Abstract
Restrictive dermopathy (RD) is a rare and extremely severe congenital genodermatosis, characterized by a tight rigid skin with erosions at flexure sites, multiple joint contractures, low bone density and pulmonary insufficiency generally leading to death in the perinatal period. RD is caused in most patients by compound heterozygous or homozygous ZMPSTE24 null mutations. This gene encodes a metalloprotease specifically involved in lamin A post-translational processing. Here, we report a total of 16 families for whom diagnosis and molecular defects were clearly established. Among them, we report seven new ZMPSTE24 mutations, identified in classical RD or Mandibulo-acral dysplasia (MAD) affected patients. We also report nine families with one or two affected children carrying the common, homozygous thymine insertion in exon 9 and demonstrate the lack of a founder effect. In addition, we describe several new ZMPSTE24 variants identified in unaffected controls or in patients affected with non-classical progeroid syndromes. In addition, this mutation update includes a comprehensive search of the literature on previously described ZMPSTE24 mutations and associated phenotypes. Our comprehensive analysis of the molecular pathology supported the general rule: complete loss-of-function of ZMPSTE24 leads to RD, whereas other less severe phenotypes are associated with at least one haploinsufficient allele.
Keywords: ZMPSTE24, restrictive dermopathy, Mandibulo-acral dysplasia, progeroid syndromes, prelamin A maturation
Introduction
Restrictive dermopathy (RD, OMIM #275210) is a rare and extremely severe congenital genodermatosis, lethal in the perinatal period. The first signs include intrauterine growth retardation and fetal hypokinesia deformation sequence. At birth, a rigid and tight skin with erosions at flexure sites is noted together with epidermal hyperkeratosis, a microstomia with a peculiar shape in an open ‘O' position, a small pinched nose, microretrognathism, sparse or absent eyelashes and eyebrows. Typical orthopedic features are bone mineralization defects, thin dysplastic clavicles and generalized arthrogryposis, which usually lead to a characteristic phenotype. Early neonatal death is usually due to severe pulmonary insufficiency.1, 2 In the original study, we were able to identify that this early lethal disorder is caused by lamin A-specific defects either due to dominant LMNA mutations or, in most cases, to recessive ZMPSTE24 null mutations.1, 3 Lamins are ubiquitous nuclear proteins constituting the type V intermediate filaments subfamily; they are subdivided in A- and B-type lamins, respectively encoded by the LMNA, LMNB1 or LMNB2 genes (OMIM reference #: 150330, 150340, 150341).4, 5 Lamins are major components of the nuclear lamina, a filamentous meshwork underlying the inner nuclear membrane involved in nuclear shape, nuclear pore complex spacing and resistance to mechanical stress. Moreover, by their numerous interactions and nucleoplasmic localization in the nuclear matrix, lamins have been involved in many other major nuclear functions, including regulation of gene expression, chromatin organization and DNA replication and repair (for review, see Broers et al6 and Prokocimer et al7).
Lamins A and C are the major isoforms expressed by the LMNA gene through alternative splicing. Although lamin C is directly translated as a mature isoform from mRNAs, lamin A is first synthesized as a precursor called prelamin A, which undergoes four post-translational processing steps.8, 9 These include: farnesylation of a Cys residue localized in a C-terminal ‘CaaX' farnesylation motif (CSIM amino acids); proteolytic cleavage removing the SIM tripeptide, methylation of the C-terminal farnesylated Cystein residue and finally, a last proteolytic cleavage removing the C-terminal 15 amino acids, performed by the zinc metalloprotease STE24 (ZMPSTE24), generating a non-farnesylated mature lamin A.
LMNA mutations are known to cause Hutchinson–Gilford progeria syndrome (HGPS, OMIM #176670), because of the accumulation of an unprocessed lamin A deleted precursor. Clinically, HGPS is characterized by segmental features of premature aging including alopecia, loss of subcutaneous fat and early death (usually in the second decade), mostly due to myocardial infarction.10 After the identification of the main genetic cause of HGPS as a de novo LMNA mutation (c.1824C>T, Gly608Gly) affecting splicing and prelamin A maturation,11, 12 mutations in ZMPSTE24 were then identified in patients affected by severe Mandibulo-acral dysplasia associated with B-type lipodystrophy (MAD-B),13 and later in RD.1, 3, 14 Clinically, MAD is characterized by skeletal abnormalities including hypoplasia of the mandible and clavicles, acro-osteolysis of distal phalanges, cutaneous atrophy and lipodystrophy. MAD-A, linked to LMNA mutations, is associated with a partial lipodystrophy of the extremities. On the opposite, in typical MAD-B, lipodystrophy is generalized, an earlier age of onset is observed (often during the first year of life) and the median age of death is 30 years. Other mutations were found in additional cases of RD15, 16, 17, 18, 19, 20, 21 and progeroid syndromes phenotypically overlapping with MAD and HGPS that can be nosologically classified as MAD-B, given the common molecular basis, involving ZMPSTE24 deficiency.13, 22, 23, 24, 25, 26, 27, 28 RD, MAD-B and HGPS share a common pathophysiological mechanism based on the abnormal accumulation of a wild type or modified lamin A precursors.
Here, we report the results obtained in 19 families referred for molecular diagnosis to our genetic laboratories on the basis of RD or MAD phenotypes. Among them, we identified five new compound heterozygous mutations in ZMPSTE24 and two novel homozygous mutations. In three unrelated cases clinically diagnosed as RD at birth, no mutation was observed in either ZMPSTE24 or LMNA. In addition, in order to determine whether the common c.1085dupT mutation was associated with a founder haplotype, we explored six intragenic ZMPSTE24 SNPs in homozygous patients and their heterozygous parents. Bioinformatic approaches regarding the mutations' impact at the RNA and protein levels are described as useful tools to predict the pathogenicity of new mutations. Finally, based on a complete review of the literature and the novel patients reported here, the physiopathology of premature aging syndromes linked to ZMPSTE24 mutations is discussed, as well as clinical and molecular diagnostic implications.
Materials and methods
Informed consent and DNA/RNA extractions
Informed consent for genetic testing was obtained from the parents of all the patients included in this study, complying with the ethical guidelines of the institutions involved. Genomic DNA was extracted from peripheral blood lymphocytes by standard procedures. RNA was obtained either from lymphoblastoid or fibroblast cell lines. Lymphoblastoid cell lines were established from patients' or their parents' lymphocytes by EBV standard immortalization procedures. Fibroblasts were obtained from skin biopsies and cultured in a DMEM medium containing 10% fetal calf serum, 2 mm/ml L-glutamine and 100 U/ml penicillin–streptomycin (Invitrogen-Life Technologies, Carlsbad, CA, USA). RNA extraction was performed using TRIzol following the manufacturer's recommendations (Invitrogen-Life Technologies).
Genomic and transcriptional analysis
Reverse transcription was performed using a Superscript II reverse transcriptase (Invitrogen-Life Technologies) following the manufacturer's recommendations. ZMPSTE24 mRNA sequence reference is RefSeq NM_005857.4 and the following RT-PCR primers, used to indicate the splicing defect in the patient from family 6, were located in exon 6: 5′-TGCCTGAGGGAAAGCTTAAA-3′ (forward) and in exon 9: 5′-CCAATAAGAGTGGGTTGGCTA-3′ (reverse). All the other primers were previously described in Navarro et al.1 The sequencing reactions were performed with a dye terminator procedure, and loaded on a capillary automatic sequencer CEQ 8000 (Beckman Coulter, Brea, CA, USA) according to the manufacturer's recommendations. They were controlled using a different dye terminator procedure and loaded onto an ABI 3130XL automatic sequencer (Applied Biosystems-Life Technologies, Carlsbad, CA, USA). All sequence variations are described according to the Human Genomic Variation Society recommendations available at http://www.hgvs.org/mutnomen and the nomenclature of each mutation was checked using the Mutalyzer software http://www.lovd.nl/mutalyzer/.
SNP primers amplification and PCR annealing temperatures
NCBI references for the amplified SNPs were rs7514099, rs7516571, rs6600323, rs10489430, rs1015354, rs6664294, respectively. The sequences of the primers used are shown in Supplementary Table S1. In all, 50 μl of the PCR reaction mixture contained 5 μl of buffer 10X, 2 μl MgCl2 (50 mM), 0.5 μl dNTP (25 mM), 5 pmoles of each primer (forward and reverse), Taq Polymerase 1 U, 50 ng of genomic DNA. The volume was completed to 50 μl with sterile water. All PCR reagents were from Invitrogen-Life Technologies. Cycling conditions were a 3-min initial denaturation step at 94 °C, followed by 35 cycles of: 30 s at 94 °C, 30 s at 61 °C, 30 s at 72 °C and a last final 3-min elongation step at 72 °C. To amplify exon 9, a proof reading (high fidelity) Pfu polymerase (Fermentas, Villebon sur Yvette, France) was used.
Bioinformatic tools
All web sites and databases addresses used in the manuscript are detailed in Supplementary Table S2.
Results
Molecular findings
The genomic analysis of the entire ZMPSTE24/FACE1 coding sequence and intronic boundaries, allowed the identification of causative mutations in 16 of 19 families with one or two affected children, referred to the laboratories for molecular diagnosis of RD or Mandibulo-acral phenotypes, based on clinical and, in some cases, histopathological evaluation.
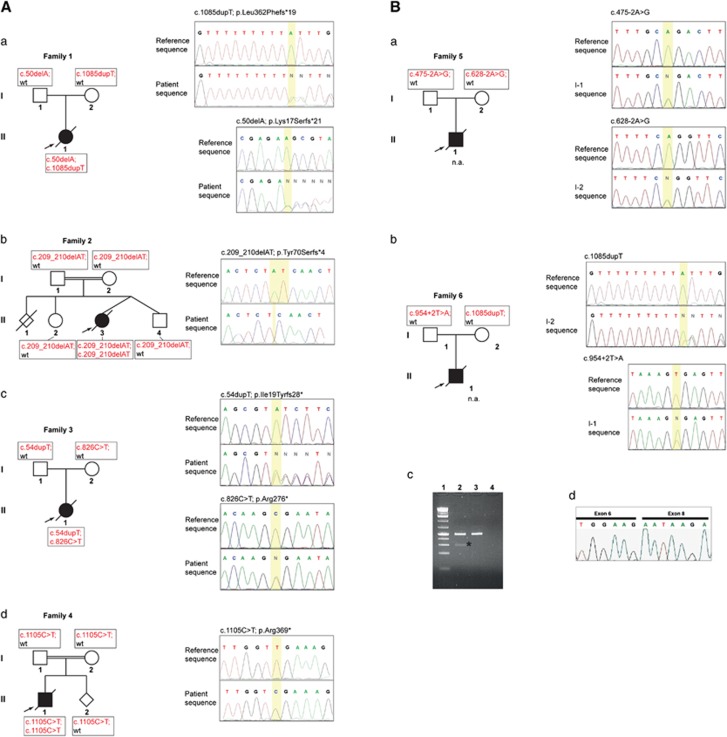
Two deletions, one of them novel, were observed in two different families (Figure 1 and Table 1). In family 1 (Figure 1Aa), the index case presented an adenine deletion located in exon 1, c.50delA (previously described in Smigiel et al20), in a compound heterozygous state with the common c.1085dupT duplication. In family 2 (Figure 1Ab), the child of a consanguineous Turkish couple, presented a novel homozygous deletion of two nucleotides in exon 2, c.209_210delAT. In a third family (Figure 1Ac), the compound heterozygous mutations c.54dupT, previously described in Moulson et al14 and a novel nonsense mutation located in exon 7 (c.826C>T) were observed. A further novel homozygous mutation, c.1105C>T located in exon 9, causing a stop gain, led to RD in a consanguineous German family 4 (Figure 1Ad). All the frame shift mutations resulted in premature termination codons. The predicted effects on translation are p.Lys17Serfs*21, p.Tyr70Serfs*4, p.Ile19Tyrfs*28, p.Arg276* and p.Arg369*, respectively (Table 1).
Figure 1.
Segregation of ZMPSTE24 mutations leading to RD. (A) Segregation of novel ZMPSTE24 frame-shift mutations leading to RD. The pedigrees show women as circles, men as rectangles, affected children as black-filled symbols and prenatally diagnosed fetuses as rhomboid symbols. ZMPSTE24 mutations are shown right to the pedigrees. The critical mutant regions in the sequences are underlined in yellow. (a) The affected child of a Dutch family carried a heterozygous adenine deletion in exon 1 (c.50delA) with the common thymidine duplication located in exon 9 (c.1085dupT). Sequence pherograms of exon 1 (forward direction) show a frame-shift. (b) The affected child of a consanguineous Turkish family carried a homozygous deletion of two base pairs in exon 2, c.209_210AT. (c) The affected child of a Swedish family was compound heterozygous for a c.826C>T transition and the previously reported c.54dupT duplication. (d) In a consanguineous German family, the novel homozygous transition c.1105C>T resulted in RD. (B) Segregation of splice site mutations in two RD families. (a) Genomic splice site mutations identified in both parents of a RD fetus in family 5 (NA, tissue samples not available). (b) Genomic splice site mutation found in the genomic DNA of the index cases' father in family 6. (c) Left panel: RT-PCR products amplified from cDNAs of the father and control samples separated by agarose gel electrophoresis. Lane 1: molecular size ladder; Lane 2: RT-PCR father; Lane 3: RT-PCR positive control; Lane 4: negative control. Besides the expected 475-bp amplicon, an additional shorter amplicon of 290-bp was found in lane 2 as indicated by an asterisk. Right panel: sequencing pherogram of the 290-bp amplicon showing exon 7 skipping.
Table 1. Summary of patients affected with progeroid syndromes carrying pathogenic ZMPSTE24 mutations.
| Patients | Mutations (allele 1; allele 2) | Position | Protein prediction | Protein function | Prelamin A accumulation | Reference |
|---|---|---|---|---|---|---|
| Restrictive dermopathy | ||||||
| RD—family 1 | c.50delA; c.1085dupT | Exon1; exon 9 | p.Lys17Serfs*21/p.Leu362Phefs*19 | NA | NA | Present report and Smigiel et al20 |
| RD—family 2 (consanguineous Turkish) | c.209_210delAT; c.209_210delAT | Exon 2 | p.Tyr70Serfs*4 | NA | NA | Present report |
| RD—family 3 | c.54dupT; c.826 C>T | Exon 1; exon 7 | p.Ile19Tyrfs*28/p.Arg276* | NA | NA | Present report and Moulson et al14 |
| RD—family 4 (consanguineous German) | c.1105C>T; c.1105C>T | Exon 9 | p.Arg369* | NA | NA | Present report |
| RD—family 5 | c.475-2A>G; c.628-2A>G | Intron 4; intron 5 | p.Thr159_Leu209del/p.Val210Aspfs*21 | NA | NA | Present report |
| RD—family 6 | c.954+2 T>A; c.1085dupT | Intron 7; exon 9 | p.Gly256Glufs*2/p.Leu362Phefs*19 | NA | NA | Present report |
| Nine additional RD—families | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362fs*19 | NA | NA | Present report |
| Patient P2 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362PhefsX19 | Null | Complete | Navarro et al3 (patient P2) |
| Patient P3 | c.1085dupT; r.271_627del | Exon 9; exon 3-5del | p.Leu362Phefs*19/p.Leu91_Leu209del | Null | Complete | Navarro et al3 (P3) |
| Patient P5 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | Null | Complete | Navarro et al3 (P5) |
| Patient P6 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | Null | Complete | Navarro et al3 (P6) |
| Patient P7 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | Null | Complete | Navarro et al3 (P7) |
| Patient P8 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Navarro et al3 (P8) |
| Patient P9 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Navarro et al3 (P9) |
| Patient P10 | c.1085dupT; c.1249C>T | Exon 9; exon 10 | p.Leu362Phefs*19/p.Gln417* | NA | NA | Navarro et al3 (P10) |
| Patient P11 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Navarro et al3 (P11) |
| Patient P12 | c.1085dupT; c.295delC | Exon 9 | p.Leu362Phefs*19/p.Pro99Leufs*38 | NA | NA | Navarro et al3 (P12) |
| Consanguineous Dutch family | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Moulson et al14 |
| American family | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | Null | Complete | Moulson et al14 |
| Consanguineous Guatemalan family | c.591dupT; c.591dupT | Exon 5 | p.Ile198fs*19 | Null | Complete | Moulson et al14 |
| Mennonite kindred | c.54dupT; c.54dupT | Exon 1 | p.Ile19Tyrfs*28 | NA | NA | Moulson et al14 |
| Isolated Dutch case | c.1085dupT; c.591dupT | Exon 9 | p.Leu362Phefs*19/p.Ile198fs*19 | NA | NA | Moulson et al14 |
| Case report—Iranian patient | c.627 +1G>C; c.627 +1G>C | Intron 5 | p.Thr159_Leu209del | Null | Presence | Sander et al16 |
| Case report | c.1385C>T;? | Exon 10 | p.Leu462Arg/? | NA | NA | Thill et al17 |
| Chinese patient | c.715G>T; c.715G>T | Exon 6 | p.Glu239* | NA | NA | Chen et al15 |
| Case report 1 | c.691G>T; c.691G>T; | Exon 6 | p.Glu231* | NA | NA | Jagadeesh et al19 |
| Case report 2 | c.691G>T; c.691G>T; | Exon 6 | p.Glu231* | NA | NA | Jagadeesh et al19 |
| Case report Iranian | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Kariminejad et al44 |
| Caucasian case report | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Morais et al45 |
| Case report Mennonite Kindred | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Li et al46 |
| RD-600 | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Ahmad et al18, 23 |
| RD-500—consanguineous Mexican family | c.1020G>A; c.1020G>A | Exon 8 | p.Trp340* | NA | NA | Ahmad et al18, 23 |
| Two affected sibling | c.50delA; c.584_585delAT | Exon1; exon 5 | p.Lys17Serfs*21/p.Tyr195Phefs*22 | NA | NA | Smigiel et al20 |
| Case report Turkish | c.1085dupT; c.1085dupT | Exon 9 | p.Leu362Phefs*19 | NA | NA | Yesil et al47 |
| Two sibling | c.715G>T; c.715G>T | Exon 6 | p.Glu239* | NA | NA | Lu et al21 |
| Mandibuloacral dyslplasia (MAD-type B) and other progeroïde syndromes | ||||||
| MAD-B—Family 7 (Turkish) | c.1204-1G>A; c.794A>G | Intron 9; exon 7 | p.Val402Phefs*6; p.Asn265Ser | present report | ||
| MAD-B (MAD600) | c.1085dupT; c.1018T>C | Exon 9; exon 8 | p.Leu362Phefs*19/p.Trp340Arg | Null/residual activity | NA | Agarwal et al13 |
| HGPS/MAD | c.1085dupT; c.794A>G | Exon 9; exon 7 | p.Leu362Phefs*19/p.Asn265Ser | Null/residual activity | Partial | Shackleton et al28 |
| Australian HGPS/MAD | c.1085dupT; c.794A>G | Exon 9; exon 7 | p.Leu362Phefs*19/p.Asn265Ser | NA | NA | Agarwal et al22 |
| HGPS/MAD | c.1204_1225del22; c.1204_1225del22a | Exon 10 | p.Val402Serfs*1 | Null | Complete b | Denecke et al26 |
| Severe MAD—two affected sister | c.121C>T; c.743C>T | Exon 1; Exon 6 | p.Gln41*/p.Pro248Leu | Null/residual activity | Partial | Miyoshi et al27 |
| Early onset MAD—(MAD4700) | c.743C>T; c.1349G>A | Exon 6; exon 10 | p.Pro248Leu/p.Trp450* | NA | NA | Ahmad et al18, 23 |
| MAD—with severe skeletal phenotype | c.794A>G; c.207_208delCT | Exon 7; exon 2 | p.Asn256Ser/p.Tyr70Serfs*4 | NA | NA | Cunningham et al25 |
| MAD-B with muscular defects—case report | c.281T>C; c.281T>C | Exon 3 | Leu94Pro/Leu94Pro | Residual | Partial | Ben Yaou et al24 |
Abbreviation: NA, non available.
Patients previously described and reported here (shown in italic) and their associated mutations are detailed and resumed according to their phenotypic features.
ZMPSTE24 mutations associated with a heterozygous LMNA mutation, p.R654*.
One part of the (pre)lamin A pool, is not farnesylated because of the presence of the p.R654* mutation.
We also identified four novel splice site mutations in three families (Figure 1B; Table 2). Regarding family 5, we only received DNA from both parents, as biological samples from the affected child were not available. In view of a presumed prenatal diagnosis, the analysis was performed in search of heterozygous pathogenic mutations, inferring their transmission to the affected child. Indeed, we observed two heterozygous novel splice site mutations, each carried by one parent: one at the acceptor splice site of intron 4 (c.475-2A>G) in the father, the other at the acceptor splice site of intron 5 (c.628-2A>G) in the mother (Figure 1Ba and Table 1). We could not derive cell lines from the parents, in order to experimentally investigate on the pathogenic effect of these mutations at the transcript level. Nevertheless, as both mutations directly affect acceptor splice sites, they are predicted to be dramatically deleterious.
Table 2. Splice site calculations using HSF, BDGP and NetGene2 for splice site mutations found in the present study.
| DNA variationa | Amino-acid exchange |
HSFb |
NetGene2c |
BDGPd |
|||
|---|---|---|---|---|---|---|---|
| Wt | Mut | Wt | Mut | Wt | Mut | ||
| c.475-2A>G | p.Thr159_Leu209del | 93.01 | — | 0.83 | — | 0.99 | — |
| c.627+1G>C | p.Thr159_Leu209del | 94.09 | 67.26 | 0.47 | — | 0.98 | — |
| c.628-2A>G | p. Val210Aspfse21 | 95.05 | 66.11 | 0.92 | — | 0.95 | — |
| c.954+2 T>A | p. Gly257Glufse2 | 87.31 | — | 0.91 | — | 0.98 | — |
| c.1204-1G>A | p. Val402Phefse6 | 96.47 | 67.53e | 0.77 | — | 0.99 | —f |
Transcript reference: NM_005857.4.
New splice site considered with 83.93.
New splice site considered with 0.9.
In family 6, one mutation affecting the exon 7 donor splice site (c.954+2T>A) was inherited from the father whereas the common mutation c.1085dupT was inherited from the mother (Figure 1Bb and Table 1). The father's transcriptional exploration by RT-PCR, using a forward primer in exon 6 and a reverse in exon 9, showed a shorter amplicon of about 290 bp (Figure 1Bc), compared with the normal amplicon of 475 bp. The shorter PCR product was gel-extracted and sequenced, showing a complete deletion of exon 7 (Figure 1Bd). This deletion is predicted to cause a frame shift leading to a premature termination codon (p.Gly256Glufs*2).
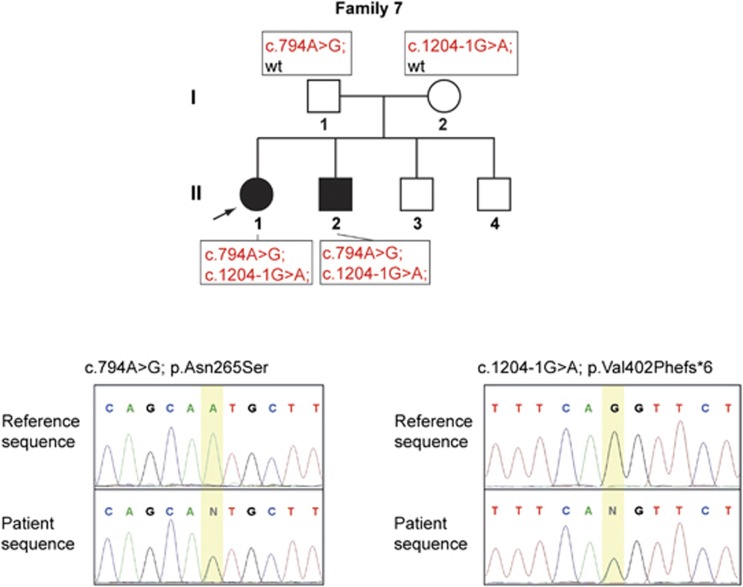
The index case of family 7 showed a severe clinical phenotype of Mandibulo-acral dysplasia (MAD) with onset before age 2. Mutational analysis in ZMPSTE24 revealed the compound heterozygous mutations c.1204-1G>A and c.794A>G in a Turkish family (Figure 2). Although the novel splice site mutation c.1204-1G>A leads to a predicted truncated ZMPSTE24 p.Val402Phefs*6, the previously described missense transversion c.794A>G results in a mutant ZMPSTE24 p.Asn265Ser classifying the affected siblings in family 7 as MAD-B.25
Figure 2.
ZMPSTE24 compound heterozygous mutations c.794A>G and c.1204-1G>A leading to MAD-B in a Turkish family. Segregation of the mutations ZMPSTE24 c.794A>G and c.1204-1G>A in family 7 and sequencing pherograms of ZMPSTE24 c.794A>G and c.1204-1G>A in the index case.
Nine additional families with one or more affected children were also investigated: each index patient carried the homozygous common null c.1085dupT mutation (data not shown, Table 1). This mutation was previously identified as the most frequent one involved in RD.3, 14 Based on molecular genetics findings, seven families asked for prenatal diagnosis of RD after genetic counseling. As a result, two homozygous wild type, six heterozygous and two homozygous or compound heterozygous RD fetuses were identified.
In three patients diagnosed as being affected with RD, no potentially pathogenic sequence variation was observed in the coding regions and intronic boundaries of the ZMPSTE24 and LMNA genes. However, clinical or histopathological findings in the children turned out to present several but not all features of typical RD (ie, atresia, total absence of dermis, abdominal inflammatory processes, advanced bone age or atypical dysmorphic features). Thus, the patients were probably affected with clinically similar forms of congenital arthrogryposis because of other molecular genetic defects.
In a larger general screening of patients affected with progeroid syndromes other than classical RD, we identified three new and most likely non-pathogenic heterozygous ZMPSTE24 variants. The heterozygous c.990C>T (p.Leu330Leu) in exon 8 was only identified in the healthy father of a child affected with a De Barsy-like syndrome (OMIM #219150), who subsequently died due to a concurrent infection, whereas both the child and the healthy mother carried a heterozygous c.770-131C>T variant in intron 6. Both are rare polymorphic variants with a frequency of 0.4% and 1.2% respectively, in a healthy reference population (rs116284141, rs78985808, http://browser.1000genomes.org/index.html). No second potentially pathogenic variant was observed for this patient in the coding regions and intronic boundaries of both ZMPSTE24 and LMNA genes. Furthermore, mutations in PYCR1 have been subsequently identified as described in De Barsy syndrome.29, 30 In addition, no prelamin A was detected by western blot in the patient's skin fibroblasts. In all, 60% of his fibroblasts' nuclei showed morphological abnormalities, including large aggregates or misdistribution of lamin A/C. However, no blebs or herniations lacking lamin B, which are usually observed in ZMPSTE24-deficient cells, were found.1, 3, 14, 31 The nuclear abnormalities observed in this patient could be due to mutations of lamin A partners or other nuclear proteins. Another child of a consanguineous couple was affected with a complex progeroid phenotype, including neonatal insulin resistance, lipodystrophy, facial dysmorphism as well as growth and psychomotor retardation. The c.1035A>G (p.Thr345Thr) and c.1356C>G (p.Phe452Leu) heterozygous variants were observed in exons 8 and 10 of the ZMPSTE24 gene, respectively. Both had been transmitted, in cis, from the healthy mother, excluding them as being causative per se of the phenotype. In addition, the child carried these variants in association with a de novo LMNA variant in exon 2 (c.438C>T; p. Ala146Ala). The human splicing factor (HSF) calculation tool predicted a SF2/ASF site to be broken by this mutation, which could be associated with a frame-shift exon 2 skipping. Nonetheless, this variant has been found with a frequency of 3% in a healthy reference population (rs80356805, http://browser.1000genomes.org/index.html) and no shorter lamin A isoform nor reduced levels of lamin A/C were found in western blot analyses in the child's fibroblasts. Instead, a slight prelamin A accumulation was observed (data not shown). Given the latter observation, we assume that the digenic-3 variants combination probably concur to determine part of the child's features, that is, lipodystrophy and insulin resistance. Nonetheless, the phenotypic features of the patient are not typical of a ZMPSTE24-related progeroid syndrome and additional genetic or environmental factors must be involved in the determination of the phenotype. In particular, this patient is affected with psychomotor retardation, which has never been reported in lamin A-linked progeroid syndromes, and is issued from a consanguineous union, suggesting the probable occurrence of identical-by-descent homozygous mutations in one or more genomic loci that would concur to determine her complex phenotype.
Is there a founder effect of the common c.1085dupT mutation?
In order to check whether the common mutation c.1085dupT is associated with a common founder haplotype, we explored the haplotypes of all the family members carrying at least one c.1085dupT allele. Haplotypes were built on six intragenic SNPs reported in the NCBI database (http://www.ncbi.nlm.nih.gov/snp). We analyzed 15 families with one or two affected children carrying the c.1085dupT mutation. All the patients were either described by our team in previous publications,1, 3 or reported in this study. All individuals studied were Caucasians although they had different geographic origins. Several haplotypes were found to be associated with the c.1085dupT insertion (data not shown), indicating absence of a founder effect and suggesting independent events of a sporadic de novo elongation of a T stretch in a region of microsatellite instability.32 However, an ancestral haplotype was recently found for this mutation in an old colony of Mennonites and Hutterites.33
Discussion
Diagnostic strategies
As concluded by previous studies and confirmed by the cases reported here, the classic RD phenotype is linked to ZMPSTE24 null mutations whereas LMNA can be mutated in some less severe dominant RD-like phenotypes, which can overlap with severe neonatal HGPS forms. Thus, in classical RD phenotypes, whose clinical features are very typical and recognizable, physicians should be prompted to first screen the ZMPSTE24 gene in the context of genetic counseling. Exon 9, which harbors the major mutation, should be explored first, associating direct sequencing (for the PCR of exon 9, a polymerase with proof reading activity should be used) and fragment length analysis, because of the difficulties linked to the study of this region,3 followed by the complete screening of the gene if the most common mutation has not found. As a second step, LMNA should be screened, based on the existence of some extremely severe lamin-associated progeroid syndromes that could be clinically diagnosed at birth as RDs. This was the case regarding a patient initially characterized as RD carrying a LMNA splice mutation (c.1968+1G>A) reported in previous studies.1, 34 Thus, initial clinical expertise is crucial to direct the appropriate mutational analysis. In this study, all patients referred to our laboratory for ZMPSTE24 screening in whom no mutation was detected, were proven not to be affected with classical RD after subsequent clinical investigation.
In our first publication associating RD to ZMPSTE24 mutations published in 2004, we reported a patient carrying the heterozygous c.1085dupT mutation.1 However, we later discovered that the patient was homozygous for that mutation as corrected by us and confirmed in other patients.3 We take advantage of this publication to definitively clarify this point; indeed the original erroneous data are still sometimes discussed in the literature. We thus wish to highlight the fact that all classical RD patients carry homozygous or compound heterozygous null ZMPSTE24 mutations.
Pathogenicity of splice site mutations
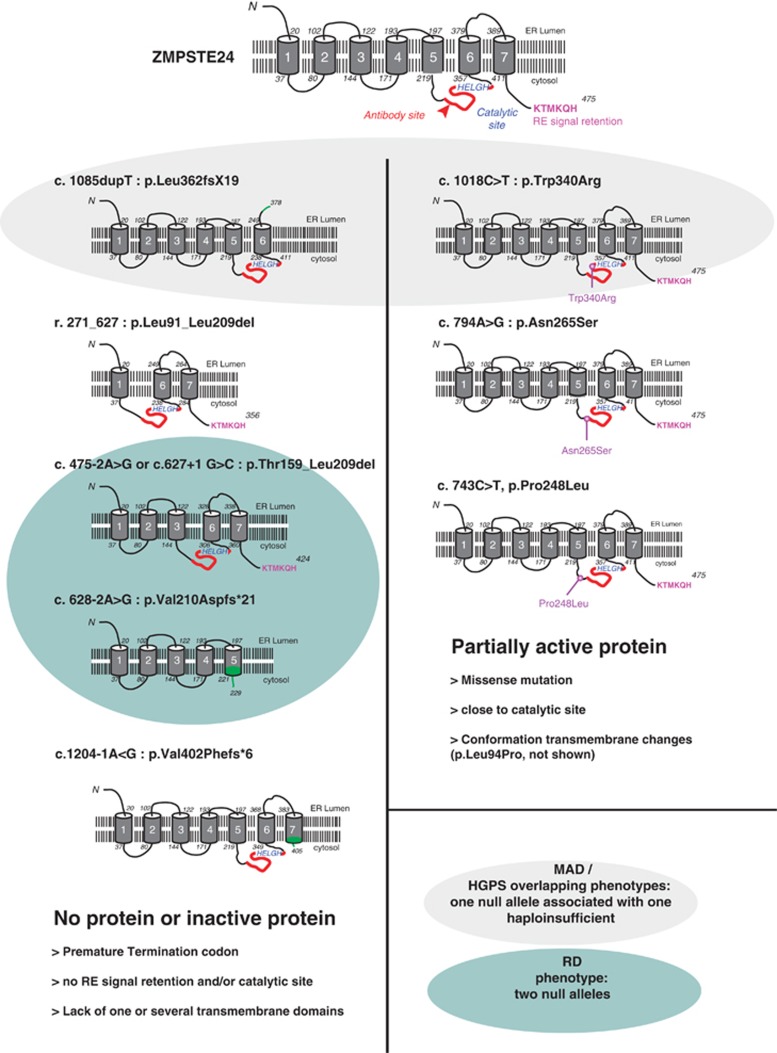
Five ZMPSTE24 mutations collected in this study represent splice site mutations (Table 2). Unfortunately, no biological material was available for additional RT-PCR studies. However, all of them affect the nucleotides of the canonical positions (−2, −1, +1 or +2) and therefore strongly influence the consensus value (CV). The CV was calculated with three different prediction tools and all of them predict a complete loss or a reduction about 30% of the initial CV (Table 2).35 Thus, CVs smaller than 70% are associated with potentially non-functional splice sites. For the c.1204-1G>A mutation, two of three tools predicted a newly generated splice site leading to a frame-shift of one base, which results in a predicted premature stop codon (Table 2). For the remaining four mutations no cryptic or new splice sites are predicted, most likely leading to exon skipping. Two of these mutations, c.628-2A>G and c.954+2 T>A (Table 2), would cause a frame-shift. The predicted effect of the mutations c.475-2A>G and c.627+1G>C is the complete in-frame skipping of exon 5. If this protein was produced, deletion of amino acids 159–209 would eliminate ZMPSTE24 transmembrane domains 4 and 5, likely modifying the protein folding (Figure 3). Moreover, Sander et al16 published in 2008 a homozygous splice site mutation located at the donor splice site of intron 6 (c.627 +1G>C), which induced skipping of the complete exon 5. The authors proved the exclusive expression of the shorter transcript carrying an in-frame deletion and concluded that this transcript probably encoded a less stable or less active protein, as demonstrated by prelamin A accumulation.
Figure 3.
Examples of predicted mutant ZMPSTE24 forms. Left panel—null mutations observed in RD patients; Right panel—missense mutations involved in milder progeroid syndromes. Antibody recognition site is indicated in red, endoplasmic reticulum retention signal is detailed in purple and catalytic site in blue characters. Protein sequences modified by frame-shift mutations are indicated in green.
We previously showed for the r.271_627del mutation the appearance of a shorter in-frame transcript skipping exons 3–5, whose corresponding protein was undetectable by western blot analyses.3 All these arguments suggest that mutations affecting ZMPSTE24 splice sites may result in null mutations even when they predict transcript in-frame deletions, at least as far as the region surrounding exon 5 is affected.
Genotype–phenotype correlation
All the ZMPSTE24 mutations described in patients affected with RD or MAD-B were homozygous or compound heterozygous. Based on the recessive inheritance of the disease, heterozygous mutations identified in healthy RD parents are apparently not deleterious. This was also observed in Zmpste24+/− mice, which presented with normal phenotypes, compared with Zmpste24−/− mice, presenting with progeroid features and shortened lifespans.31 It can thus be postulated that one ZMPSTE24 wild-type allele is necessary and sufficient for normal processing of prelamin A to mature lamin A, and consequently compensate the null allele. Nonetheless, recently, a heterozygous (c.1312C>T, p.Leu438Phe) mutation was identified in a patient affected with a metabolic syndrome.36 In this patient, slight prelamin A accumulation was detected by immunofluorescence analyses in fibroblasts, associated with nuclear abnormalities. In this case, processing of prelamin A was not complete as demonstrated by ELISA. Furthermore, a reduced enzymatic activity for this variant was confirmed by an in vitro essay.37 It is still not clear if this mutation is acting per se or in combination with other unknown predisposing factors. It would be interesting to clinically investigate the prevalence of metabolic syndrome in heterozygous carriers of other ZMPSTE24 missense mutations. Indeed, to our knowledge, none of the RD parents nor grandparents developed such a syndrome.
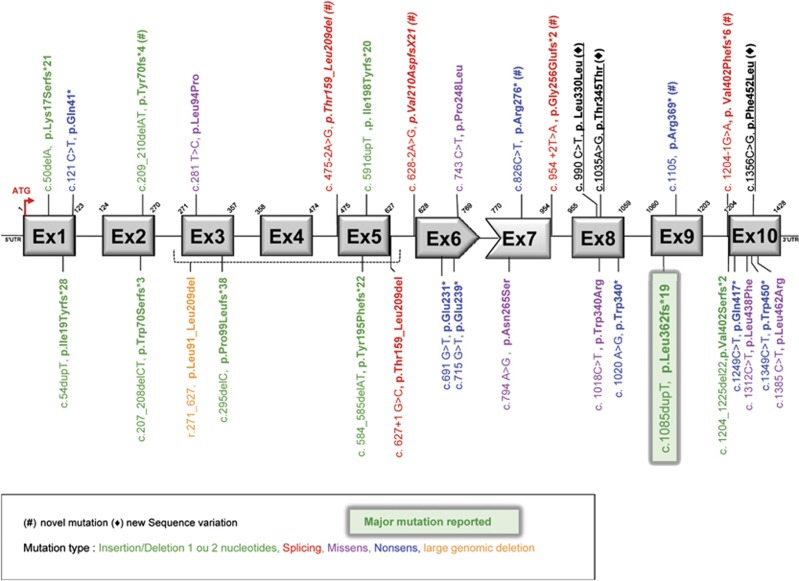
Disease-causing mutations have been observed throughout the gene, as shown in Figure 4. However, a recurrent Thymine duplication (c.1085dupT) located in exon 9 has been frequently observed. Considering all ZMPSTE24 mutations reported (Table 1), the common thymine duplication occurs in 52.9%, of all progeroid syndromes including RD, MAD and overlapping progeroid syndromes, reported in the literature and in the present study (Supplementary Table S3). Regarding mutation distribution classified by phenotypes, this mutation represents 59.1% of all ZMPSTE24 mutations in association with the RD phenotype and 18.8% of the MAD-B or overlapping syndromes HGPS/MAD.
Figure 4.
Distribution of pathogenic mutations and sequence variations in ZMPSTE24. Exons are shown as gray rectangles. A red arrow indicates the ATG start and adenine is numbered as nucleotide 1. Frame-shift mutations are shown in green, missense mutations leading to amino-acid changes in purple and nonsense mutation in blue. One large genomic deletion is shown in orange and mutations affecting consensus splice sites are shown in red. Novel mutations are indicated by (#); (♦) indicates new sequence variations identified during our screening.
Previously, 22 different mutations were reported in RD or other severe progeroid syndromes, so that with the data included in this report, 29 mutations have been described, to date. A total of 52 families were linked to ZMPSTE24 mutations in the literature. Each of them presented one or two affected children and 44 were associated with RD phenotypes. Eight additional families with patients affected with MAD or MAD/HGPS overlapping phenotypes were also associated with mutations in this gene. Thus, all disorders associated with ZMPSTE24 mutations can be classified as secondary progeroid laminopathies.
Interestingly, except for the mutation c.1385C>T (p.Leu462Arg) described by Thill et al17 and discussed below; all mutations involved in RD are null mutations. The recurrent c.1085dupT results in the complete absence of ZMPSTE24 in all patients tested, as shown by western blot analysis.3 Moreover, no maturation of the yeast a-factor was detected by enzymatic tests.13 Other null mutations are nonsense mutations or frame-shifting splice site mutations leading to premature stop codons. However, some null mutations could be exon skipping mutations regarding the mRNA's open reading frame but resulting in degraded or inactive proteins. In contrast, all patients with ‘non-RD' progeroid syndromes are compound heterozygotes carrying one null mutation and one missense mutation. Missense mutations such as p.Trp340Arg or p.Asn265Ser lead to a reduced catalytic ZMPSTE24 activity.13, 27 All the reported cases of MAD-B phenotypes followed this pathophysiological rule.22, 23, 25, 27 An apparent exception to this general rule has been reported by Denecke et al26 but is explained by a digenic mechanism involving both ZMPSTE24 and LMNA. The case reported in this study was described as a HGPS patient but, according to molecular findings and our current knowledge, it should be classified as a severe MAD-B. The patient carried a homozygous ZMPSTE24 frame-shift mutation (p.Val402Serfs*2) associated with a LMNA nonsense mutation (p.Arg654*) acting as a salvage alteration alleviating the clinical picture from RD to HGPS-like.26 Thill et al17 presented an exception to the rule. They described a typical RD phenotype in a patient carrying only a heterozygous missense mutation (p.Leu462Arg). The authors discussed the fact that they could have missed a second mutation, most probably because it was not detectable by conventional PCR-based mutational analysis, limited to coding regions (excluding heterozygous deletions, or pathogenic point mutations localized in introns, promoters or UTR regions). However, all missense mutations reported in ZMPSTE24 so far, except the latter, were associated with less severe phenotypes than typical RD, with residual ZMPSTE24 catalytic activity. The authors argued that mutations involved in less severe phenotypes are not located that close to the RE retention signal. No tissues from the aborted fetus were available for western blot analysis to validate the complete accumulation of prelamin A that is always associated with a RD phenotype. Barrowman et al37 published a proteolytic activity test concerning a wide list of ZMPSTE24 variants, unfortunately Leu462Arg was not included in that study but this could be an elegant way to demonstrate clearly a suggested null effect of this mutation.
The major pathophysiological mechanism of RD is the dramatic accumulation of prelamin A in its farnesylated form, because of the complete absence of ZMPSTE24's processing activity. Instead, a residual enzymatic activity, with partial lamin A maturation, allows longer life span. This was observed in MAD or ‘HGPS' patients carrying compound heterozygous loss-of-function and null ZMPSTE24 mutations and recently confirmed with in vitro studies in which enzymatic analyses performed on a series of ZMPSTE24 variants showed that residual proteolytic activity can be correlated with disease severity.37 A patient carrying a homozygous loss-of-function ZMPSTE24 mutation (c.281C>T, Leu94Pro) belongs to the clinical continuum of this phenotypic group, as the milder phenotype associated with ZMSPTE24 mutations, which correlated to slight prelamin A accumulation.24
Finally, progeroid disorders caused by ZMPSTE24 recessive mutations are always severe disorders. They are included in the nosologic spectrum of lamin-associated progeroid syndromes, sharing a common pathophysiologic pathway and being observed in MAD, HGPS or RD patients. Each of these diseases can be caused either by LMNA or ZMPSTE24 mutations; indeed, despite some clinical differences, abnormal prelamin A processing and accumulation is a common and central point of their pathophysiology. Nonetheless, HGPS patients carrying ZMPSTE24 mutations, are not affected by the ‘classic' form of HGPS, defined by the heterozygous recurrent LMNA mutation (p.Gly608Gly) as well as by specific and highly reproducible clinical and developmental features. Both the farnesylated state of the precursor and its levels seem to be crucial points. Several studies clearly demonstrated that the levels of accumulated prelamin A are related to the severity of the phenotype. Indeed, double knock out Zmpste24−/− Lmna+/− mice were phenotypically entirely normal, lacking all disease features, compared with Zmpste24 −/− mice.38, 39 Finally, Moulson et al34 and Reunert et al40 found larger amounts of progerin in neonatal forms of progeria compared with several classic ones and, conversely, lower progerin accumulation was observed in mild progeria/atypical Werner syndromes.41
RD samples are extremely rare, moreover primary RD fibroblasts are not easy to maintain in culture, because of their intrinsic pathology. Consequently, the only available material is DNA in most cases. In this context, in silico analyses concerning both RNA and protein impacts of novel mutations are very useful. For this purpose, electronic tools like HSF, BDGP and NetGene2 can help to predict mutation impacts on the splicing machinery. The use of more than one of such prediction tools is recommended as they use different calculation algorithms, making common predictions more reliable (Table 2). However, only functional expression analysis at the RNA level on patients' cells can validate the predictions. In addition, several protein prediction programs can be used to explore the hypothetical structure of a mutant in order to gain insight into the pathophysiological mechanisms. As an example, c.1085dupT in ZMPSTE24 was predicted to produce a mutant protein lacking the last transmembrane domain and the ER retention signal. In contrast, we detected ZMPSTE24 mRNA by full length RT-PCR in patient 4 from our previous studies1, 3 carrying both c.1085dupT and r.271_627del mutations, but no ZMPSTE24 protein was found by western blot analyses. In this patient, ZMPSTE24 proteins lacking the ER retention signal and/or one or several transmembrane domains may be identified as misfolded proteins, being degraded by the proteaseome (Figure 3). In patients carrying ZMPSTE24 mutations for whom no RNA or proteins were available, bioinformatic tools can help to predict the enzyme's localization, conformation and consequently its activity or fate. Very recently, two ZMPSTE24 structural studies proved that mutations predicted to reduce ZMPSTE24 activity were mapped in an unexpected voluminous peptide-binding chamber. These new structural data will be helpful to predict pathogenicity of novel missense mutations in this gene.42, 43
Acknowledgments
MW was supported by a grant from the German Network of Muscular Dystrophies (MD-NET, 01GM0302) funded by the German Ministry of Education and Research (BMBF) and an EU grant Euro-Laminopathies contract #018690. PM was supported by the Wellcome Trust (grant number WT087244) and TDN was funded by the National Foundation for Science and Technology Development (NAFOSTED, grant 106.06-2010.62) Vietnam. The support of LTTH by the Joint Graduate Education Program of Deutscher Akademischer Austauschdienst (DAAD, VNM 04/A17) is acknowledged. CLN was supported by ANR (Agence Nationale de la Recherche, grant R08190AS) and subsequently by French Association against Myopathies (AFM, grant AFMNL). We thank Dr Andrée Robaglia-Schlupp, in charge of the Biological Resource Center (CRB) of the Department of Medical Genetics, La Timone Children's hospital, Marseille, and Cécile Mouradian and Karine Bertaux for excellent technical support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Navarro CL, De Sandre-Giovannoli A, Bernard R, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- Smitt JH, van Asperen CJ, Niessen CM, et al. Restrictive dermopathy. Report of 12 cases. Dutch Task Force on Genodermatology. Arch Dermatol. 1998;134:577–579. doi: 10.1001/archderm.134.5.577. [DOI] [PubMed] [Google Scholar]
- Navarro CL, Cadinanos J, De Sandre-Giovannoli A, et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum Mol Genet. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA. 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- Prokocimer M, Davidovich M, Nissim-Rafinia M, et al. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med. 2009;13:1059–1085. doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz D, Tanaka RA, Hartwig J, McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989;59:969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci. 1994;107 (Pt 1:61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Go G, Gardner JM, et al. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125:913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Kuo HH, Huang YC, et al. A case of restrictive dermopathy with complete chorioamniotic membrane separation caused by a novel homozygous nonsense mutation in the ZMPSTE24 gene. Am J Med Genet A. 2009;149A:1550–1554. doi: 10.1002/ajmg.a.32768. [DOI] [PubMed] [Google Scholar]
- Sander CS, Salman N, van Geel M, et al. A newly identified splice site mutation in ZMPSTE24 causes restrictive dermopathy in the Middle East. Br J Dermatol. 2008;159:961–967. doi: 10.1111/j.1365-2133.2008.08772.x. [DOI] [PubMed] [Google Scholar]
- Thill M, Nguyen TD, Wehnert M, et al. Restrictive dermopathy: a rare laminopathy. Arch Gynecol Obstet. 2008;278:201–208. doi: 10.1007/s00404-008-0676-6. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Phadke S, Arch E, Glass J, Agarwal A, Garg A. Homozygous null mutations in ZMPSTE24 in restrictive dermopathy: evidence of genetic heterogeneity. Clin Genet. 2010;81:158–164. doi: 10.1111/j.1399-0004.2010.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesh S, Bhat L, Suresh I, Muralidhar SL. Prenatal diagnosis of restrictive dermopathy. Indian Pediatr. 2009;46:349–351. [PubMed] [Google Scholar]
- Smigiel R, Jakubiak A, Esteves-Vieira V, et al. Novel frameshifting mutations of the ZMPSTE24 gene in two siblings affected with restrictive dermopathy and review of the mutations described in the literature. Am J Med Genet A. 2010;152A:447–452. doi: 10.1002/ajmg.a.33221. [DOI] [PubMed] [Google Scholar]
- Lu CS, Wu SC, Hou JW, Chu CP, Tseng LL, Lue HC. Restrictive dermopathy: report of two siblings. Pediatr Neonatol. 2013;54:198–201. doi: 10.1016/j.pedneo.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Zhou XJ, Hall RK, et al. Focal segmental glomerulosclerosis in patients with mandibuloacral dysplasia owing to ZMPSTE24 deficiency. J Investig Med. 2006;54:208–213. doi: 10.2310/6650.2006.05068. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Zackai E, Medne L, Garg A. Early onset mandibuloacral dysplasia due to compound heterozygous mutations in ZMPSTE24. Am J Med Genet A. 2010;152A:2703–2710. doi: 10.1002/ajmg.a.33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yaou R, Navarro C, Quijano-Roy S, et al. Type B mandibuloacral dysplasia with congenital myopathy due to homozygous ZMPSTE24 missense mutation. Eur J Hum Genet. 2011;19:647–654. doi: 10.1038/ejhg.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham VJ, D'Apice MR, Licata N, Novelli G, Cundy T. Skeletal phenotype of mandibuloacral dysplasia associated with mutations in ZMPSTE24. Bone. 2010;47:591–597. doi: 10.1016/j.bone.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Denecke J, Brune T, Feldhaus T, et al. A homozygous ZMPSTE24 null mutation in combination with a heterozygous mutation in the LMNA gene causes Hutchinson-Gilford progeria syndrome (HGPS): insights into the pathophysiology of HGPS. Hum Mutat. 2006;27:524–531. doi: 10.1002/humu.20315. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Akagi M, Agarwal AK, et al. Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin Genet. 2008;73:535–544. doi: 10.1111/j.1399-0004.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton S, Smallwood DT, Clayton P, et al. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J Med Genet. 2005;42:e36. doi: 10.1136/jmg.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernsey DL, Jiang H, Evans SC, et al. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am J Hum Genet. 2009;85:120–129. doi: 10.1016/j.ajhg.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Escande-Beillard N, Dimopoulou A, et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- Pendas AM, Zhou Z, Cadinanos J, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- Mori Y, Yin J, Rashid A, et al. Instabilotyping: comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res. 2001;61:6046–6049. [PubMed] [Google Scholar]
- Loucks C, Parboosingh JS, Chong JX, et al. A shared founder mutation underlies restrictive dermopathy in Old Colony (Dutch-German) Mennonite and Hutterite patients in North America. Am J Med Genet A. 2012;158A:1229–1232. doi: 10.1002/ajmg.a.35302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulson CL, Fong LG, Gardner JM, et al. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum Mutat. 2007;28:882–889. doi: 10.1002/humu.20536. [DOI] [PubMed] [Google Scholar]
- Desmet F-O, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutour A, Roll P, Gaborit B, et al. High prevalence of laminopathies among patients with metabolic syndrome. Hum Mol Genet. 2011;20:3779–3786. doi: 10.1093/hmg/ddr294. [DOI] [PubMed] [Google Scholar]
- Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum Mol Genet. 2012;21:4084–4093. doi: 10.1093/hmg/dds233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- Fong LG, Ng JK, Meta M, et al. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci USA. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reunert J, Wentzell R, Walter M, et al. Neonatal progeria: increased ratio of progerin to lamin A leads to progeria of the newborn. Eur J Hum Genet. 2012;20:933–937. doi: 10.1038/ejhg.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisama FM, Lessel D, Leistritz D, et al. Coronary artery disease in a Werner syndrome-like form of progeria characterized by low levels of progerin, a splice variant of lamin A. Am J Med Genet A. 2011;155A:3002–3006. doi: 10.1002/ajmg.a.34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S, Hrycyna CA. Biochemistry. A protease for the ages. Science. 2013;339:1529–1530. doi: 10.1126/science.1236764. [DOI] [PubMed] [Google Scholar]
- Quigley A, Dong YY, Pike AC, et al. The structural basis of ZMPSTE24-dependent laminopathies. Science. 2013;339:1604–1607. doi: 10.1126/science.1231513. [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Goodarzi P, Thanh HuongleT, Wehnert MS. Restrictive dermopathy. Molecular diagnosis of restrictive dermopathy in a stillborn fetus from a consanguineous Iranian family. Saudi Med J. 2009;30:150–153. [PubMed] [Google Scholar]
- Morais P, Magina S, Ribeiro Mdo C, et al. Restrictive dermopathy: a lethal congenital laminopathy. Case report and review of the literature. Eur J Pediatr. 2009;168:1007–1012. doi: 10.1007/s00431-008-0868-x. [DOI] [PubMed] [Google Scholar]
- Li C. Homozygosity for the common mutation c.1085dupT in the ZMPSTE24 gene in a Mennonite baby with restrictive dermopathy and placenta abruption. Am J Med Genet A. 2010;152A:262–263. doi: 10.1002/ajmg.a.33163. [DOI] [PubMed] [Google Scholar]
- Yesil G, Hatipoglu L, Esteves-Vieira V, Levy N, De Sandre-Giovannoli A, Tuysuz B. Restrictive dermopathy in a Turkish newborn. Pediatr Dermatol. 2011;28:408–411. doi: 10.1111/j.1525-1470.2010.01296.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.