach activities, including transportation to clinic staff. After enrollment, an FM made one home visit within 2 weeks, and then, after notification of the delivery, the FM conducted home visits to assess the mother and newborn on postpartum days 1, 4, 10, and 28.
Ethical and political clearance.
The University of Zambia Research Ethics Committee and Boston University Institutional Review Board approved the protocols and informed consent forms for the formative qualitative study and the cluster-randomized, controlled trial. The MoH provided guidance on items to be included in the newborn and maternal health messages to pregnant and postpartum women. Because of limited human resources, the ZamCAT team agreed with the MoH to avoid poaching medical staff from the healthcare system. All hired trainers were already retired from their government service; additionally, all hired FMs were not taken away from positions in the public health service. This political good will strengthened the relationship between the team and the MoH. All community sensitization and research activities were conducted in close collaboration with study consultants from the MoH, the SPMO, and the DMOs of each study district.
Results and Discussion
Conducting a community-based trial of this size and scope was logistically and technically challenging given Zambia's dispersed population (population density of 18.8/km2) relative to densely populated countries in south Asia (e.g., Bangladesh has a population density of 1,034/km2). Working in rural, sparsely populated health center catchment areas presented major recruitment and follow-up challenges. These included transportation, study supply chain management, notification of community-based deliveries, supervision of data collectors, follow-up of participants, ascertainment of maternal and newborn vital status, and cultural and human resource barriers. Involving the community and the political and cultural leaderships at multiple levels was essential to overcome these barriers.
Our focus on community sensitization and engagement helped to inform the implementation process and sustain study participation. Specifically, this process helped us identify and recruit FMs, facilitate home visits, and strengthen birth notification systems. In addition, community sensitization helped the study team address contentious issues (e.g., myths, suspicions, fears, and misinformation) and facilitated local acceptability of study procedures.
Impact of the ZamCAT implementation strategy.
The cluster-randomized, controlled trial initiated enrollment in February of 2011. With continued community engagement and use of women from the community as FMs, we have had very successful enrollment and follow-up. More than 92% of pregnant women screened were enrolled, resulting in a total of 39,679 pregnant women enrollees. Only 3.7% of the participants were withdrawn or lost to follow-up before delivery; 98 of 37,856 (0.2%) neonates were lost to follow-up. This low rate compares favorably to chlorhexidine application trials conducted in south Asia (5% lost to follow-up in Bangladesh, and 2.5% lost to follow-up in Nepal) and newborn health trials in Africa.7,8,22,23
Structural barriers.
In Zambia, like many African countries, rural communities are widely dispersed, and families often travel great distances to reach primary health centers. During the rainy season (November to March), roads and bridges are often washed out, creating even more difficulty in reaching clinics and enrolled women. Use of cell phone networks, when available, dramatically improved study implementation by reducing the time required for birth notification and facilitated regular communication between FMs, field supervisors, and district managers. Given the large land mass covered by ZamCAT (85,283 km2), seven cars with over one million kilometers driven, five motorcycles, and 270 bicycles were necessary to transport all required study goods, including data collection forms, clean delivery kits, chlorhexidine, and other study supplies, to 90 health centers. Each cluster had three FMs, and each was equipped with a bicycle with readily available and affordable spare parts in their communities. On recruitment, the first visit undertaken within 2 weeks of consenting was designed to trace and confirm the address of a participant, build trust, and reaffirm the study messages in addition to collect data on the danger signs and perceptions and deliver some study supplies (CDKs). This contributed to the improved success rates of future home visits and hence, the high follow-up rate observed under ZamCAT. Recruitment and placement of FMs were based on distance and resources availability. The cluster sample size was the same for all clusters; hence, each FM was expected to enroll and follow-up an equal number of study participants (N = 158).
Cultural context.
Understanding and addressing community belief systems before initiation of a clinical trial is important for ensuring successful recruitment and retention and high levels of participant follow-up.17 The baseline formative research was critically important to determine appropriate visitors and their timing in the first 24 hours of a child's life. Collaborating with the community on the FM selection helped foster a sense of ownership and participation from traditional leaders, village headmen, and other community members. Using their suggestions, an incentive system was developed that allowed the ZamCAT FMs to reach nearly all (96%) newborns within the first 48 hours after delivery. FMs followed individual women from enrollment to study completion, therefore establishing a rapport between the study team and participants.
Community and health system acceptance.
Despite a challenging logistical and cultural environment, we were able to successfully recruit nearly 40,000 pregnant women and maintain a high follow-up rate by engaging with three tiers of stakeholders who helped us obtain community support.
At the community level, we ensured that traditional leadership was supportive of research activities and had an opportunity to help shape the design of study procedures, such as birth notification systems and FM selection. Because FMs were selected for characteristics that made it culturally acceptable for them to enter the home of a newborn, they were rarely prohibited from data collection, although they were conducting interviews during a sensitive time.
Equally important was obtaining the support of health center staff. For example, we strove to develop a data collection system that complemented rather than burdened already stretched government health workers by basing FMs at health centers for enrollment and study coordination. FMs alleviated some of the health workers' administrative duties by helping with routine management of clinic attendees and recording health data.
To align the study goals and outcomes with the national child health agenda, ZamCAT was included in the official MoH child health annual plan. At the district level, we worked in close consultation with the SPMO on cluster selection before randomization. We also regularly requested access from district officials before contacting chiefs. Adhering closely to the statutory requirements of the ethical review board, the Zambia Medicines Regulatory Authority (ZMRA), and the MoH was also necessary. Although we prioritized community benefits in district-level discussions, the potential impacts of study results were key topics when we engaged with national-level MoH representatives.
Conclusions
Although large-scale, community-based trials in rural areas present formidable practical challenges, low rates of participant withdrawal and loss to follow-up can be achieved. Working closely with traditional authorities to limit the social disruption and suspicion that might result when people from outside the community conduct research is critically important to the success of community-based research. Our experience shows that participants can be successfully followed through completion of data collection as long as there is intensive and sustained commitment to engaging the host communities. By conducting qualitative research with a variety of stakeholders to inform the trial design, working with various levels of the political and health systems and traditional leadership, ensuring extensive community sensitization about study purpose and procedures, hiring local research staff in consultation with community leaders, and using community input on birth notification systems, we were able to successfully enroll and follow large numbers of pregnant women and newborns in the community, despite major structural (e.g., geographic and environmental) and cultural (e.g. religious and traditional gender roles) barriers.
ACKNOWLEDGMENTS
This study is registered at Clinical Trials.gov (NCT01241318).
Disclaimer: The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests.
Footnotes
Financial support: This manuscript is based on research funded by the Bill & Melinda Gates Foundation.
Authors' addresses: Davidson H. Hamer, Julie M. Herlihy, Donald M. Thea, Jonathon L. Simon, Kojo Yeboah-Antwi, Caroline Grogan, and Katherine E. A. Semrau, Center for Global Health and Development, Boston University, Boston, MA, E-mails: dhamer@bu.edu, herlihyj@bu.edu, dthea@bu.edu, jonsimon@bu.edu, kyantwi@bu.edu, cgrogan@bu.edu, and ksemrau@ariadnelabs.org. Kebby Musokotwane, Ministry of Community Development, Mother and Child Health, Lusaka, Zambia, E-mail: kebbymusokotwane@yahoo.com. Bowen Banda, Chipo Mpamba, Boyd Mwangelwa, and Portipher Pilingana, Zambia Chlorhexidine Application Trial Field Office, Zambia Center for Applied Health Research and Development (ZCAHRD), Choma, Zambia, E-mails: bowenbanda@gmail.com, chipo.mp@gmail.com, boyd.mwangelwa@gmail.com, and ppilingana@yahoo.co.uk.
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Oestergaard MZ, Inoue M, Yoshida S, Mahanani WR, Gore FM, Cousens S, Lawn JE, Mathers CD. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8:e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 5.Soofi S, Cousens S, Imdad A, Bhutto N, Ali N, Bhutta ZA. Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. Lancet. 2012;379:1029–1036. doi: 10.1016/S0140-6736(11)61877-1. [DOI] [PubMed] [Google Scholar]
- 6.Imdad A, Mullany LC, Baqui AH, El Arifeen S, Tielsch JM, Khatry SK, Shah R, Cousens S, Black RE, Bhutta ZA. The effect of umbilical cord cleansing with chlorhexidine on omphalitis and neonatal mortality in community settings in developing countries: a meta-analysis. BMC Public Health. 2013;13:S15. doi: 10.1186/1471-2458-13-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S, Adhikari R, Tielsch JM. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367:910–918. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR, Begum N, Al-Kabir A, Darmstadt GL, Santosham M, Black RE, Baqui AH. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet. 2012;379:1022–1028. doi: 10.1016/S0140-6736(11)61848-5. [DOI] [PubMed] [Google Scholar]
- 9.Mullany LC, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25:665–675. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imdad A, Bautista RM, Senen KA, Uy ME, Mantaring JB, 3rd, Bhutta ZA. Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database Syst Rev. 2013;5:CD008635. doi: 10.1002/14651858.CD008635.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam MA, Ali NA, Sultana N, Mullany LC, Teela KC, Khan NU, Baqui AH, El Arifeen S, Mannan I, Darmstadt GL, Winch PJ. Newborn umbilical cord and skin care in Sylhet District, Bangladesh: implications for the promotion of umbilical cord cleansing with topical chlorhexidine. J Perinatol. 2008;28((Suppl 2)):S61–S68. doi: 10.1038/jp.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry UK. Traditional practices of women from India: pregnancy, childbirth, and newborn care. J Obstet Gynecol Neonatal Nurs. 1997;26:533–539. doi: 10.1111/j.1552-6909.1997.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill Z, Tawiah-Agyemang C, Okeyere E, Manu A, Fenty J, Kirkwood B. Improving hygiene in home deliveries in rural Ghana: how to build on current attitudes and practices. Pediatr Infect Dis J. 2010;29:1004–1008. doi: 10.1097/INF.0b013e3181f5ddb1. [DOI] [PubMed] [Google Scholar]
- 14.Moran AC, Choudhury N, Uz Zaman Khan N, Ahsan Karar Z, Wahed T, Faiz Rashid S, Alam MA. Newborn care practices among slum dwellers in Dhaka, Bangladesh: a quantitative and qualitative exploratory study. BMC Pregnancy Childbirth. 2009;9:54. doi: 10.1186/1471-2393-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maimbolwa MC, Yamba B, Diwan V, Ransjo-Arvidson AB. Cultural childbirth practices and beliefs in Zambia. J Adv Nurs. 2003;43:263–274. doi: 10.1046/j.1365-2648.2003.02709.x. [DOI] [PubMed] [Google Scholar]
- 16.Arenas-Lopez S, Fajardo C, Valls i Soler A, Garcia-Corzo JR, Lima-Rogel MV, Calle G, Leite R, Lobos E, Hume-Wright Q, MacLeod S. Pediatric clinical trials in Latin America and Guyana: present views of local practitioners and ways to embrace the future. Paediatr Drugs. 2011;13:257–265. doi: 10.2165/11590350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Idoko OT, Kochhar S, Agbenyega TE, Ogutu B, Ota MO. Impact, challenges, and future projections of vaccine trials in Africa. Am J Trop Med Hyg. 2013;88:414–419. doi: 10.4269/ajtmh.12-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambia Central Statistical Office . Zambia 2010 Census of Population and Housing: Population Summary Report. Lusaka, Zambia: Zambia Central Statistical Office; 2012. [Google Scholar]
- 19.Ministry of Health . Health Management Information System. Lusaka, Zambia: Ministry of Health; 2009. [Google Scholar]
- 20.Campbell ML, Gregor FM. Mapping Social Relations: A Primer in Doing Institutional Ethnography. Aurora, Canada: Rowman Altamira; 2002. [Google Scholar]
- 21.Herlihy J, Shaikh A, Mazimba A, Gagne N, Grogan C, Mpamba C, Sooli B, Simamavwa G, Mabeta C, Shankoti P, Messersmith LJ, Semrau K, Hamer DH. Local perceptions, cultural beliefs and practices that shape umbilical cord care: a qualitative study in Southern Province, Zambia. PLoS ONE. 2013;8:e79191. doi: 10.1371/journal.pone.0079191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill CJ, Phiri-Mazala G, Guerina NG, Kasimba J, Mulenga M, MacLeod WB, Waitolo N, Knapp AB, Mirochnick M, Mazimba A, Fox MP, Sabin LL, Seidenberg P, Simon JL, Hamer DH. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. Brit Med J. 2011;342:d346. doi: 10.1136/bmj.d346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood BR, Manu A, ten Asbroek AH, Soremekun S, Weobong B, Gyan T, Danso S, Amenga-Etego S, Tawiah-Agyemang C, Owusu-Agyei S, Hill Z. Effect of the Newhints home-visits intervention on neonatal mortality rate and care practices in Ghana: a cluster randomised controlled trial. Lancet. 2013;381:2184–2192. doi: 10.1016/S0140-6736(13)60095-1. [DOI] [PubMed] [Google Scholar]