Abstract
Rationale
Relapse is an important problem in substance dependence treatment. When drug users try to abstain from drug use, they often report strong temptations to use drugs. Temptation episodes have commonalities with relapse episodes, and assessment of temptation episodes may help to identify individuals at risk of relapse.
Objectives
This study aims to examine affect and cognition prior to and during temptation episodes by administering self-report and implicit cognitive assessments on a handheld computer (PDA) using Ecological Momentary Assessment.
Methods
Heroin-dependent patients (N=68) attending a drug detoxification unit completed up to four random assessments (RAs) per day on a PDA for 1 week. They also completed an assessment when they experienced a temptation to use drugs (temptation assessment; TA).
Results
Participants completed 1,482 assessments (353 TAs, 1,129 RAs). The rate of TAs was maximal during the first 2 days. Participants reported higher levels of negative affect, anxiety, and difficulty concentrating, and more positive explicit attitudes to drugs, at TAs compared to RAs. In addition, they exhibited elevated attentional bias to drug cues (assessed using the modified Stroop task) at TAs compared to RAs. Implicit affective associations with drug cues (assessed using the Implicit Association Test) were not different at TAs compared to RAs. Attentional bias was elevated in the 1 h prior to the entry of a temptation episode.
Conclusions
Elevated attentional bias may be a harbinger of temptation episodes. Interventions that target cognitions prior to or during temptation episodes may reduce the probability or severity of a temptation episode.
Keywords: Ecological momentary assessment, Implicit cognition, Implicit association test, Modified stroop, Temptation, Attentional bias
Relapse is an important problem in substance dependence treatment. When attempting to abstain from drug use, many drug users report strong temptations to use drugs (e.g., Shiffman et al. 1997; Epstein et al. 2009). It is important to study temptation episodes because assessment of temptation episodes may help to identify individuals at risk of relapse.
A temptation episode can be defined as an occasion when a drug user, attempting to abstain from drug use, experiences an acute increase in the urge to use drugs or an occasion when the user feels that he or she has come to the brink of using drugs without actually doing so (Shiffman et al. 1996). The characteristics of temptation episodes have been examined using Ecological Momentary Assessment (EMA). EMA involves assessing phenomena at the moment they occur in a person’s natural environment. Assessments may be done at random times (“random assessments”; RAs) and/or when participants experience heightened emotions or motivational states (e.g., temptations). Data from EMA studies are highly detailed and can reveal longitudinal patterns of change within a few hours (e.g., Shiffman and Waters 2004; Epstein et al. 2009).
The psychological processes that underlie temptation episodes and relapse episodes likely share some similarities (Shiffman et al. 1996). In smokers, research using EMA has shown that self-reported negative affect is most elevated just prior to lapse episodes, and it is higher just prior to temptation episodes than at random assessments (Shiffman et al. 1996). In addition, characteristics of temptation episodes have been associated with relapse. For example, duration (though not frequency) of temptations has been associated with relapse (Shiffman et al. 1997). In lapsers, the peak reported urge during temptations (a measure of temptation intensity) increased in the days prior to lapse (Shiffman et al. 1997).
EMA has also been used to study craving episodes in heroin- and cocaine-abusing outpatients treated with methadone (Epstein et al. 2009). Participants were instructed to report on a handheld computer whenever they craved heroin or cocaine without using them. In the hours preceding episodes of heroin (but not cocaine) craving, there were significant increases in endorsements (at random assessments) of a number of negative affective items, such as “feeling sad” and “feeling angry”. Endorsement rates were typically maximal within 1 h of the craving episode.
The aforementioned studies relied on self-report data to examine the precipitants of temptation episodes. Self-report measures have two important limitations. First, it is easy for people to misrepresent or “fake” their mood and cognitions on self-report measures (Hammersley 1994). Second, automatic (or implicit) processes cannot be adequately assessed using self-report measures. Automatic processes are psychological processes that are fast, parallel, effortless, and may not engage conscious awareness (Schneider and Shiffrin 1977). Beginning with Tiffany (1990), a number of researchers have highlighted the role of automatic cognitive processes in addiction (Baker et al. 2004; Robinson and Berridge 1993; Wiers and Stacy 2006). Meta-analyses have confirmed that measures of automatic/implicit cognition are associated with substance use (Rooke et al. 2008; Cox et al. 2006).
Two widely studied automatic processes are (1) automatic attention capture (Cox et al. 2006) and (2) automatic affective associations in memory (e.g., Wiers et al. 2002). The importance of automatic attention capture (or “attentional bias”) is highlighted in Franken’s model (2003). Franken (2003) posits that attentional bias reflects the incentive salience of drug cues (Robinson and Berridge 1993). The model further assumes that attentional bias can cause or increase craving, and that craving can cause or increase attentional bias. A meta-analysis has confirmed that measures of automatic/implicit cognition are associated with self-reported craving (Field et al. 2009b). The model also assumes that both craving and attentional bias can cause relapse. A number of studies have reported that attentional bias prospectively predicts outcomes in the addictions (e.g., Carpenter et al. 2006; Cox et al. 2002, 2007; Janes et al. 2010; Marissen et al. 2006; Powell et al. 2010; Waters et al. 2003).
Automatic affective associations in memory are another implicit cognitive mechanism that is important in addiction. The assumption is that drug-related choices are often influenced by associations in memory that are spontaneously activated under certain conditions (Stacy and Wiers 2010). Implicit associations are memory associations with the substance of abuse that are not revealed through introspection, self-reflection, or causal attribution (Stacy and Wiers 2010). Research has demonstrated that drug users have tended to exhibit more positive (less negative) implicit associations with drug-related cues than non-users [see Roefs et al. 2010 for review of studies using the Implicit Association Test (IAT)].
In the current study, we administered both self-report and implicit cognitive assessments in temptations in heroin- and cocaine-abusing participants undergoing drug detoxification. The rationale for using this population and setting was as follows. First, attentional bias is typically robust in heroin and cocaine abusers (e.g., Franken et al. 2000; Hester et al. 2006; Liu et al. 2010). Second, there is evidence that the association between implicit cognition and craving-related variables is stronger in illicit drug users than licit drug users (Field et al. 2009b). Last, EMA methods could plausibly be used in a drug detoxification setting to determine the association between implicit and craving-related variables in a clinical setting, to determine the time course of these variables in this specific setting, and perhaps to identify those individuals at risk of treatment failure.
The goals of the study were as follows. First, we wanted to describe the natural history of temptation episodes during drug detoxification using EMA methods. Second, we examined whether self-reported negative affect, craving, and explicit attitudes were elevated in temptation episodes. Third, we examined implicit cognitive processes (attentional bias and automatic affective associations) during temptation episodes. We hypothesized that these implicit cognitions would be elevated during a temptation episode. Last, we also examined whether implicit and explicit cognitions were elevated in the hours preceding a temptation episode.
Method
Participants
Participants were 68 heroin-dependent inpatients recruited from an addiction treatment center (Bouman GGZ) in Rotterdam, The Netherlands (Table 1). Inclusion criteria were (1) aged between 18 and 65 years; (2) meeting the DSM-IV criteria for heroin dependence; and (3) the ability to speak, read, and write in Dutch at an eight-grade literacy level. Exclusion criteria were (1) indications of severe psychopathology (psychosis, severe mood disorder) as assessed by a physician, (2) self-reported color blindness or (non-corrected) defective vision, and (3) pregnant or breast-feeding. Although cocaine dependence was not an inclusion criterion, most patients attending the treatment center were dependent on cocaine. We therefore used heroin and cocaine versions of all behavioral tasks and questionnaires (see below). In the current sample, 88.1% of participants were dependent on cocaine (Table 1), and the eight participants who did not meet criteria for cocaine dependence reported that they had used cocaine regularly for an average of 10.1 years. The study was approved by the Institutional Review Board of the Erasmus Medical Center, Rotterdam, The Netherlands.
Table 1.
Summary statistics for demographic variables, sub-stance use variables, and methadone use
| Mean | SD | |
|---|---|---|
| Demographic variables | ||
| Age | 40.87 | 7.72 |
| Males (%) | 85.3 | |
| Education (%)a | ||
| Primary education | 14.9 | |
| Junior secondary education | 56.7 | |
| Senior secondary education | 25.4 | |
| Higher education | 3.0 | |
| Race (%) | ||
| Caucasian | 51.5 | |
| Other | 48.5 | |
| DSM GAF scorea | 47.01 | 5.97 |
| Substance use variables | ||
| Heroin dependence (%) | 100.0 | |
| Cocaine dependence (%)a,b | 88.1 | |
| Heroin use 1 week prior to intake (%)c | 95.0 | |
| Age of first heroin use | 22.34 | 6.72 |
| Age of first cocaine use | 22.32 | 8.50 |
| Total years of heroin usea | 14.13 | 8.73 |
| Total years of cocaine usea | 12.40 | 7.87 |
| Number of heroin use days in last montha | 21.28 | 9.10 |
| Number of cocaine use days in last montha | 19.00 | 10.08 |
| Smokingd as main heroin administration route (%) | 85.3 | |
| Smokinge as main cocaine administration route (%) | 86.8 | |
| Alcohol dependence (%)a | 17.9 | |
| Nicotine dependence (%) | 100.0 | |
| Methadone variables | ||
| Methadone treatment (%)f | 95.5% | |
| Starting dose of methadone (mg)g | 58.9 | 26.8 |
| No. of days of methadone use during PDA studyh | 6.57 | 1.51 |
Values are means (N=68) unless otherwise indicated
n=67
One participant with missing data reported they had used cocaine regularly for 12 years, and that they had used cocaine for 12 days in the past month
n=60
Smoking by means of heating the substance on tin foil (“chasing the dragon”)
Smoking with a crack pipe
n=66 (two participants had missing data)
n=63 (participants with data who started on methadone)
n=63 (participants with data who used methadone during study)
Treatment setting
The study took place at an inpatient detoxification unit of a large, urban, addiction treatment center. The inpatient detoxification unit consists of living and dining rooms, a smoking room, a small kitchen, and a garden. Patients had their own private bedroom. Detoxification treatment generally lasts 3 weeks. The goal of this treatment is to reduce physical dependency on the substances used by the patient. Ninety-five percent of the participants were placed on methadone maintenance at admission (Table 1). Methadone reduces acute heroin withdrawal and craving for heroin. Antidepressants, and/or sedative and anti-anxiety medications were administered as required. The usual procedure is that, after the detoxification treatment, patients start a follow-up treatment which is a rehabilitation program that can last from 1 month to a couple of years, depending on the severity of the problems. The goal of rehabilitation treatment is to prepare the patients for reintegration into society.
Procedure
All patients were informed about the study on the second day of their detoxification treatment. They had 24 h to decide whether they wanted to participate. Participants signed an informed consent form. They were trained how to use the PDA and completed a practice assessment. Participants carried around the PDA for 1 week. The PDA beeped at random times up to four times per day (RAs). Participants were instructed to press a button on the PDA whenever they experienced a temptation to use heroin or cocaine, defined as acute rise in urge to use heroin or cocaine or an occasion when they felt they were on the brink of acquiring and using heroin or cocaine (Temptation Assessment; TA). At each assessment (RA or TA), participants first responded to items assessing subjective (e.g., mood), pharmacological (e.g., use of coffee, alcohol, and cigarettes), and contextual variables (e.g., location). They subsequently completed either a drug Stroop task or an Implicit Association Test (IAT). The PDA was programmed to administer the two tasks in an alternating sequence to each participant. After day 7 of the study, the participant returned the PDA to the researcher and received financial compensation, which was proportional to the number of RAs completed (maximum compensation was €50; approximately $65).
During treatment, a patient was permitted to go on leave for a couple of hours if the treatment staff approved. Although this was of course unwanted, participants could lapse (take heroin or cocaine) while offsite (away from the clinic). Participants were not permitted to take the PDA with them offsite. Therefore, all PDA assessments were completed at the detoxification unit. Thus, although the study involved many features of a typical EMA study (such as the use of a PDA to deliver random and participant-initiated assessments), unlike the majority of EMA studies, the data were collected in an inpatient setting rather than the patients natural environment.
Interview measure
The alcohol and drug section of the Addiction Severity Index (ASI) was used to assess drug use history and severity (McLellan et al. 1980).
PDA measures
Subjective measures
Participants were asked to respond according to how they feel “at this moment”. Unless otherwise indicated, participants made their responses on seven-point Likert scales (1= strongly disagree to 7= strongly agree). Craving for heroin, craving for cocaine, hunger, and difficulty concentrating were assessed with single items. Explicit attitude toward heroin (“At this moment, please indicate your overall attitude to heroin”; 1= strongly negative to 7= strongly positive), and explicit attitudes toward cocaine were also assessed with single items (these two items were only administered on the assessments when an IAT was administered). A six-item version of the State-Trait Anxiety Inventory (STAI) (upset, worried, frightened, calm, secure, self-confident) was administered (Sayette et al. 2001). A state anxiety rating was computed from the mean of the items, reverse-scoring where appropriate (ratings from individual items are not reported). Six affect items (enthusiastic, happy, relaxed, bored, sad, angry) were presented. Two additional items assessed overall mood (My overall mood/feeling is…1= strongly negative to 7= strongly positive) and energy/arousal levels (My energy/arousal level is…1= very low to 7= very high). A negative affect rating was computed from the mean of seven items (enthusiastic, happy, relaxed, bored, sad, angry, and overall mood, reverse-scoring where appropriate; ratings from individual items are not reported).1
Pharmacological and contextual measures
Items assessed the number of cigarettes smoked in the past 2 h, the amount of alcohol consumed in the past 2 h, and the amount of coffee consumed in the past 2 h. One item assessed social context, and two items assessed location. After each IAT or modified Stroop assessment, the participant was asked to report the number of times that he or she was interrupted while performing the task. Response options for these items are shown in Table 2.
Table 2.
Summary statistics on subjective, implicit, pharmacological, and contextual variables
| TAs (n=353) |
RAs (n=1,129) |
All assessments (N=1,482) |
|
|---|---|---|---|
| Craving | |||
| Heroin craving (1–7) | 3.89 (2.20) | 2.65 (1.82) | 2.95 (1.99) |
| Cocaine craving (1–7) | 3.68 (2.47) | 2.41 (1.90) | 2.71 (2.12) |
| Subjective variables | |||
| Negative affect (1–7)a | 4.19 (1.24) | 3.55 (1.22) | 3.70 (1.25) |
| State anxiety (1–7)a | 4.01 (1.22) | 3.36 (1.22) | 3.52 (1.25) |
| Mood (1–7) | 3.63 (1.70) | 4.40 (1.59) | 4.22 (1.65) |
| Energy level (1–7) | 3.93 (1.85) | 4.17 (1.66) | 4.11 (1.71) |
| Difficulty concentrating (1–7) | 4.41 (1.87) | 3.86 (1.81) | 3.99 (1.84) |
| Hunger (1–7) | 2.88 (1.71) | 2.56 (1.62) | 2.63 (1.64) |
| Heroin explicit attitude (1–7) | 3.33 (1.90) | 2.61 (1.74) | 2.78 (1.81) |
| Cocaine explicit attitude (1–7) | 3.15 (2.10) | 2.43 (1.85) | 2.61 (1.93) |
| Implicit variables | |||
| Heroin Stroop (ms) | 64.8 (196) | 34.2 (150) | 42.5 (165) |
| Cocaine Stroop (ms) | 67.8 (152) | 36.4 (165) | 42.7 (163) |
| Heroin IAT (ms) | 208 (844) | 79.8 (642) | 107 (692) |
| Cocaine IAT (ms) | 120 (832) | 109 (594) | 112 (664) |
| Heroin IAT (D score) | 0.16 (0.61) | 0.12 (0.57) | 0.13 (0.58) |
| Cocaine IAT (D score) | 0.11 (0.61) | 0.16 (0.56) | 0.14 (0.58) |
| Pharmacological variables | |||
| No. of cigarettes smoked | |||
| No cigarettes (%) | 10.5 | 11.2 | 11.0 |
| 1 cigarette (%) | 20.4 | 26.4 | 25.0 |
| Many cigarettes (%) | 69.1 | 62.4 | 64.0 |
| Alcohol | |||
| No alcohol (%) | 97.7 | 98.6 | 98.4 |
| Small amount (%) | 1.7 | 1.2 | 1.3 |
| Large amount (%) | 0.6 | 0.3 | 0.3 |
| Coffee | |||
| No coffee (%) | 31.7 | 37.2 | 35.9 |
| Small amount (%) | 52.7 | 49.3 | 50.1 |
| Large amount (%) | 15.6 | 13.5 | 14.0 |
| Contextual variables | |||
| Social context | |||
| Alone (%) | 54.7 | 46.1 | 48.1 |
| With others (%) | 45.3 | 53.9 | 51.9 |
| Location 1 | |||
| Living room (%) | 19.8 | 27.1 | 25.4 |
| Dining room (%) | 22.4 | 21.8 | 21.9 |
| Bedroom (%) | 37.4 | 30.5 | 32.1 |
| Medication room (%) | 2.6 | 1.0 | 1.4 |
| Somewhere else in clinic (%) | 17.9 | 19.7 | 19.2 |
| Location 2 | |||
| Outside (%) | 15.3 | 10.3 | 11.5 |
| Indoors (%) | 84.7 | 89.7 | 88.5 |
| Interruptions | |||
| No times (%) | 48.5 | 50.5 | 50.0 |
| One time (%) | 21.7 | 21.7 | 21.7 |
| Two times (%) | 17.2 | 16.4 | 16.6 |
| Three times (%) | 9.3 | 8.1 | 8.4 |
| Four or more times (%) | 3.3 | 3.3 | 3.3 |
Values are means (SD) unless otherwise indicated (N=1,482 assessments). Means and SDs are computed by aggregation across assessments. Mixed-model based estimates of the data, which account for the fact that participants differ in the number of observations they contribute, are available on request. Significant between-assessment type differences are bolded. Participants completed 381 heroin-Stroop and 404 cocaine-Stroop assessments, making 785 Stroop assessments in total (185 TAs, 600 RAs). Participants completed 342 heroin-IAT and 355 cocaine-IAT assessments, making 697 IAT assessments in total (168 TAs, 529 RAs). Explicit attitude ratings were assessed at IAT assessments
RA random assessment, TA temptation assessment
State anxiety is mean of six items, and negative affect is mean of seven items (see text)
Drug Stroop task
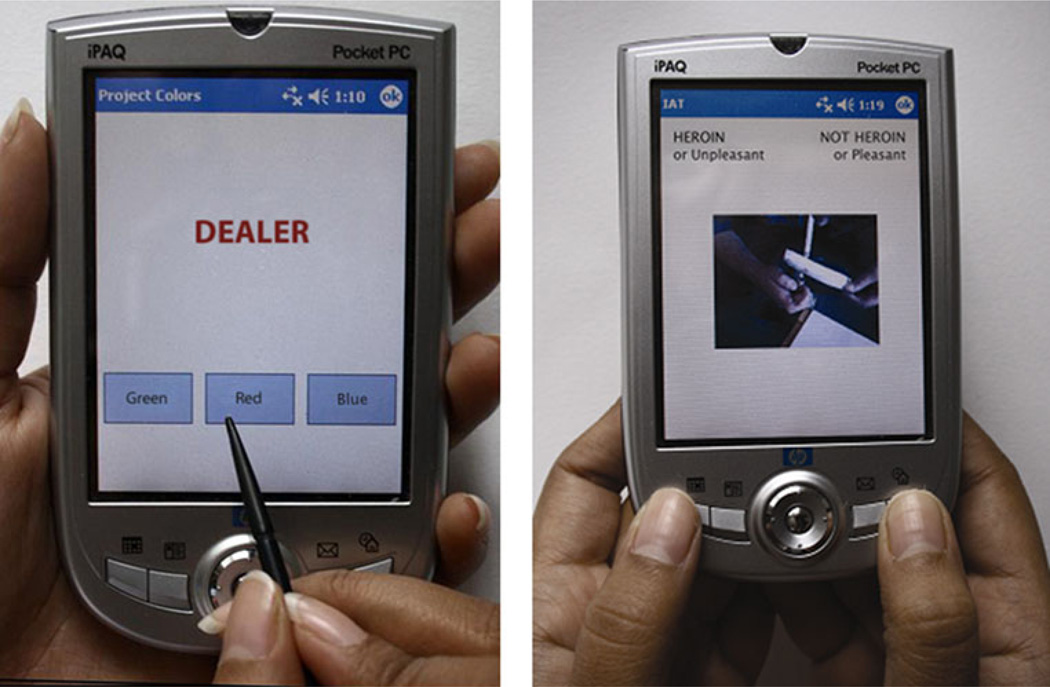
Participants were instructed that words written in different colors would be presented on the PDA screen one after the other and that the task was to indicate as rapidly and as accurately as possible which color the word was written in by pressing one of the three response buttons on the PDA using the stylus (see Fig. 1). Participants were instructed that they should ignore the meaning of the (target) word and to focus on the color. At each assessment, participants responded to a practice sequence of letter strings (e.g., MMMM; 33 trials), followed by two test blocks of 33 trials each, separated by a 5-s break. Each word was presented in capital letters and remained on the screen until either (1) the participant responded or (2) 3 s had elapsed. If the participant made an incorrect response or failed to respond, there was a tone. Five-hundred milliseconds after a response (or 500 ms after the 3-s time window in case of a non-response), a new word was presented. During the inter-stimulus interval, the screen was blank.
Fig. 1.
PDA versions of the modified Stroop task (left) and the IAT (right) (shown in English). In the Stroop task, the response buttons are boxes with color names within them (red, green, and blue). The positions of the response buttons on the screen varied across lists (e.g., on list 1 they were ordered [Blue] [Green] [Red]; on list 2 they were ordered as [Green] [Red] [Blue]). Participants make responses using the stylus. On the IAT, a picture or word is presented on each trial. There are labels on top of the screen to remind participants of the categories assigned to each key for the current task. Participants perform the categorization task by pressing either the left or the right key of the PDA. For the heroin IAT, on two blocks of trials (task 1), heroin is paired with pleasant, and not heroin is paired with unpleasant. On another two blocks (task 2), heroin is paired with unpleasant, and not heroin is paired with pleasant (shown in Fig. 1). The IAT effect is the difference in response times on the categorization task in these two tasks. Faster performance when heroin is paired with pleasant reflects a more positive implicit association with drug cues (see text for details)
Stimulus materials
At those assessments at which the Stroop task was administered, the participant completed either a heroin Stroop or a cocaine Stroop. The Stroop task was randomly selected (without replacement) from one of 24 sequences of words (“lists”), and therefore the selection of a heroin- or a cocaine-Stroop task was independent of the relative magnitude of reported heroin and cocaine craving. Twelve lists contained heroin words and matched neutral words (heroin Stroop), and the other 12 contained cocaine words and matched neutral words (cocaine Stroop). The order of presentation of neutral and drug words was counterbalanced across lists. For each list, a random sequence was generated that determined the order of presentation of words for that list, under the constraint that the same color did not occur on two consecutive trials.
In the heroin version, each list contained 11 heroin-related words (Dutch equivalent of score, flash, smack, dope, dealer, junk, shot, ball, heroin, inhale, high) and 11 neutral words drawn from the category “transport” (Dutch equivalent of ticket, metro, tram, moped, bike path, scooter, zebra crossing, asphalt, gasoline, freeway, racing; see Franken et al. 2000). The heroin and neutral words were matched on word length (5.7 vs. 6.1 letters, respectively) and word frequency (3.3 vs. 5.0, respectively) using the CELEX database (Baayen et al. 1995). In the cocaine version, each list contained 11 cocaine words (Dutch equivalent of pipe, puff, crack, smoke, cocaine, blow, line, coke, snort, powder, base) and 11 neutral words drawn from the category “indoor features” (rug, blanket, sofa, oven, lamp, attic, cabinet, armchair, tap, couch, stove; word length 5.4 vs. 5.6, respectively; word frequency 11.2 vs. 12.9, respectively).
Scoring
Reaction times (RTs) from incorrect responses were discarded (3.5% of trials), as were RTs <100 ms (0.01% of trials). To reduce the influence of RT outliers caused by interruptions, we discarded RTs from assessments with interruptions as previously described (Waters and Li 2008) (0.68% of trials). The drug Stroop effect was computed on each assessment by taking the difference score between mean RT on drug Stroop words and mean RT on linked neutral words. Using a split-half approach (odd and even trials), the estimated internal reliabilities of mean RT on neutral words (heroin lists), mean RT on heroin words, and the heroin Stroop effect were r=0.96, r=0.97, and r=0.72 respectively. The estimated internal reliabilities of mean RT on neutral words (cocaine lists), mean RT on cocaine words, and the cocaine Stroop effect were r=0.96, r=0.96, and r=0.68, respectively.
Implicit association test (IAT)
The IAT consists of two tasks. On task 1, participants are required to respond rapidly with a key press to items representing two concepts (e.g., heroin + pleasant) and with a different key press to items from two other concepts (e.g., not heroin + unpleasant). In task 2, the assignment of one concept is switched. For example, in the current case, not heroin + pleasant would share a response, and heroin + unpleasant would share the other response. The main idea is that it is easier to perform the key presses when the two concepts are strongly associated in memory than when the two concepts are unrelated. The IAT effect is an index of the relative strength of automatic associations. In the example above, it indicates whether associations are stronger between heroin and pleasant, and not heroin and unpleasant, than between not heroin and pleasant, and heroin and unpleasant.
At those assessments at which the IAT was administered, the participant completed either a heroin IAT or a cocaine IAT. The PDA was programmed to present the heroin and cocaine IAT in an alternating sequence; the selection of a heroin or a cocaine IAT was independent of the relative magnitude of reported heroin and cocaine craving. The heroin IAT consisted of four blocks: (1) first block of combined categorization task (task 1) (e.g., heroin + pleasant/not heroin + unpleasant), (2) second block for task 1, (3) first block of alternative combined categorization task (task 2) (e.g., not heroin + pleasant/heroin + unpleasant), and (4) second block for task 2. At each assessment, participants were randomly assigned to one of four IATs: (a) heroin + pleasant first, pleasant on left; (b) heroin + pleasant first, unpleasant on left; (c) heroin + unpleasant first, pleasant on left; and (d) heroin + unpleasant first, unpleasant on left. Analogous procedures were used for cocaine IAT. There were no practice blocks.
On each trial, a stimulus (picture or word) was presented in the center of the Pocket PC screen. On the top of the screen were labels to remind participants of the categories assigned to each key for the current task. Participants performed the categorization task by pressing either the left or the right key of the Pocket PC (Fig. 1). They were instructed to respond as quickly and as accurately as possible. The program randomly selected items such that the sequence of trials alternated between the presentation of a (heroin/not heroin or cocaine/not cocaine) picture and the presentation of a (pleasant/unpleasant) word. If the participant responded correctly, the program proceeded to the next trial. If the participant made an error, a red “X” appeared below the stimulus and remained there until the participant responded correctly. Participants were instructed to correct their errors as quickly as possible. The inter-trial interval was 150 ms.
IAT stimulus materials
We used 10 heroin and 10 neutral pictures in the heroin version of the IAT (see Franken et al. 2003) and 10 cocaine and 10 (different) neutral pictures in the cocaine version (van de Laar et al. 2004). We used 12 words to capture the pleasant concept (Dutch equivalent of nice, pleasant, cool, relaxing, soothing, restful, smooth, peaceful, positive, friendly, satisfying, calm) and 12 words to capture the unpleasant concept (nasty, unpleasant, dirty, foul, smelly, unhealthy, ugly, negative, antisocial, depressing, harmful, revolting).
Scoring
The error rate on the IAT was 13.0%. The scoring algorithm recommended by Greenwald and colleagues was used to derive the IAT effect (Greenwald et al. 2003). Data from all four blocks were used to compute the IAT effect. RTs>10,000 ms were eliminated (0.98% of RTs). The algorithm eliminates assessments on which a participant had RTs of less than 300 ms on more than 10% of the trials (30 assessments; 4.1% of completed assessments). The computed IAT effect, D, is similar to an effect-size measure (Greenwald et al. 2003). The untransformed IAT effect (in milliseconds) is also reported to assist in interpretation. (For the ms score, the difference score is not divided by pooled SD of RTs.) The estimated split-half internal reliability was 0.64 (ms score) and 0.72 (D score) for the heroin IAT and 0.72 (ms score) and 0.77 (D score) for the cocaine IAT.
PDA hardware and software
Study procedures were implemented on a HP iPAQ Pocket PC running the Microsoft Windows Pocket PC operating system. The iPAQ uses a pen-based, touch-screen system. Participants could prevent the PDA from presenting RAs for up to 2 h (“suspend” function). Participants could also delay RAs by 5 min (up to four times per RA). Because use of the suspend and delay functions may cause the data to become less representative of daily experience, participants were encouraged to use these functions as infrequently as possible.
Data reduction and analysis
Of the 68 participants, 64 participants contributed data to the study (353 TAs, 1,129 RAs).2 Linear mixed models (LMM) were used for the primary analyses involving continuous outcome variables using SAS proc mixed. LMM analyses take into account the dependence between observations due to clustering of the data by participants, and allow for different numbers of observations across participants. To select an appropriate working correlation structure, we first ran LMM analyses under two commonly used correlation structures (compound symmetry and first-order autocorrelation) and compared the resulting Akaike/Schwartz information criteria (AIC/BIC). Based on the reported AIC/BIC (smaller is better), we selected the more appropriate working correlation structure for each dependent variable. To analyze dichotomous (e.g., Social Context) or categorical outcome variables (e.g., Location, see Table 2), we used proc glimmix in SAS (using maximum likelihood with adaptive quadrature estimation). This procedure permits the analysis of dichotomous and multinomial outcome variables, and also takes into account the clustering of the data by participants.
For analyses on the natural history of temptations over time, days (n=414) served as the unit of analysis. To assess the effect of Assessment Type (TAs vs. RAs), assessments (n=1,482) were the unit of analysis. Assessment Type (TA vs. RA) was entered as a class (categorical) variable (reference category=RA). To control for the effect of time, day in study was entered as a continuous variable. Number of assessments within each day was entered as a continuous variable. For analyses on the drug Stroop effect or drug IAT, drug material type (heroin vs. cocaine Stroop; heroin vs. cocaine IAT) was entered as a class variable. Following the recommendations of Hedeker et al. (2009), if a significant effect of Assessment Type was observed, we added temptation rate [i.e., number of TAs divided by the total number of assessments (TAs + RAs)] as a subject-level covariate to the model. This allowed us to examine (1) whether subjects who reported more TAs exhibited higher scores (averaged over TAs and RAs) on the dependent variable and (2) the effect of Assessment Type controlling for temptation rate. A significant effect for the latter would bolster the conclusion that the effect of Assessment Type is a truly within-subject effect (Hedeker et al. 2009), that is, when subjects experience a TA they have higher scores on the dependent variable at TAs than at RAs.
If a significant between-assessment difference (TAvs. RA) was observed in the above analyses, we used LMM to examine whether mood and cognitions were elevated in RAs in the 1 h preceding a TA (1 h pre-TA, n=61; “proximal” RAs) compared to RAs occurring more than 3 h prior to a TA and more than 3 h after a TA (n=1,232; “control RAs”) (reference category = control RAs). In secondary analyses, we used LMM to examine whether mood and cognitions were elevated in RAs occurring 1 h to 3 h prior to a TA (“distal” RAs) compared to control RAs. As above, day in study and number of assessments within each day were entered as continuous variables in all analyses.
For all analyses, alpha was set at 0.05. All tests were two-tailed.3
Results
On average, the 64 participants participated in the study for 6.47 days (SD=1.80). Participants completed 77.9% of presented RAs. They completed an average of 17.6 RAs (SD=7.3) and an average of 5.5 TAs (SD=6.1). Most participants (92.2%) completed at least one TA. The mean completion time was 5.7 min (SD=1.6) for assessments during which the Stroop task was administered and 8.2 min (SD=3.8) for assessments during which the IAT was administered.
Natural history of temptations
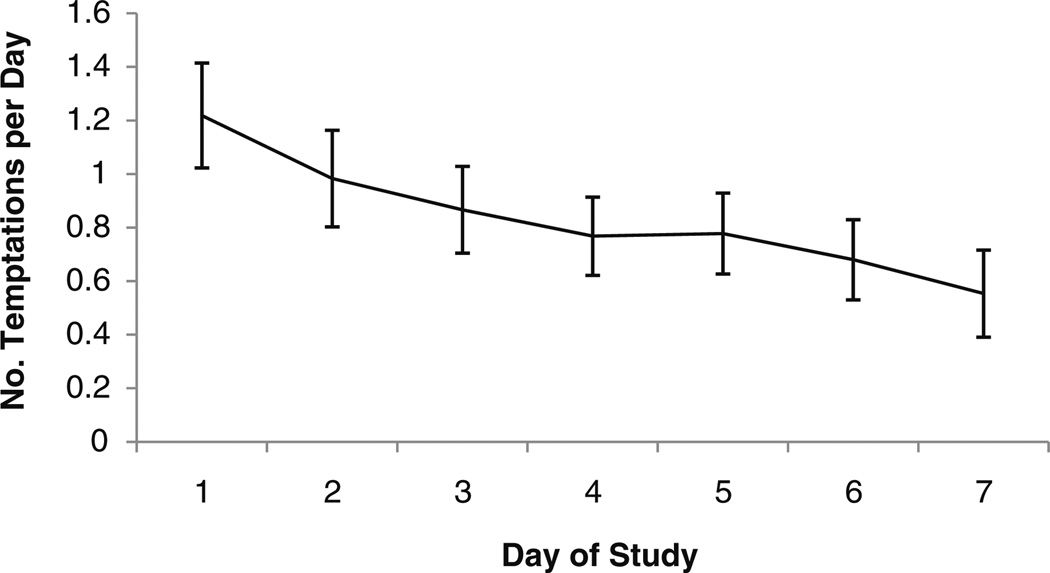
On average, there were 0.82 (SD=1.27) TAs recorded per day. The number of reported TAs per day declined over the course of the week [parameter estimate (PE)=−0.10, SE=0.03, p<0.01; Fig. 2). In contrast, the number of completed RAs per day did not decline over time, e.g., from days 2 to 7 (whole study days) (p>0.1). The distribution of TAs and completed RAs over the day was similar: 20.0% of RAs and 24.7% of TAs occurred before 12:00pm (midday), 29.1% (RAs) and 26.6% (TAs) occurred between 12:00pm and 4:00pm, 33.7% (RAs) and 31.4% (TAs) occurred between 4:00pm and 8:00pm, and 17.2% (RAs) and 17.3% (TAs) occurred after 8:00pm. The mean time of day for TAs (3:28pm; SD=4:05) was not significantly different (PE=0.43, SE=0.23, p=0.06) from the mean time of day for RAs (3:50pm; SD=3:44).
Fig. 2.
Mean number of temptations reported per day (1 SE) by day in study
Craving during temptations
Ratings for craving heroin (PE=0.86, SE=0.09, p<0.01) and cocaine (PE=1.02, SE=0.08, p<0.01) were both higher at TAs than RAs (Table 2). When temptation rate was added to the models, temptation rate was not associated with heroin craving (p>0.1) or with cocaine craving (p>0.1). Assessment type remained significant in these models (ps<0.01).
Participants’ mean ratings (over all observations) for craving heroin and craving cocaine were strongly correlated (n=64, r=0.67, p<0.01). A within-subject correlation for craving heroin and craving cocaine could be computed for 53 participants; the within-subject correlations also suggested a strong association between heroin and cocaine craving (mean r=0.69, SE=0.36, p<0.01). There was evidence that ratings for craving heroin (PE=−0.049, SE=0.024, p<0.05) and craving cocaine (PE=−0.035, SE=0.020, p=0.08) declined over days in RAs. Ratings for craving heroin and craving cocaine did not decline over days in TAs (ps>0.1).
Subjective variables during temptations
Participants reported higher levels of negative affect (PE=0.36, SE=0.06, p<0.01), anxiety (PE=0.42, SE=0.05, p<0.01), and difficulty concentrating (PE=0.26, SE=0.09, p<0.01) at TAs vs. RAs. When temptation rate was added to the models, temptation rate was associated with negative affect (PE=1.11, SE=0.47, p<0.05), state anxiety (PE=1.24, SE=0.53, p<0.05), and difficulty concentrating (PE=1.48, SE=0.74, p<0.05). Assessment type remained significant in these models (ps<0.01). Participants reported more positive explicit attitudes to heroin (PE=0.74, SE=0.13, p<0.01) and cocaine (PE=0.94, SE=0.13, p<0.01) at TAs vs. RAs. When temptation rate was added to the models, temptation rate was not associated with explicit attitude to heroin (p>0.1) or with explicit attitude to cocaine (p>0.1). Assessment type remained significant in these models (ps<0.01). There were no significant between-assessment differences for energy level or hunger (Table 2).
Implicit cognitions during temptations
Participants exhibited a robust drug Stroop effect (slower responses on drug words than neutral words, indicative of higher attentional bias) at both TAs (PE=67.7, SE=14.0, p<0.01) and RAs (PE=35.6, SE=7.2, p<0.01). However, the drug Stroop effect was significantly elevated at TAs (PE=31.7, SE=14.2, p<0.05) vs. RAs (Table 2). When temptation rate was added to this model, temptation rate was not associated with the drug Stroop effect (p>0.1). Assessment type remained significant in this model (p<0.05). There was no effect of Stroop type (heroin vs. cocaine Stroop) on the drug Stroop effect (p>0.1), and Stroop type did not moderate the effect of assessment type on the drug Stroop effect (p>0.1). As noted above, heroin craving and cocaine craving were higher at TAs vs. RAs. The drug Stroop effect was significantly elevated at TAs (PE=29.6, SE=14.9, p<0.05) vs. RAs when controlling for both heroin craving and cocaine craving.4
Participants tended to exhibit a positive IAT effect (faster performance when heroin/cocaine paired with pleasant compared to unpleasant, indicative of an automatic positive memory association) at both TAs (ms score—PE=185.8, SE=104.8, p=0.08; D score—PE=0.16, SE=0.08, p<0.05) and RAs (ms score—PE=97.7, SE=47.3, p<0.05; D score—PE=0.14, SE=0.05, p<0.01). However, no significant between-assessment (RAs vs. TAs) differences on the IAT effect (ms or D score) were observed. There was no effect of IAT type (heroin vs. cocaine IAT) on the IAT effect (p>0.1), and IAT type did not moderate the effect of assessment type on the drug IAT effect (p>0.1).5
Pharmacological and contextual variables
TAs were more likely to be reported when participants were outside the facility (in the garden; PE=−0.48, SE=0.21, p<0.05). There were no other significant associations (Table 2).
Mood and cognition in the hours before temptations
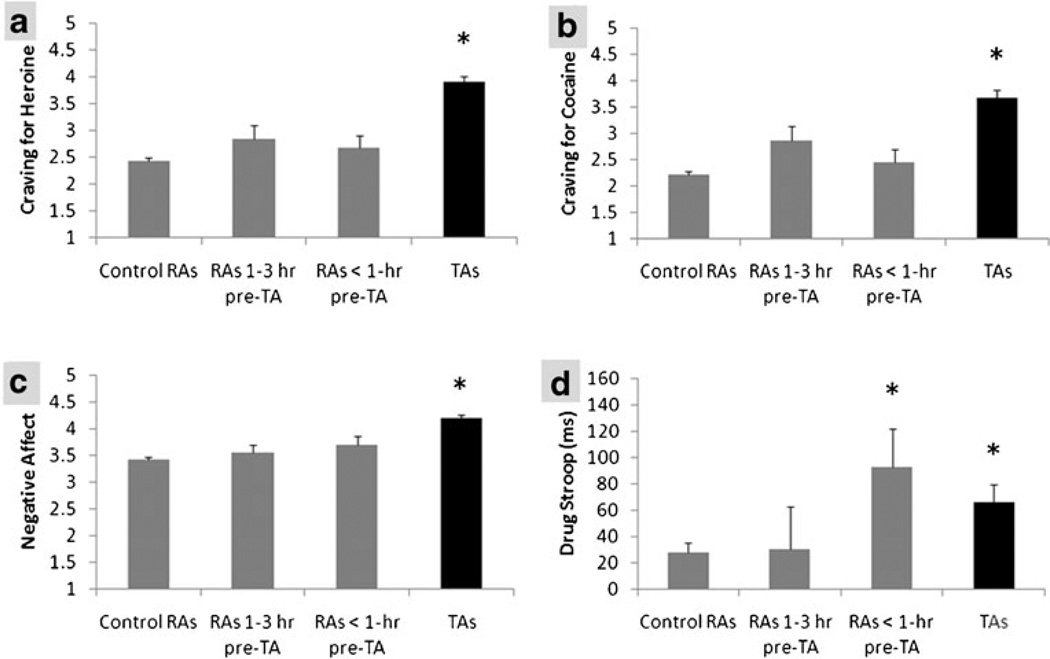
Neither craving for heroin (p>0.1) nor craving for cocaine (p>0.1; Fig. 3 shows raw data) was significantly elevated in proximal RAs (RAs that occurred less than 1 h prior to TAs) vs. control RAs. Negative affect (p>0.1) (Fig. 3), anxiety (p>0.1), difficulty concentrating (p>0.1), and explicit attitudes to heroin (p>0.1) and cocaine (p>0.1) were also not significantly elevated in proximal RAs (vs. control RAs). However, the drug Stroop effect (PE=62.4, SE=29.5, p<0.05; Fig. 3) [but not the IAT effect (D score or ms score, ps>0.1)] was significantly elevated in proximal RAs (vs. control RAs; Fig. 3d). There were no significant differences when comparing distal RAs (RAs occurring 1 h to 3 h prior to a TA) and control RAs (all ps>0.1).
Fig. 3.
Mean (1 SE) reported craving for heroin (a), reported craving for cocaine (b), reported negative affect (c), and drug Stroop effect (ms) (d) as a function of assessment type (RA vs. TA) and time before TA (see text for details). Data shown are raw (uncorrected) means. RA random assessment, TA temptation assessment. *Significant difference vs. control RAs (p<0.05)
Discussion
The main findings were as follows. First, participants reported on average around five and a half temptation episodes over the course of a week while in a clinical drug detoxification setting. Reports were maximal at the outset of the week. Second, negative affect was elevated when a participant reported a temptation episode. Explicit attitudes to heroin and cocaine were also elevated (more positive). Third, attentional bias to drug cues was elevated during temptation episodes, but implicit affective associations with drug cues were not more positive. Last, and perhaps of greatest interest, attentional bias—but not negative affect or explicit attitudes—was elevated in the 1 h prior to the report of a temptation episode. Thus, elevated attentional bias at random assessments may be a harbinger of temptation episodes.
The number of temptations declined during the week, suggesting improvement over time. However, participants may have become tired of completing study assessments over time, meaning that the decline reflected study fatigue rather than a genuine change in drug use motivation. Note, however, that craving ratings tended to decline in random assessments over time, which suggests a genuine decline in drug use motivation. If real, the decline in reported temptations and heroin craving may reflect recovery from acute heroin withdrawal because methadone does not completely alleviate craving for heroin (Fareed et al. 2011). Speculatively, a decline in heroin craving may promote a reduction in cocaine craving due to cross-drug priming of craving (e.g., Epstein et al. 2010). These points notwithstanding, one should note that craving ratings and temptation rate might increase later in detoxification treatment when participants were withdrawn from methadone (Gossop et al. 1987; Glasper et al. 2008).
Overall, temptation episodes seem problematic for patients during detoxification treatment. The negative affect experienced during temptation episodes presumably impairs quality of life during drug detoxification and may increase the risk of relapse and/or treatment dropout. In addition, individuals who reported more temptations reported generally higher levels of negative affect and state anxiety (averaged across temptations and random assessments). These points notwithstanding, we do not know whether temptation episodes cause negative affect, whether negative affect causes temptation episodes, or whether a third variable underlies the association.
More importantly, the study revealed that attentional bias was elevated at temptation episodes. This finding is consistent with Franken’s (2003) model. In this model, attentional bias results from incentive sensitization. Attentional bias to external or imaginal cues can cause increased craving and, presumably, temptations episodes (when craving is acutely elevated). Conversely, craving (and, presumably, temptations) can cause attentional bias. Either way, an association between assessment type and attentional bias should be and was observed.
Interestingly, however, the association between assessment type and attentional bias persisted when controlling for self-reported heroin and craving. Thus, the association between assessment type and attentional bias was not accounted for, or mediated by, craving. This finding suggests that there is another attribute of temptation episodes that is associated with elevated attentional bias. Berridge (2009) has noted that the attribution of incentive salience may occur to the mental representations of drug-related actions (“action salience”) as well as the mental representations of drug-related stimuli. As Berridge (2009, p. 9) puts it, an addict “might urgently “want” to act”. The attribution of incentive salience to stimuli and actions might occur in parallel, and the latter process may be subjectively detected in the absence of subjective craving. Speculatively, attentional bias may be more closely associated with the subjective correlate of action salience (wanting to act) than with the subjective correlate of incentive salience (craving).
We do not know whether the attentional bias causes the temptation episode or whether the temptation episode causes attentional bias (or whether a third factor underlies the association). However, attentional bias (but not negative affect or other mood measures) was elevated prior to the onset of a temptation episode. This is consistent with the idea that attentional bias can cause a temptation episode.
To definitively examine the causal relationship between attentional bias and temptation episodes, it is necessary to experimentally manipulate attentional bias. If attentional bias causes temptation episodes, then an attentional retraining intervention should influence the number of reported temptation episodes. The effect of attentional retraining on temptation episodes has not hitherto been examined. Laboratory studies have examined the effect of attentional retraining on self-reported craving. These studies have yielded mixed findings, with some studies reporting an effect (Field and Eastwood 2005; Attwood et al. 2008, males only), and others reporting no effect (Field et al. 2007; 2009a; Schoenmakers et al. 2007).
Whether or not the association between attentional bias and temptation episodes is causal, the study has implications for treatment. If elevated attentional bias is indeed a harbinger of temptation episodes, it may be possible to intervene (when attentional bias is elevated) to reduce the risk that a temptation episode is subsequently experienced. An ecological momentary intervention (EMI) could be delivered on a PDA, just in time, when the individual is most in need of that intervention (Shiffman et al. 2008). Interventions that reduce the risk of temptation episodes may improve quality of life during drug detoxification and, perhaps, reduce the risk of relapse. If the association between attentional bias and temptation episodes is shown to be causal, then an attentional retraining intervention, delivered on the PDA, would be warranted.
The study had a number of limitations. First, to avoid overburdening participants, we did not ask them to report when each temptation episode had concluded. Thus, we did not collect data on the duration of temptation episodes. In addition, it is likely that some of the RAs occurred during the temptation episode (e.g., those within 1 h of the temptation onset). Second, we did not directly assess whether each temptation was primarily directed toward heroin use or cocaine use (or both). Third, a relatively small number of RAs occurred within 1 h prior to a temptation episode. Thus, the null effects for negative affect and explicit attitudes (for the comparison between proximal and control RAs) should be treated with caution. Fourth, in common with other EMA studies, assessment type (RA vs. TA) is confounded with assessment initiation method (person-initiated vs. PDA-initiated). In future studies, it may be useful to collect data from person-initiated assessments when participants are not experiencing a temptation. Fifth, the generalizability of the findings to unmedicated users in outpatient or more naturalistic settings is not known. If the findings generalized to other detoxification settings, this would be of significant clinical interest. Future research should investigate also the time course of temptations throughout the entire detoxification period. Last, as noted earlier, the direction of causality of the observed relationships remains uncertain.
The study also had strengths. We administered both subjective and cognitive assessments on the PDA. The data revealed that there were robust between-assessment differences on a number of subjective and cognitive measures. We were also able to examine the time course of mood and cognition prior to participants’ entries of temptation episodes.
In sum, the data revealed that attentional bias—but not subjective measures—was elevated both prior to the entry of a temptation episode and during a temptation episode. Interventions that target cognitions prior to or during temptation episodes may reduce the probability or the duration of a temptation episode and, perhaps, reduce the risk of relapse.
Acknowledgement
This study was funded by ZonMw grant 31180001 (Franken) and R01 DA020436-S3 (Waters).
Footnotes
Given that positive affect can be independent of negative affect (e.g., Watson 2000), it could be argued that two affect factors (positive affect and negative affect) might be derived. Exploratory factor analysis did not provide compelling evidence for two factors. If two factors were extracted, positive affect was significantly lower at TAs than RAs, and vice versa for negative affect (ps<0.01).
Data from two participants were lost due to PDA error, one participant dropped out of the study immediately following the training because he did not comprehend the procedures, and one participant dropped out of treatment prior to completing any assessments. Of the 64 participants, 10 relapsed (n=9) or dropped out of treatment (n=1) during the PDA study. Analyses on relapse are not reported in the present paper.
The primary analyses were conducted on data from all available days (n=414) and assessments (n=1,482). Secondary analyses were conducted on data derived from days (n=400) and assessments (n=1,437) that occurred before reported relapses. These analyses revealed very similar findings and are not reported here.
The current paper focuses on between-assessment differences. A detailed analysis of the associations between craving ratings and implicit cognitions is beyond the scope of the current paper. However, when craving for heroin or cocaine is entered into the model (including day, number of assessments, and Stroop type, but excluding assessment type), the parameter estimates were non-significant (heroin —PE=2.41, SE=3.14, p>0.1; cocaine—PE=3.74, SE=3.01, p>0.1).
One may question whether the drug-related pictures in the IAT could provoke temptations. Of the 529 RAs at which an IAT was administered, 30 (5.7%) were followed by a TA within an hour. For RAs in which a Stroop task was administered, 31 (5.2%) were followed by a TA within an hour. Therefore, most IAT RAs (94.3%) were not followed by a TA, and the post-assessment TA rate was not higher (PE=0.11, SE=0.26, p>0.66, using proc glimmix) following IAT vs. Stroop assessments (the Stroop task did not include pictures).
Contributor Information
Andrew J. Waters, Email: andrew.waters@usuhs.mil, Department of Medical and Clinical Psychology, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD, 20814, USA.
Reshmi Marhe, Institute of Psychology, Erasmus University Rotterdam, Rotterdam, The Netherlands.
Ingmar H. A. Franken, Institute of Psychology, Erasmus University Rotterdam, Rotterdam, The Netherlands
References
- Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafo MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX lexical database [CDROM] Philadelphia: University of Pennsylvania, Linguistic Data Consortium; 1995. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Wanting and liking: observations from the neuroscience and psychology laboratory. Inquiry. 2009;52:378–398. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-Stroop test: theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology. 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis. 2011;30:27–38. doi: 10.1080/10550887.2010.531672. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M. Experimental manipulation of attentional biases in heavy drinkers: do the effects generalise? Psychopharmacology. 2007:593–608. doi: 10.1007/s00213-007-0760-9. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Tyler E, Schoenmakers T. Experimental attentional bias modification in tobacco smokers. Nicotine Tob Res. 2009a;11:812–822. doi: 10.1093/ntr/ntp067. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IHA. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009b;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroine dependence. J Psychopharmacol. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Stam CJ, Hendriks VM, Brinks W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroine dependence. Psychopharmacology. 2003;170:205–212. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Glasper A, Gossop M, de Wet C, Reed L, Bearn J. Influence of the dose on the severity of opiate withdrawal symptoms during methadone detoxification. Pharmacology. 2008;81:92–96. doi: 10.1159/000109982. [DOI] [PubMed] [Google Scholar]
- Gossop M, Bradley B, Phillips GT. An investigation of withdrawal symptoms shown by opiate addicts during and subsequent to a 21-day in-patient methadone detoxification procedure. Addict Behav. 1987;12:1–6. doi: 10.1016/0306-4603(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MA. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Hammersley R. A digest of memory phenomena for addiction research. Addiction. 1994;89:283–293. doi: 10.1111/j.1360-0443.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation associated with smoking: an application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli SR, Richardt S, Frederick BD, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Moeller FG, Lane SD, Schmitz JM, Waters AJ, Cunningham KA. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2010;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The addiction severity index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Powell JH, Dawkins L, West R, Powel J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roefs A, Huijding J, Smulders FTY, MacLeod CM, de Jong PJ, Wiers RW, Jansen ATM. Implicit measures of association in psychopathology research. Psychol Bull. 2010;137:149–193. doi: 10.1037/a0021729. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: a meta-analysis. Addict Behav. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Perrott MA, Wertz JM, Hufford MR. A test of the appraisal-disruption model of alcohol and stress. J Stud Alcohol Drugs. 2001;62:247–256. doi: 10.15288/jsa.2001.62.247. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing:1. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Schoenmakers T, Wiers RW, Jones BT, Bruce G, Jansen ATM. Attentional retraining decreases attentional bias in heavy drinkers without generalization. Addiction. 2007;102:399–405. doi: 10.1111/j.1360-0443.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RA. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- van de Laar MC, Licht R, Franken IHA, Hendriks VM. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology. 2004;177:121–129. doi: 10.1007/s00213-004-1928-1. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Li Y. Evaluating the utility of administering a reaction time task in an ecological momentary assessment study. Psychopharmacology. 2008;197:25–35. doi: 10.1007/s00213-007-1006-6. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Mood and temperament. New York: Guilford; 2000. [Google Scholar]
- Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks: Sage; 2006. [Google Scholar]
- Wiers RW, van Woerden N, Smulders FTY, de Jong PJ. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. J Abnorm Psychol. 2002;111:648–658. doi: 10.1037/0021-843X.111.4.648. [DOI] [PubMed] [Google Scholar]