Abstract
Objective
The aim of this study was to assess the predictive value of functional performance and range of motion measures on outcomes after total knee arthroplasty.
Design
This is a secondary analysis of two pooled prospective randomized controlled trials. Sixty-four subjects (32 men and 32 women) with end-stage knee osteoarthritis scheduled to undergo primary total knee arthroplasty were enrolled. Active knee flexion and extension range of motion, Timed Up and Go (TUG) test time, and 6-min walk test distance were assessed.
Results
Preoperative measures of knee flexion and extension were predictive of long-term flexion (β = 0.44, P < 0.001) and extension (β = 0.46, P < 0.001). Acute measures of knee flexion and extension were not predictive of long-term flexion (β= 0.09, P = 0.26) or extension (β = 0.04, P = 0.76). Preoperative TUG performance was predictive of long-term 6-min walk performance (β = −21, P < 0.001). Acute TUG performance was predictive of long-term functional performance on the 6-min walk test, after adjusting for the effects of sex and age (P = 0.02); however, once adjusted for preoperative TUG performance, acute TUG was no longer related to long-term 6-min walk performance (P = 0.65).
Conclusions
Acute postoperative measures of knee range of motion are of limited prognostic value, although preoperative measures have some prognostic value. However, acute measures of functional performance are of useful prognostic value, especially when preoperative functional performance data are unavailable.
Keywords: Osteoarthritis, Older Adults, Rehabilitation, Physical Function, Total Knee Arthroplasty, Range of Motion
Total knee arthroplasty (TKA) is the most commonly performed musculoskeletal procedure in the United States, with close to 700,000 performed annually.1,2 Future projections of TKA rates anticipate 3.5 million procedures being performed annually by the year 2030.3
In the United States, rehabilitation after TKA can consist of several different practice settings depending on the patient, healthcare system, and decisions made by both the healthcare team and the patient. Rehabilitation begins in the acute inpatient setting after TKA, where decisions are made for the most appropriate discharge location for the patient. Possible discharge locations for a patient after TKA are a skilled nursing facility, an inpatient rehabilitation facility, home with home health physical therapy, home with a prescribed home exercise program, or outpatient physical therapy.4 The proper discharge location depends on the patient’s current functional ability at the time of anticipated discharge, physical demands of his/her home environment, social support, the patient’s overall goals, and the patient’s prognosis. Despite the high frequency at which TKA is being performed, there is sparse information on expected outcomes in the early postoperative period and how these outcomes relate to prognosis and long-term outcomes. This information is vital for clinicians to assist in discharge planning, establishing prognosis, and identifying patients at high risk for a poor outcome.
Knee range of motion (ROM) and physical functional performance are major outcomes after TKA. Preoperative measures of joint function and functional performance are robust predictors of postoperative outcomes.5–10 However, clinicians often do not have access to preoperative outcome measures in the postoperative clinical setting. To date, no study has compared acute postoperative outcomes with preoperative and long-term postoperative outcomes. Therefore, the purpose of this study was to assess the predictive value of functional performance and ROM measures taken during the preoperative and acute time points on long-term postoperative outcomes after TKA.
METHODS
This study was a secondary analysis of two pooled prospective randomized clinical trials.11,12 All patients were assessed 1–2 wks preoperatively (preoperative time point) and 6 mos postoperatively (long-term time point) in the Clinical and Translational Research Center at the University of Colorado Hospital. The patients were also assessed 48 hrs after surgery (acute time point) on the inpatient orthopedic floor of the University of Colorado Hospital. Postoperative day 2 was chosen because the mean hospital length of stay is 3 days, and this enabled all patients to be tested; some were discharged on postoperative day 2. The 6-mo time point was chosen as the long-term time point because recovery after TKA usually plateaus by this time point.13–15 Informed consent was obtained from all participants. Both studies were approved by the Colorado Multiple Institutional Review Board.
Participants and Interventions
Sixty-four patients (mean [SD] age, 64.6 [8.5] yrs; 32 men and 32 women; body mass index, 30.6 [4.8] kg/m2) were recruited from the community and with assistance of three participating orthopedic surgeons between June 2006 and June 2010. Eligible volunteers were those between the ages of 50 and 85 yrs and scheduled to receive a primary unilateral TKA secondary to end-stage osteoarthritis of the knee. Patients were excluded for any of the following criteria that could affect long-term functional performance: significant cardiac or neurologic impairments, contralateral knee osteoarthritis (as defined by pain greater than 5/10 with activity), other unstable lower extremity orthopedic conditions, body mass index of 40 kg/m2 or greater, uncontrolled diabetes, or uncontrolled hypertension.
All patients underwent surgery in the same hospital using a tricompartmental, cemented TKA with a medial parapatellar surgical approach. After surgery, the patients participated in a standardized rehabilitation protocol beginning on postoperative day 1 as previously described.16 The patients were seen twice daily in the acute setting for 2–4 days before discharge to home. In the acute setting, rehabilitation consisted of patient education, passive and active ROM, gait training, transfer training, and stair training, if necessary. The patients were then seen in the home and outpatient setting for a total of 16–18 visits during 8 wks. Rehabilitation in the home and outpatient settings consisted of passive and active ROM, lower extremity flexibility exercises, patellofemoral mobilization, incision mobilization, gait training, functional training (transfers and stairs), weight-bearing and non–weight-bearing exercises, and modalities (ice and heat) as needed. All patients were given a home exercise program to be completed twice daily until discharge from therapy.
Outcomes
Range of Motion
Active ROM was measured with a long-arm goniometer with the patient lying in supine, as described by Norkin and White.17 For active knee extension ROM, a raised block was placed under the patient’s heel, and he/she was cued to actively extend his/her knee. Throughout this article, negative values of knee extension represent hyperextension. For active flexion ROM, the participant was cued to maximally flex the knee while keeping his/her heel on the supporting surface. The reliability of active knee flexion goniometric measurement after TKA is good18 in the acute19 (intraclass correlation coefficient [ICC], 0.89) and outpatient20 settings (ICC, 0.81–0.87). The reliability of active knee extension goniometric measurement is moderate18 in the acute setting19 (ICC, 0.64) and good in the outpatient20 setting (ICCs ranging from 0.86 to 0.87). Passive knee extension goniometric reliability is poorer than that of active knee extension, yet both passive and active knee flexion demonstrate good reliability.19,20 Therefore, only active measurement ROM measurements were examined for the purposes of this article.20
Functional Performance
Measures of functional performance included the Timed Up and Go (TUG) and 6-min walk (6MW) tests. The TUG measures the time to rise from a chair, walk 3 m, turn around, and return to sitting in the same chair without physical assistance.21 The same chair (seat height of 46 cm) was used for both inpatient and outpatient testing, and the patients were allowed to use an assistive device to complete the test if needed. The TUG test demonstrates good reliability22 (ICC, 0.75) in the patients awaiting TKA. The 6MW test is a commonly used test to assess walking speed and endurance after TKA.14,22–24 The patients completed this test in an indoor hall-way with a 30.5-m (100 ft) walkway and were instructed to walk back and forth to cover as much distance as possible in the 6-min period. The patients were allowed rest breaks if needed and the use of an assistive device, if necessary. The 6MW test demonstrates good reliability22 (ICC, 0.94) in the patients awaiting TKA. However, the 6MW is too difficult for the patients to complete in the acute setting. Therefore, only the TUG was used to measure acute functional performance, whereas both the TUG and 6MW tests were used to measure outpatient functional performance at both the preoperative and long-term time points.
Statistical Analysis
The SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses. Data from the two clinical trials were examined before data pooling. Trial 1 examined the use of neuromuscular electrical stimulation on the recovery of strength and function after TKA. Only the patients from the control group in trial 1 were included because functional outcomes in the experimental group were significantly better.12 The patients in the control group participated in a standardized rehabilitation program that did not use neuromuscular electrical stimulation. Trial 2 examined the use of minimally invasive surgery on the recovery of strength and function after TKA. Both the control group (TKA with a traditional surgical approach) and the experimental group (TKA with a minimally invasive surgical approach) were included because there was no difference between groups over time in ROM or function.11 All patients in trial 2 participated in the same standardized rehabilitation program as the patients in trial 1. Differences in age and body mass index between the two clinical trials were analyzed using an independent samples t test. Differences in sex between the two clinical trials were analyzed using the χ2 test. Differences in the TUG and 6MW tests over time between the groups were examined using a repeated-measures linear mixed model. There were no differences in age, body mass index, sex, and TUG or 6MWoutcomes over time between the two clinical trials.
After data pooling, basic descriptive statistics were calculated, and the distribution of continuous outcomes was examined for evidence of skew. The TUG test times were log transformed secondary to the presence of positive skew to ensure proper statistical inference in the linear regression analyses. Linear regression was used to examine the relationship between outcome variables over time. Study group was also assessed as a potential confounder; however, no evidence of confounding was found, and therefore, all results report the crude, unadjusted beta coefficients. With 64 subjects, the central limit theorem ensures the robustness of statistical inference (P values and confidence intervals [CIs]) using standard linear regression methods. Linear regression was also used to assess the contribution of changes in acute TUG performance and preoperative TUG performance to changes in long-term 6MW performance after adjusting for age and sex, which have been shown to be related to functional outcomes after TKA.8 A two-tailed α level was set at 0.05 for the regression analyses.
RESULTS
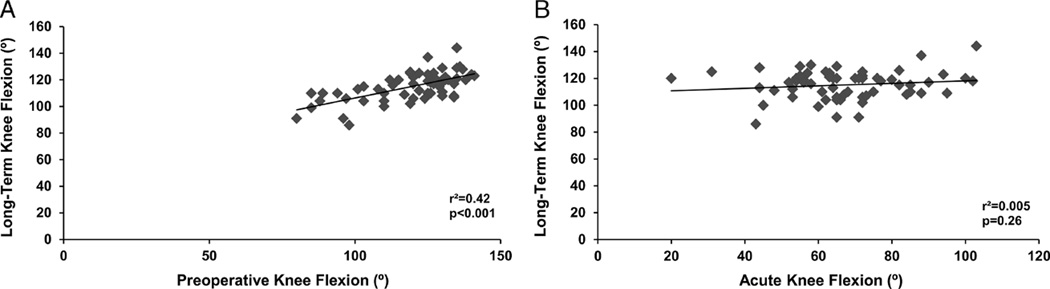
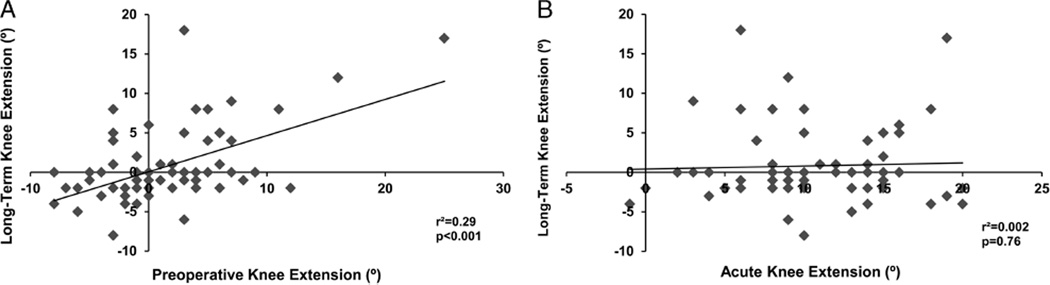
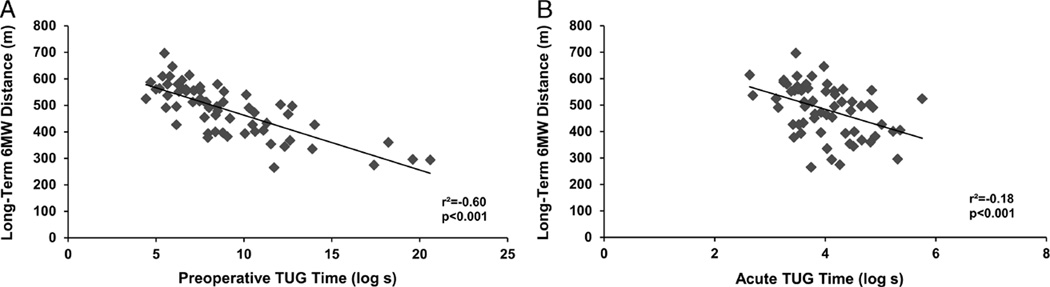
Outcomes by time point are presented in Table 1. Preoperative knee flexion was predictive of long-term knee flexion (β = 0.44; 95% CI, 0.31 to 0.58; r2 = 0.42; P < 0.001). Acute knee flexion was not related to either preoperative (β = 0.03; 95% CI, −0.20 to 0.26; r2 = 0.001; P = 0.80) or long-term (β = 0.09; 95%CI, −0.07 to 0.26; r2 = 0.005; P = 0.26) knee flexion (see Figs. 1A, B). Preoperative knee extension was predictive of long-term knee extension (β = 0.46; 95% CI, 0.27 to 0.64; r2 = 0.29; P < 0.001). Acute knee extension demonstrated no relationship with either preoperative (β = 0.05; 95% CI, −0.25 to 0.35; r2 = 0.002; P = 0.76) or long-term (β = 0.04; 95% CI, −0.22 to 0.30; r2 = 0.002; P = 0.76) knee extension (see Figs. 2A, B). Preoperative TUG performance was predictive of long-term 6MW performance (β = −211; 95% CI, −255 to −167; r2 = 0.60; P < 0.001). Acute TUG performance was related to preoperative (β = −61; 95% CI, −107 to −14; r2 = 0.10; P = 0.01) and long-term(β = −62; 95%CI, −97 to −28; r2 = 0.18; P < 0.001) 6MW performance (see Figs. 3A, B).
TABLE 1.
Outcomes by time point
| Variable | Preoperative, Mean (SD) (Range) |
Acute, Mean (SD) (Range) |
Long-term, Mean (SD) (Range) |
|---|---|---|---|
| Involved knee flexion, degrees | 119.7 ± 15.2 (80.0 to 141.0) | 66.6 ± 16.7 (20.0 to 103.0) | 115.1 ± 10.5 (86.0 to 144.0) |
| Involved knee extension, degrees | 1.5 ± 5.8 (−8.0 to 25.0) | 10.4 ± 4.8 (−1.0 to 20.0) | 0.8 ± 4.9 (−8 to 18) |
| TUG test, secs | 9.0 ± 3.5 (4.4 to 20.6) | 69.0 ± 54.3 (13.9 to 315.0) | 7.9 ± 2.3 (4.5 to 14.9) |
| 6MW test, meters | 452.7 ± 124 (106.7 to 707.1) | — | 483.3 ± 95.8 (265.2 to 696.5) |
The 6MW test was not performed at the acute postoperative time point. For knee extension, negative values represent hyperextension.
FIGURE 1.
Relation between preoperative (A) and acute (B) knee flexion with long-term knee flexion.
FIGURE 2.
Relation between preoperative (A) and acute (B) knee extension with long-term knee extension. Negative values of knee extension represent hyperextension.
FIGURE 3.
Relation between preoperative (A) and acute (B) TUG time with long-term 6MW distance. TUG time has been log transformed.
To assess the contribution of changes in acute TUG performance and preoperative TUG performance to changes in long-term 6MW performance, a hierarchical linear regression was performed (see Table 2). In step 1, age was added to the model and was significantly associated with long-term 6MW performance (P = 0.04). In step 2, sex was added to the model and was significantly associated with long-term 6MW performance (P = 0.008). In step 3, acute TUG time was added to the model and was significantly associated with long-term 6MW performance (P = 0.02). In step 4, preoperative TUG time was added to the model and was significantly associated with long-term 6MW performance (P < 0.001). However, in the overall model, once preoperative TUG time was added, acute TUG time was no longer predictive of long-term 6MW performance (P = 0.65; see Table 3).
TABLE 2.
Results of the hierarchical regression on 6MW distance 6 mos after TKA
| Model | r2 | Change in r2 | Change in F | Significant F Change |
|---|---|---|---|---|
| Age | 0.07 | — | 4.48 | 0.04 |
| Age + sex | 0.17 | 0.10 | 7.41 | 0.008 |
| Age + sex + acute TUG time | 0.24 | 0.07 | 5.42 | 0.02 |
| Age + sex + acute TUG time + preoperative TUG time | 0.63 | 0.40 | 62.84 | <0.001 |
TABLE 3.
Parameter estimates for the overall regression on 6MW distance 6 mos after TKA
| Variable | Parameter Estimate | SE | t Value | P |
|---|---|---|---|---|
| Intercept | 952.0 | 69.4 | 13.72 | <0.001 |
| Age, yrs | −1.3 | 0.98 | −1.28 | 0.21 |
| Sex (1 = male) | 34.2 | 16.2 | 2.11 | 0.04 |
| Acute TUG time (log sec) | 6.7 | 14.7 | 0.45 | 0.65 |
| Preoperative TUG time (log sec) | −202.3 | 25.5 | −7.93 | <0.001 |
DISCUSSION
This was the first study to assess the predictive value of functional performance and ROM measures on acute and long-term outcomes after TKA. Acute measures of knee ROM were not related to long-term ROM outcomes, whereas preoperative measures of ROM were. Both preoperative and acute measures of functional performance were predictive of long-term functional performance. However, preoperative functional performance was a stronger predictor of long-term functional performance than acute functional performance.
Factors affecting ROM after TKA have been examined in several studies. Preoperative ROM has been the strongest predictor of postoperative ROM, with contributory factors of age, sex, obesity, a history of knee surgery, presence of an extensor lag, diagnosis, intraoperative ROM, use of a posterior capsule release, and postoperative tibiofemoral angle.10,25–27 In the current study, preoperative ROM was also found to be a significant predictor of long-term ROM. However, acute ROM, measured at a time when discharge planning had begun, was not related to long-term ROM outcomes. This suggests that ROM measurements on postoperative day 2 are not indicative of how a patient will do long-term. Ideally, limited preoperative ROM should be used to identify individuals who will require more intensive, supervised rehabilitation to minimize poor ROM outcomes or subsequent need for manipulation.
The poor correlation of acute ROM measures with long-term ROM measures could be contributed to several potential variables. For example, high levels of pain immediately after surgery may cause patients to be tentative about challenging their limit of mobility. Therefore, differences in pain tolerance, anesthesia type, and postoperative dosing of pain medication may have led to increased ROM variability at this time point and a lack of relationship to long-term ROM. Time since surgery may also play a role. The measurements on postoperative day 2 in this study were not related to long-term outcomes. However, in Australian studies28,29 in which hospital stays are longer, inpatient ROM measurements of extension performed at discharge on postoperative days 6–8 were related to long-term extension ROM; the same relationship was not observed for flexion.
Functional performance after TKA is known to be related to preoperative functional performance, comorbid conditions, age, sex, quadriceps strength in the involved and uninvolved limbs, and postoperative rehabilitation.5–9,24,30–32 In this study, both preoperative and acute performance on the TUG were predictive of long-term functional performance on the 6MW test. However, after adjusting for the effects of age, sex, and preoperative functional performance, acute TUG performance was no longer predictive of long-term functional performance. This suggests that preoperative functional performance is a better measure for determining prognosis when both preoperative and acute postoperative outcomes are available. However, because clinicians do not always have access to preoperative functional data, acute TUG performance can be used to guide discharge planning in the acute setting and predict long-term functional performance.
The primary limitation of this study was that the inclusion criteria for the two pooled clinical trials were fairly strict. Thus, the results cannot be generalized to the population of all individuals who undergo TKA. Comorbidities are known to influence ROM and functional performance, and patients with significant comorbidities were excluded from both clinical trials. An additional limitation was that the rehabilitation intervention used in this study may not be representative of practice patterns in other regions of the United States, which may have affected long-term outcomes.
CONCLUSIONS
For discharge planning in the acute setting, preoperative ROM and functional performance should ideally be used to identify patients who may require close monitoring or intensive rehabilitation. If preoperative measures are not available, acute postoperative performance on the TUG test can be useful for establishing a prognosis. However, acute postoperative ROM measurements have very limited prognostic utility.
Acknowledgments
Disclosures:
Supported by the National Institutes of Health (NIH) (K23 AG029978, R03 AR054538, T32 AG00279, UL1 RR025780) and the Foundation for Physical Therapy Promotion of Doctoral Studies II Scholarship. The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which they are associated, and, if applicable, the authors certify that all financial and material support for this research (e.g., NIH or NHS grants) and work are clearly identified in the title page of this article.
Footnotes
Presented at the APTA Combined Sections Meeting in San Diego, CA, in January 2013.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Health Care Cost and Utilization Project: HCUP facts and figures: Statistics on hospital-based care in the United States 2008. [Accessed January 18, 2011]; Available at: http://www.hcup-us.ahrq.gov/reports/factsandfigures/2008/exhibit3_1.jsp.
- 2.American Academy of Orthopaedic Surgeons: Most commonly performed musculoskeletal-related procedures. [Accessed June 27, 2009]; Available at: www.aaos.org/research/stats/top_hospitalization_visits.pdf. [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Lingard EA, Berven S, Katz JN. Management and care of patients undergoing total knee arthroplasty: Variations across different health care settings. Arthritis Care Res. 2000;13:129–136. [PubMed] [Google Scholar]
- 5.Fortin PR, Clarke AE, Joseph L, et al. Outcomes of total hip and knee replacement: Preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Brown K, Kachelman J, Topp R, et al. Predictors of functional task performance among patients scheduled for total knee arthroplasty. J Strength Cond Res. 2009;23:436–443. doi: 10.1519/JSC.0b013e318198fc13. [DOI] [PubMed] [Google Scholar]
- 7.Lavernia C, D’Apuzzo M, Rossi MD, et al. Is postoperative function after hip or knee arthroplasty influenced by preoperative functional levels? J Arthroplasty. 2009;24:1033–1043. doi: 10.1016/j.arth.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy DM, Hanna SE, Stratford PW, et al. Preoperative function and gender predict pattern of functional recovery after hip and knee arthroplasty. J Arthroplasty. 2006;21:559–566. doi: 10.1016/j.arth.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingard EA, Katz JN, Wright EA, et al. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ritter MA, Harty LD, Davis KE, et al. Predicting range of motion after total knee arthroplasty. Clustering, log-linear regression, and regression tree analysis. J Bone Joint Surg Am. 2003;85-A:1278–1285. doi: 10.2106/00004623-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Stevens-Lapsley JE, Bade MJ, Shulman BC, et al. Minimally invasive total knee arthroplasty improves early knee strength but not functional performance: A randomized controlled trial. J Arthroplasty. 2012;27:1812–1819. doi: 10.1016/j.arth.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: A randomized controlled trial. Phys Ther. 2012;92:210–226. doi: 10.2522/ptj.20110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ethgen O, Bruyere O, Richy F, et al. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy DM, Stratford PW, Riddle DL, et al. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther. 2008;88:22–32. doi: 10.2522/ptj.20070051. [DOI] [PubMed] [Google Scholar]
- 15.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 16.Mintken PE, Carpenter KJ, Eckhoff D, et al. Early neuromuscular electrical stimulation to optimize quadriceps muscle function following total knee arthroplasty: A case report. J Orthop Sports Phys Ther. 2007;37:364–371. doi: 10.2519/jospt.2007.2541. [DOI] [PubMed] [Google Scholar]
- 17.Norkin CC, White DJ. Measurement of Joint Motion. A Guide to Goniometry. ed 3. Philadelphia, PA: FA Davis Co; 2003. [Google Scholar]
- 18.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. ed 2. Upper Saddle River, New Jersey: Prentice-Hall Inc; 2000. [Google Scholar]
- 19.Lenssen AF, van Dam EM, Crijns YH, et al. Reproducibility of goniometric measurement of the knee in the in-hospital phase following total knee arthroplasty. BMC Musculoskelet Disord. 2007;8:83. doi: 10.1186/1471-2474-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen TL, Christensen M, Christensen SS, et al. Reliability of knee joint range of motion and circumference measurements after total knee arthroplasty: Does tester experience matter? Physiother Res Int. 2010;15:126–134. doi: 10.1002/pri.450. [DOI] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy DM, Stratford PW, Wessel J, et al. Assessing stability and change of four performance measures: A longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord. 2005;6:3. doi: 10.1186/1471-2474-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther. 2010;40:559–567. doi: 10.2519/jospt.2010.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: A randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 25.Schurman DJ, Matityahu A, Goodman SB, et al. Prediction of postoperative knee flexion in Insall- Burstein II total knee arthroplasty. Clin Orthop Relat Res. 1998;353:175–184. doi: 10.1097/00003086-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Katz MM, Hungerford DS, Krackow KA, et al. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69:225–233. [PubMed] [Google Scholar]
- 27.Shoji H, Solomonow M, Yoshino S, et al. Factors affecting postoperative flexion in total knee arthroplasty. Orthopedics. 1990;13:643–649. doi: 10.3928/0147-7447-19900601-08. [DOI] [PubMed] [Google Scholar]
- 28.Naylor JM, Ko V, Rougellis S, et al. Is discharge knee range of motion a useful and relevant clinical indicator after total knee replacement? Part 1. J Eval Clin Pract. 2011;18:644–651. doi: 10.1111/j.1365-2753.2011.01655.x. [DOI] [PubMed] [Google Scholar]
- 29.Naylor JM, Ko V, Rougellis S, et al. Is discharge knee range of motion a useful and relevant clinical indicator after total knee replacement? Part 2. J Eval Clin Pract. 2011;18:652–658. doi: 10.1111/j.1365-2753.2011.01656.x. [DOI] [PubMed] [Google Scholar]
- 30.Farquhar S, Snyder-Mackler L. The Chitranjan Ranawat Award: The nonoperated knee predicts function 3 years after unilateral total knee arthroplasty. Clin Orthop Relat Res. 2010;468:37–44. doi: 10.1007/s11999-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizner RL, Petterson SC, Stevens JE, et al. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–1539. [PubMed] [Google Scholar]
- 32.Jones CA, Voaklander DC, Suarez-Alma ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83:696–706. [PubMed] [Google Scholar]