Abstract
Survival for mesothelioma has been shown to be poor, with marginal improvement over time. Recent advances in the understanding of pathophysiology and treatment of mesothelioma may impact therapy to improve survival that may not be evident from available clinical trials that are often small and not randomized. Therapies may affect survival differently based on mesothelioma location (pleural vs peritoneal). Data are conflicting regarding the effect of asbestos exposure on mesothelioma location. OBJECTIVES: We examined survival in a large cohort of mesothelioma subjects analyzed by tumor location and presence and mode of asbestos exposure. METHODS: Data were analyzed from cases (n = 380) diagnosed with mesothelioma from 1992 to 2012. Cases were either drawn from treatment referrals, independent medical evaluation for medical legal purposes, or volunteers who were diagnosed with mesothelioma. Subjects completed an occupational medical questionnaire, personal interview with the examining physician, and physician review of the medical record. RESULTS: This study reports better survival for mesothelioma than historical reports. Survival for peritoneal mesothelioma was longer than that for pleural mesothelioma (hazard ratio = 0.36, 95% confidence interval = 0.24-0.54, P < .001) after adjusting for gender and age at diagnosis. Non-occupational cases were more likely to be 1) diagnosed with peritoneal mesothelioma, 2) female, 3) exposed, and 4) diagnosed at a younger age and to have a 5) shorter latency compared to occupational cases (P < .001). CONCLUSION: Peritoneal mesothelioma was more likely associated with non-occupational exposure, thus emphasizing the importance of exposure history in enhancing early diagnosis and treatment impact.
Introduction
Asbestos exposure has been recognized as a significant risk factor in the development of mesothelioma. A history of asbestos exposure can be found in > 80% of mesothelioma cases [1]. Exposure can occur in both an occupational and a non-occupational setting, such as through contact with occupationally contaminated individuals and/or their belongings, exposure to products in the environment, such as floor or ceiling tiles, insulation, sheet rock, cement, brake dust or through air currents, such as from nearby factories [2], [3], [4]. Individuals exposed in the non-occupational setting can have similar asbestos fiber lung burden [5] to those exposed in the occupational setting, presenting a significant mesothelioma risk [6], [7], [8]. Mesothelioma can arise in various locations—most commonly the pleura and the peritoneum. Early reports suggest that individuals with the heaviest exposure more often developed peritoneal mesothelioma [9], [10], [11]. Results of more recent reports are mixed [12], [13].
Recent advances in the understanding of mesothelioma pathophysiology may impact therapy and survival [14], [15]. Despite these advances, reported survival is poor according to the SEER database and improving only slowly over time [16]. Factors reported to be important in mesothelioma development are age at first exposure, latency, a personal and family history of cancers [17], and genetic predisposition [14], [18], [19], [20]. Recent significant innovations have affected treatment of mesothelioma, especially peritoneal mesothelioma where hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery have become standard of care [21], [22], [23], [24], [25], [26]. HIPEC appears to increase survival but can typically be used to treat only peritoneal mesothelioma. This produces an increased survival effect that could be misinterpreted as resulting from non-occupational exposures that are more likely to produce peritoneal tumors. If exposure type (occupational vs non-occupational) impacts location of the tumor, then these new treatments may appear to impact survival based on type of exposure.
Clinical observation has led to a hypothesis that peritoneal mesothelioma patients live longer than pleural patients and individuals exposed in a non-occupational setting also appear to live longer than those exposed in an occupational setting. We set out to test these hypotheses and to determine current mesothelioma survival.
Material and Methods
Study Population and Data Acquisition
Study subjects (n = 380) were obtained from several sources, including treatment referrals for peritoneal mesothelioma surgery (n = 21), independent medical evaluations for medical legal purposes (n = 316), and volunteers diagnosed with mesothelioma (n = 43). All study participants gave informed consent for their participation, and the protocol was approved by the Institutional Review Boards at Saint Louis University and Wake Forest School of Medicine.
Study entry criteria included a completed occupational medical questionnaire and either a death certificate (n = 2) or pathology report, including immunohistochemical staining documenting the presence and location of mesothelioma from a CLIA certified hospital laboratory within the United States or Canada (n = 377). One patient was a volunteer from a mesothelioma support group and the diagnosis of mesothelioma was by self report. In most cases, subjects also underwent a full physical examination (n = 301), personal interview with the examining physician or study coordinator to clarify questionnaire responses (n = 380), and physician review of the full medical record (n = 301). Legal cases frequently had independent pathologic review in addition to the clinical pathologic diagnostic workup noted above. Demographic data, such as gender, age, age at first exposure to asbestos, occupational history, and personal and family health history, were collected and archived from the questionnaire/interview. For respondents that reported an occupational exposure, occupations were categorized into groups based on the Rice et al. classification [27]. Since there are no large randomized controlled trials comparing the various mesothelioma therapies [systemic chemotherapy, extrapleural pneumonectomy (EPP), pleurectomy, radiation, cytoreductive surgery, and/or HIPEC], treatment modality was not a variable that was analyzed in this study.
Data and Statistical Analysis
Survival rate was determined for both peritoneal versus pleural and occupationally versus non-occupationally exposed mesothelioma subjects using the Kaplan-Meier method and the log-rank test. Multivariate Cox proportional hazards regression models were used to determine predictors of survival. Statistical significance was defined as a P value < .05. All analyses were performed using SAS 9.2 (Cary, NC). Survival in months was calculated from the date of mesothelioma diagnosis, taken from the biopsy pathology report, to the date of death. The closing date was set at 1 March 2012.
Demographic variables (ages at first exposure and diagnosis, gender, latency, personal and family history of malignancy) were compared for both peritoneal versus pleural and occupationally versus non-occupationally exposed mesothelioma subjects. Frequencies and proportions were calculated for categorical data. Significance of differences between two groups was determined by t test or chi square.
Results
Survival
Peritoneal mesothelioma had significantly better survival (hazard ratio = 0.36, 95% confidence interval = 0.24-0.54, P < .001) compared to pleural mesothelioma, after adjusting for age at diagnosis and gender (Figure 1). The median survival for pleural and peritoneal mesothelioma cases was 18.4 (range 0.26-142.69) and 75.7 (0.79-326.30) months, respectively (Table 1). The percent survival at 1, 3, 5, and 10 years for pleural mesothelioma cases was 73.1%, 22.9%, 12.0%, and 4.7%, respectively, whereby the percent survival at 1, 3, 5, and 10 years for peritoneal mesothelioma cases was 91.6%, 73.8%, 65.3% and 39.4%, respectively. Subjects exposed in the non-occupational setting were more likely to develop the longer surviving peritoneal rather than pleural mesothelioma. Despite a large difference in median survival between occupational and non-occupational cases, there was no significant difference after adjusting for age at diagnosis and gender (Table 1; P = .86). The number of subjects surviving at the end of the study (1 March 2012) was 92 (48/16% pleural and 44/57% peritoneal).
Figure 1.
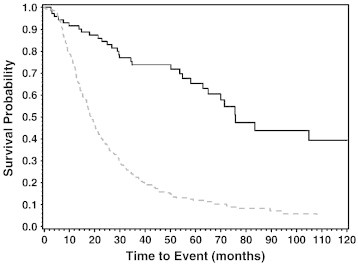
Mesothelioma survival by location. Kaplan-Meier curve representing survival in months from diagnosis of peritoneal versus pleural mesothelioma cases. There was a significant difference in survival between groups, with peritoneal mesothelioma patients having a more favorable survival outcome compared to pleural cases (log-rank P < .001). Peritoneal mesothelioma survival and pleural mesothelioma survival are shown as solid and dashed lines, respectively.
Table 1.
Survival and Demographic Data
| Age at First Exposure, Mean + SD | Age at Diagnosis, Mean ± SD | Latency (Years), Mean ± SD | Gender (%, Male/Female) | Median Survival in Months (Range) | Past Personal or Family History of Cancer, n (%) | ||
|---|---|---|---|---|---|---|---|
| Location | Pleural, n = 303 | 17.4 ± 7.8 | 66.7 ± 11.9 | 49.8 ± 11.3 | 81/19 | 18.4 (0.26-142.69) | 220 (72.6%) |
| Peritoneal, n = 77 | 12.1 ± 0.2 | 50.7 ± 15.1 | 9.1 ± 12.7 | 39/61 | 75.7 (0.79-326.3) | 55 (71.4%) | |
| Exposure type | Occupational, n = 284 | 18.7 ± 6.4 | 67.3 ± 11.2 | 49.2 ± 10.9 | 93/7 | 19.71 (0.26-144.66) | 210 (73.9%) |
| Non-occupational, n = 96 | 9.3 ± 10.2 | 51.9 ± 15.6 | 43.2 ± 15.1 | 12.5/87.5 | 53.74 (2.69-326.3) | 65 (67.7%) |
Subjects who were occupationally exposed were initially exposed to asbestos and diagnosed with mesothelioma at an older age (P < .001) and also had a longer latency (P < .001) than subjects who were exposed in a non-occupational setting.
Demographic and Occupational Data
Of the 380 mesothelioma subjects that met inclusion criteria, 303 were pleural and 77 peritoneal cases (Table 1). Most (64%) of the peritoneal cases were treated with HIPEC and cytoreductive surgery (49 HIPEC, 22 systemic chemotherapy, 6 unknown). Of the patients exposed to asbestos in an occupational setting, 257 (90.5%) developed pleural and 27 (9.5%) developed peritoneal mesothelioma. Of the non-occupational cases, 46 (47.9%) developed pleural mesothelioma and 50 (52.1%) developed peritoneal mesothelioma (Figure 2). Non-occupational cases were exposed to asbestos at a younger age, were diagnosed at an earlier age, and had a shorter latency compared to occupational cases (Table 1). The majority of occupationally exposed mesothelioma cases were men, whereas the majority of the non-occupational cases were women. Laborer/factory worker was the most common occupation for pleural mesothelioma. Construction worker was the most common occupation for peritoneal mesothelioma cases (Table 2).
Figure 2.
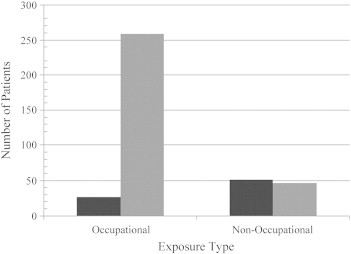
Anatomic location of mesothelioma with regard to exposure type. A total of 380 patients were enrolled in the study. There were 284 patients exposed to asbestos in an occupational setting: 257 (90.5%) developed pleural mesothelioma and 27 (9.5%) developed peritoneal mesothelioma. There were 96 patients exposed to asbestos in the non-occupational setting: 46 (47.9%) developed pleural mesothelioma and 50 (52.1%) developed peritoneal mesothelioma.
Table 2.
Exposure History
| Occupation | Pleural, n (%) | Peritoneal, n (%) |
|---|---|---|
| Occupational exposure: ship related | ||
| Military | 36 (14.0) | |
| Occupational exposure: mining and milling | ||
| Mine Worker | 3 (1.2) | |
| Occupational exposure: end users/maintenance/asbestos as a contaminant | ||
| Factory laborer | 52 (20.3) | 6 (22.2) |
| Construction | 44 (17.1) | 9 (33.4) |
| Maintenance/repair/mechanic | 28 (10.9) | 5 (18.5) |
| Pipe fitter | 14 (5.4) | 1 (3.7) |
| Shipping/receiving | 14 (5.4) | |
| Sheet metal worker | 11 (4.3) | |
| Machinist | 11 (4.3) | 1 (3.7) |
| Farming | 9 (3.5) | 2 (7.4) |
| Boiler worker | 7 (2.7) | |
| Chemical worker | 3 (1.2) | 1 (3.7) |
| Railroad worker | 2 (0.8) | |
| Other occupational exposure | ||
| Non-industrial/non-labor | 11 (4.3) | 1 (3.7) |
| Office/clerical | 5 (1.9) | |
| Other | 7 (2.7) | 1 (3.7) |
Occupations of the 284 occupationally exposed cases categorized according to Rice [27].
Discussion
This study shows that survival for peritoneal mesothelioma patients was significantly better than pleural mesothelioma patients, which may be reflective of differences in the natural history of these two forms of mesothelioma, effect of newer therapies, or both. The prognosis for peritoneal mesothelioma has improved significantly in recent years due to therapeutic advancements, especially with HIPEC coupled with cytoreductive surgery [28]. Of the reports cited [21], [22], [23], [24], [25], [26], the median survival values for peritoneal mesothelioma patients receiving cytoreductive surgery plus HIPEC falls within the range of 34 to 92 months. A more recent review of the literature [29] lists a range of 53 to 92 months for median survival, which is suggestive of survival improving over time. Heated intraoperative chemotherapy (HIOC) has also been used in conjunction with EPP with and without adjuvant radiation therapy [30], [31], [32]. It has been shown to be safe [31], [32] and effective [30]. Sugarbaker and coworkers showed that addition of HIOC to EPP improved median survival from 12.8 to 27.1 months. There was, however, no significant difference between EPP + HIOC and EPP + radiation therapy [30]. While HIOC + EPP appears to double mean survival compared with EPP alone, the mean survival for pleural mesothelioma treated with HIOC + EPP is far less than that reported for peritoneal mesothelioma treated with HIPEC + cytoreductive surgery, which suggests a difference in the natural history of pleural versus peritoneal mesothelioma regardless of treatment. Because these studies were not randomized, they must, however, be interpreted with caution.
The reported range for median survival for pleural mesothelioma in a 2009 review, regardless of stage, was between 9 and 17 months [33]. Other more recent reviews show no improvement in survival compared to this review [34], [35]. Our data are consistent with, but at the upper end of, median survival values reported by other studies for both peritoneal and pleural cases, suggesting that newer therapies do improve survival. A case series of 238 pleural and peritoneal mesotheliomas suggests that survival rates outside of therapy trials reported as recently as 2011 were still low, with a reported overall survival of 8.8 months with mean survival increasing to 11.3 months in patients receiving therapy versus 6.4 months in those that remained untreated [36]. This case series was dominated by pleural cases (94.4%) underscoring the difference in natural histories of pleural and peritoneal mesotheliomas, but the mean survival is still quite low compared with our reported median survival of 18.4 (range 0.26-142.69), further illustrating improvements in survival over time. Unfortunately, many patients with both pleural and peritoneal mesothelioma are often diagnosed at a late stage. Although the treatment of mesothelioma is evolving, these patients may benefit less from therapies because of advanced disease, highlighting the role of asbestos exposure history in early diagnosis.
Exposure type (occupational or non-occupational) did affect survival but only because subjects exposed in the non-occupational setting were more likely to develop the longer surviving peritoneal rather than pleural mesothelioma. Subjects exposed in the occupational setting had a median survival of 19.71 (0.26-144.66) months, whereby those exposed in the non-occupational setting had a median survival of 53.74 (2.69-326.30) months. When survival was adjusted for gender and age at diagnosis however, exposure type did not significantly affect survival. The non-occupational group had a significantly greater proportion of females and a younger age at first exposure and diagnosis. Report of these data is important because non-occupational subjects are more likely to be under-recognized than those with occupational exposure because of recall and gender bias. However, they are also more likely to develop peritoneal mesothelioma, which is more amenable to the newer therapies that are associated with longer survival.
This study has several limitations. The authors recognize that there is potential for the introduction of confounding variables resulting from a selection bias in the subject pool as a function of a clinician's interest in medico-legal issues or in mesothelioma treatment, per se. The overall n of 380 represents a significant number of subjects, considering that only 3000 new diagnoses are made each year, nationwide. The authors felt that the large number of subjects and histopathologic or death certificate confirmation of cases was more than sufficient to wash out any effect of variance in this study based on referral bias.
Asbestos exposure was based on patient report that can be flawed by memory.
Furthermore, no attempt was made to quantify dose or fiber type of asbestos given the reliance on patient report. While certain occupational categories would suggest a preponderance of amphibole exposure and many occupational exposures are mixed as to fiber type, all the fiber types cause pleural and peritoneal mesotheliomas, and therefore knowing the fiber type in each case is not necessary, particularly in a study like this one [37].
Additionally, most, if not all, subjects were involved in litigation, which may bias the reporting of extent of asbestos exposure. Conversely, however, it could cause bias based on court required proof of exposure and pathologic evidence of mesothelioma that is more rigorous than patient reported medical histories not involved in litigation. On the basis of the recommendation of the American Thoracic Society, subjects not involved in litigation were told at the time of evaluation that they had cause for legal action [38].
Tumor misclassification especially in peritoneal cases where ovarian tumors can be misdiagnosed as mesothelioma is unlikely in this study. Pathology reports including immunohistochemical staining were available on all peritoneal cases. Furthermore, most, if not all, had expert second and third consultative pathology reports related to litigation and treatment at a tertiary care center.
Another limitation of this study is that past personal and family history of cancer was from patient report, with confirmation, when available, from the medical record. While this is an imperfect approach in documenting this important phenotypic data, the presence of a cancer history is consistent with and further enhances the validity of the mesothelioma diagnosis [17], [39], [40], [41].
Emerging data regarding pathophysiology of mesothelioma suggest that non-occupational and occupational asbestos exposure are non-differentiating variables with regard to causality [5], [42], [43]. These studies demonstrate that asbestos exposure may be as high in the non-occupational as the occupational setting. Asbestos exposure dose is equal to fiber density times duration of exposure. While fiber dose may be lower in the non-occupational settings, duration of exposure can be much longer in the non-occupational compared with the occupational setting. Occupationally exposed individuals are generally limited to about 2000 exposure hours per year, whereas non-occupational exposure is unlimited, because the asbestos fibers permeate their environment. Therefore, cumulative exposure may be comparable between occupational and non-occupational cases [43]. As such, exposure intensity is just one factor in determining mesothelioma development.
Recent evidence suggests that genetic susceptibility is another factor for mesothelioma development. Genetic predisposition is complex in that genes can affect 1) tumorogenesis, [14], [18], [44] 2) the ability to spread and metastasize, 3) immune surveillance that aborts tumor growth and development, and 4) response to chemotherapy. This dynamic interaction between genes and asbestos exposure suggests that asbestos dose may play a less significant role in mesothelioma development than previously thought and that genetic predisposition may play a larger causative role. Further research is required to ferret out the differential impact of genetics and asbestos exposure.
Conclusion
Survival is improving for peritoneal and, to a lesser extent, pleural mesothelioma and appears to be longer than reported in historical accounts. This study emphasizes the increasing difference in survival of peritoneal versus pleural cases and the importance of obtaining a thorough occupational and non-occupational exposure history when evaluating a patient with signs or symptoms of a thoracic or abdominal malignancy.
Finally, results of this study suggest that further investigation into the role of other factors, which were beyond the scope of this study, would be of significant value. The authors suggest that future research in this area examines the role that such independent variables as 1) genetic predisposition, 2) gene environment interactions, and 3) various treatment modalities, such as intraperitoneal chemotherapy versus systemic chemotherapy, may play in patient longevity.
Acknowledgements
The authors thank Katrina Swett, MS, for performing the statistical analysis of survival data. Conflict of interest statement: J.A.O. performs occupational medicine independent medical evaluations and expert witness testimony on the part of the litigant or defense, as part of her role as Professor of Medicine at Wake Forest School of Medicine. Revenue from evaluations, depositions, and court appearances is paid to the university. She derives personal revenue from chart reviews. M.H., S.H., J.F., E.A.L., and G.C. have none to report. Funding source: Funding for this study was provided by the Mesothelioma Applied Research Foundation (MARF).
Contributor Information
Jennifer Faig, Email: johar@wakehealth.edu.
Suzanne Howard, Email: showard@wakehealth.edu.
Edward A. Levine, Email: elevine@wakehealth.edu.
Gary Casselman, Email: gcasselman@ccgteam.com.
Mary Hesdorffer, Email: info@curemeso.org.
Jill A. Ohar, Email: johar@wakehealth.edu.
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353(15):1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Donovan EP, Donovan BL, McKinley MA, Cowan DM, Paustenbach DJ. Evaluation of take home (para-occupational) exposure to asbestos and disease: a review of the literature. Crit Rev Toxicol. 2012;42(9):703–731. doi: 10.3109/10408444.2012.709821. [DOI] [PubMed] [Google Scholar]
- 3.Donovan EP, Donovan BL, Sahmel J, Scott PK, Paustenbach DJ. Evaluation of bystander exposures to asbestos in occupational settings: a review of the literature and application of a simple eddy diffusion model. Crit Rev Toxicol. 2011;41(1):52–74. doi: 10.3109/10408444.2010.506639. [DOI] [PubMed] [Google Scholar]
- 4.Ampleford EJ, Ohar J. Mesothelioma: you do not have to work for it. Diagn Cytopathol. 2007;35(12):774–777. doi: 10.1002/dc.20766. [DOI] [PubMed] [Google Scholar]
- 5.Roggli VL, Longo WE. Mineral fiber content of lung tissue in patients with environmental exposures: household contacts vs. building occupants. Ann N Y Acad Sci. 1991;643:511–518. doi: 10.1111/j.1749-6632.1991.tb24501.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson HA, Lilis R, Daum SM, Fischbein AS, Selikoff IF. Household-contact asbestos neoplastic risk. Ann N Y Acad Sci. 1976;271:311–323. doi: 10.1111/j.1749-6632.1976.tb23127.x. [DOI] [PubMed] [Google Scholar]
- 7.Magnani C, Agudo A, González CA, Andrion A, Calleja A, Chellini E, Dalmasso P, Escolar A, Hernandez S, Ivaldi C. Multicentric study on malignant pleural mesothelioma and non-occupational exposure to asbestos. Br J Cancer. 2000;83(1):104–111. doi: 10.1054/bjoc.2000.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller A. Mesothelioma in household members of asbestos-exposed workers: 32 United States cases since 1990. Am J Ind Med. 2005;47(5):458–462. doi: 10.1002/ajim.20167. [DOI] [PubMed] [Google Scholar]
- 9.Browne K, Smither WJ. Asbestos-related mesothelioma: factors discriminating between pleural and peritoneal sites. Br J Ind Med. 1983;40(2):145–152. doi: 10.1136/oem.40.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson RF, O'Sullivan MF, Huang J, Holiday DB, Hammar SP. Asbestos in extrapulmonary sites: omentum and mesentery. Chest. 2000;117(2):486–493. doi: 10.1378/chest.117.2.486. [DOI] [PubMed] [Google Scholar]
- 11.Dawson A, Gibbs AR, Pooley FD, Griffiths DM, Hoy J. Malignant mesothelioma in women. Thorax. 1993;48(3):269–274. doi: 10.1136/thx.48.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyland RA, Ware S, Johnson AR, Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health. 2007;33(4):286–292. doi: 10.5271/sjweh.1145. [DOI] [PubMed] [Google Scholar]
- 13.Skammeritz E, Omland LH, Johansen JP, Omland O. Asbestos exposure and survival in malignant mesothelioma: a description of 122 consecutive cases at an occupational clinic. Int J Occup Environ Med. 2011;2(4):224–236. [PubMed] [Google Scholar]
- 14.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astoul P, Roca E, Galateau-Salle F, Scherpereel A. Malignant pleural mesothelioma: from the bench to the bedside. Respiration. 2012;83(6):481–493. doi: 10.1159/000339259. [DOI] [PubMed] [Google Scholar]
- 16.Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The impact of underreported Veterans Affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101(7):533–536. doi: 10.1093/jnci/djn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohar JA, Ampleford EJ, Howard SE, Sterling DA. Identification of a mesothelioma phenotype. Respir Med. 2007;101(3):503–509. doi: 10.1016/j.rmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Carbone M, Korb FL, Baumann F, Napolitano A, Lum CA, Flores EG, Gaudino G, Powers A, Bryant-Greenwood P, Krausz T. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10(1):179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadby G, Mukherjee S, Musk AW, Reid A, Garlepp M, Dick I, Robinson C, Hui J, Fiorito G, Guarrera S. A genome-wide association study for malignant mesothelioma risk. Lung Cancer. 2013;82(1):1–8. doi: 10.1016/j.lungcan.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Matullo G, Guarrera S, Betti M, Fiorito G, Ferrante D, Voglino F, Cadby G, Di Gaetano C, Rosa F, Russo A. Genetic variants associated with increased risk of malignant pleural mesothelioma: a genome-wide association study. PLoS One. 2013;8(4):e61253. doi: 10.1371/journal.pone.0061253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Liewehr DJ, Kleiner DE, Alezander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21(24):4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 22.Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, Glehen O. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13(3):405–412. doi: 10.1245/ASO.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 23.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67(10):999–1003. [PubMed] [Google Scholar]
- 24.Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32(9):948–953. doi: 10.1016/j.ejso.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Yan TD, Deraco M, Elias D, Glehen O, Levine EA, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer. 2011;117(9):1855–1863. doi: 10.1002/cncr.25640. [DOI] [PubMed] [Google Scholar]
- 26.Blackham AU, Shen P, Stewart JH, Russell GB, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol. 2010;17(10):2720–2727. doi: 10.1245/s10434-010-1080-6. [DOI] [PubMed] [Google Scholar]
- 27.Rice C, Heineman EF. An asbestos job exposure matrix to characterize fiber type, length, and relative exposure intensity. Appl Occup Environ Hyg. 2003;18(7):506–512. doi: 10.1080/10473220301459. [DOI] [PubMed] [Google Scholar]
- 28.Munkholm-Larsen S, Cao CQ, Yan TD. Malignant peritoneal mesothelioma. World J Gastrointest Surg. 2009;1(1):38–48. doi: 10.4240/wjgs.v1.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Quteimat O, Al-Badaineh M. Intraperitoneal chemotherapy: Rationale, applications, and limitations. J Oncol Pharm Pract. 2013:1–12. doi: 10.1177/1078155213506244. [DOI] [PubMed] [Google Scholar]
- 30.Sugarbaker DJ, Gill RR, Yeap BY, Wolf AS, DaSilva MC, Baldini EH, Bueno R, Richards WG. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg. 2013;145(4):955–963. doi: 10.1016/j.jtcvs.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 31.Tilleman TR, Richards WG, Zellos L, Johnson BE, Jaklitsch MT, Mueller J, Yeap BY, Mujoomdar AA, Ducko CT, Bueno R. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg. 2009;138(2):405–411. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 32.Kodama K, Higashiyama M, Okami J, Tokunaga T, Fujiwara A, Imamura F, Nakayama T. Cytoreductive surgery and post-operative heated pleural chemotherapy for the management of pleural surface malignancy. Int J Hyperthermia. 2013;29(7):653–662. doi: 10.3109/02656736.2013.829247. [DOI] [PubMed] [Google Scholar]
- 33.Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009;27(12):2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas AR, Sterman DH. Malignant pleural mesothelioma: update on treatment options with a focus on novel therapies. Clin Chest Med. 2013;34(1):99–111. doi: 10.1016/j.ccm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zauderer M, Krug LM. The evolution of multimodality therapy for malignant pleural mesothelioma. Curr Treat Options Oncol. 2011:163–172. doi: 10.1007/s11864-011-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haber SE, Haber JM. Malignant mesothelioma: a clinical study of 238 cases. Ind Health. 2011;49(2):166–172. doi: 10.2486/indhealth.ms1147. [DOI] [PubMed] [Google Scholar]
- 37.Kanarek MS. Mesothelioma from chrysotile asbestos: update. J Ann Epidemiol. 2014:688–697. doi: 10.1016/j.annepidem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.American Thoracic Society Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170(6):691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 39.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13(3):153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huncharek M, Kelsey K, Muscat J, Christiani D. Parental cancer and genetic predisposition in malignant pleural mesothelioma: a case-control study. Cancer Lett. 1996;102(1–2):205–208. doi: 10.1016/0304-3835(96)04172-9. [DOI] [PubMed] [Google Scholar]
- 41.Heineman EF, Bernstein L, Stark AD, Spirtas R. Mesothelioma, asbestos, and reported history of cancer in first-degree relatives. Cancer. 1996;77(3):549–554. doi: 10.1002/(SICI)1097-0142(19960201)77:3<549::AID-CNCR18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Hillerdal G. Mesothelioma: cases associated with non-occupational and low dose exposures. Occup Environ Med. 1999;56(8):505–513. doi: 10.1136/oem.56.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metintas S, Metintas M, Ucgun I, Oner U. Malignant mesothelioma due to environmental exposure to asbestos: follow-up of a Turkish cohort living in a rural area. Chest. 2002;122(6):2224–2229. doi: 10.1378/chest.122.6.2224. [DOI] [PubMed] [Google Scholar]
- 44.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]


