SUMMARY
Hedgehog (HH) proteins are proteolytically processed into a biologically active form, which is covalently modified by cholesterol and palmitate. However, most studies of HH biogenesis have characterized protein from cells in which HH is over-expressed. We purified Sonic Hedgehog (SHH) from cells expressing physiologically relevant levels, and showed that it was more potent than SHH isolated from over-expressing cells. Furthermore, the SHH in our preparations were modified with a diverse spectrum of fatty acids on their amino-termini, and this spectrum of fatty acids varied dramatically depending on the growth conditions of the cells. The fatty acid composition of SHH affected its trafficking to lipid rafts, as well as its potency. Our results suggest that HH proteins exist as a family of diverse lipid-speciated proteins, which might be altered in different physiological and pathological contexts to regulate distinct properties of HH proteins.
Keywords: Sonic Hedgehog, purification, fatty acids, lipids, covalent modifications
INTRODUCTION
The Hedgehog (HH) family of proteins play diverse biological roles that are conserved across different classes of animals (Ingham and McMahon, 2001). HH ligands are responsible for embryonic patterning as well as the maintenance, growth and renewal of various adult structures (Beachy et al., 2004; Ingham and McMahon, 2001). HH proteins harboring missense mutations have also been implicated in human developmental disorders (Bale, 2002), and reactivation of their expression is required for the viability of many cancers (Teglund and Toftgard, 2010). Biochemically, the most extensively studied HH protein is Sonic HH (SHH) (Farzan et al., 2008), which is produced as a ~45-kDa pre-pro-protein that contains an amino-terminal signal sequence targeting SHH to the secretory pathway (Chang et al., 1994; Echelard et al., 1993; Krauss et al., 1993; Riddle et al., 1993; Roelink et al., 1994). During SHH's intracellular trafficking this signal sequence is cleaved off (Bumcrot et al., 1995). A sixteen carbon fatty acid, palmitate (C16:0), was reported to modify this newly exposed amino-terminal cysteine via a stable amide bond (Pepinsky et al., 1998), in a reaction catalyzed by the SHH acyltransferase Skinny Hedgehog (Buglino and Resh, 2008; Chamoun et al., 2001). Although this palmitoylated, full-length form of SHH has substantial activity (Tokhunts et al., 2010), the bulk of SHH undergoes additional processing to yield a ~24 kDa amino-terminal form (Chang et al., 1994; Lopez-Martinez et al., 1995; Marti et al., 1995; Roelink et al., 1995). This latter processing step occurs in an intramolecular fashion, and results in the addition of cholesterol to the newly exposed carboxyl-terminal glycine via a labile ester bond (SHH-Np) (Lee, 1995; Lee et al., 1994; Porter et al., 1995).
Very little data regarding the biogenesis of endogenous HH proteins has been published, likely because of the scarcity of endogenous HH proteins and the difficulty of purifying and analyzing such hydrophobic proteins. What is known about the biogenesis of HH proteins is derived from studies where HH has been purified from cells engineered to vastly overexpress it (Pepinsky et al., 1998; Porter et al., 1996; Taipale et al., 2000), or from the analyses of recombinant HH proteins that lack their hydrophobic modifications (Lee et al., 1994; Pathi et al., 2001; Taylor et al., 2001). Here, we describe the purification of a potent form of SHH-Np from cells that express endogenous-like levels of SHH. Further, we show that this purified SHH-Np actually consists of a family of proteins modified at its amino-terminus by a diverse spectrum of saturated and unsaturated fatty acids, and that these novel modifications dictate the biology of HH proteins.
EXPEIRMENTAL PROCEDURES
Comparison of SHH-Np levels
Fertile, certified research grade, pathogen free eggs (Charles River) were incubated at 37.5° C. At Hamburger-Hamilton (H&H) developmental stage 22, embryos were isolated and limb buds resected as previously described (Zeng et al., 2001). Resected buds were further divided into SHH producing posterior and SHH negative anterior portions, and lysed by suspending in 1% Tx-100, 10 mM sodium phosphate, 150 mM NaCl buffer, pH 7.4, supplemented with protease inhibitors (Roche). Immunoblotting was performed using anti SHH-Np polyclonal H-160 antibodies (Santa-Cruz).
Purification of SHH-Np
SHH-I cells (Taipale et al., 2000) were washed with PBS, collected by scraping, dounce homogenized, and centrifuged at 100,000 × g for 1 hr. The resultant pellet was resuspended in buffer A, recentrifuged, and the membrane enriched pellet extracted twice with buffer B by dounce homogenization and centrifugation at 16,000 × g for 30 min. The supernatants were combined, pH adjusted to 5.0 with 1 M MES, and applied to a bulk SP Sepharose Fast Flow resin. This resin was washed once with buffer C, followed by a second wash with buffer D. SHH-Np was eluted from this resin using buffer E. The eluted fractions were adjusted to pH 7.2 with 1 M HEPES, and then passed through a 5E1 monoclonal antibody (mAb) column (Ericson et al., 1996). After washing the column with buffer F the SHH-Np was eluted with buffer G, and then immediately neutralized with 1 M HEPES, pH 7.4. Please refer to the Supplementary Materials for details of buffers A-G.
Purified SHH-Np was quantified after SDS-PAGE, comparing its concentration against a standard curve of recombinant SHH-II (R&D). This gel was subsequently protein stained using a SilverQuest staining kit (Invitrogen). The optical density of each stained protein was then calculated and compared using Image J software (NIH). SHH activity measurements were performed essentially as described, using NIH-3T3 cells expressing a HH reporter gene (Light-II cells) (Singh et al., 2009). SHH-Np dependent differentiation and gene expression were assayed using C3H10T1/2 cell line as previously described (Zeng et al., 2001). All activity measurements were done in triplicate, and each experiment was repeated at least three times. The activity data presented is shown as the mean and the standard deviation (SD) of one representative experiment.
Mass Spectrometry Analyses
The identity of the purified SHH-Np was validated using microcapillary LC/MS on a ThermoFinnigan LTQ ion trap mass spectrometer. For identification of fatty acid modifications, SHH-Np was reduced with 4 mM DTT, alkylated with 15 mM iodoacetamide, and EndoLysC digested. Lipid modified peptides were identified by tandem mass spectrometry analysis on a LTQ-Orbitrap (Thermo) interfaced with an Eksigent nanoLC-2D HPLC. Tandem mass spectra (MS/MS) were searched against the SHH protein sequence using a Mascot (v 2.1) error tolerant search with 20 ppm parent mass accuracy, and Inspect/MS-Alignment run in blind modification search mode (Tanner et al., 2006). All MS/MS spectra peptide assignments were manually verified for peptide assignments.
Lipid treatments
SHH-I cells were serum deprived for 6-7 hrs in 0.5% FBS. Prior to lipid treatment the cells were washed with PBS once, and then maintained in the presence of 100 μM fatty acids or DMSO (vehicle) for 16-18 hrs (Liang et al., 2001). For embryonic tissue studies, 10-12 pathogen free Hamburger-Hamilton (H&H) developmental stage 22 chick embryos (Charles River) were collected (Zeng et al., 2001), and posterior fragments of the limb buds dissected as previously described (Zeng et al., 2001). These posterior tissues were incubated with 100 μM fatty acids or DMSO (vehicle) for 16-18 hrs in 6 well plates. These tissues were subsequently washed twice in ice cold PBS and homogenized in a 1% Tx-100, 10 mM sodium phosphate,150 mM NaCl buffer, pH 6.5.
OptiPrep density gradient ultracentrifugation
Cell or tissue extract was mixed with OptiPrep medium to obtain a 40% fraction. Then two other fractions, composed of 25% and 10% OptiPrep medium or 30% and 0% for tissue extract, sequentially layered on top followed by centrifugation at 120,000 × g, or 160,000 × g for tissue extract, for 21 hrs (Bruses et al., 2001; Chen et al., 2004; Lisanti et al., 1994). Fractions from the tubes were collected and subjected to SDS-PAGE analysis.
RESULTS
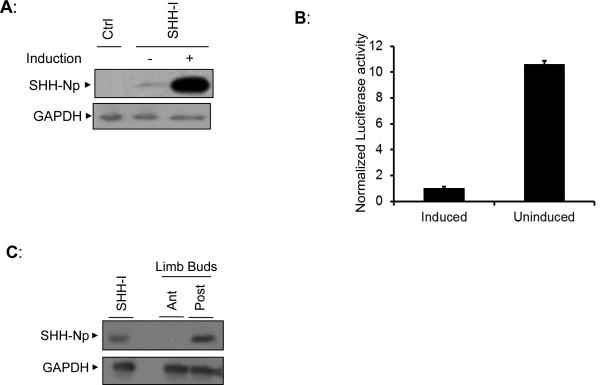
To identify a cell-line expressing low levels of SHH, we first compared the steady-state levels of SHH-Np for a previously described cell line (SHH-I cells) that expresses SHH under control of a muristerone inducible promoter (Taipale et al., 2000) (Fig. 1A and Fig S1A). SHH-I cells produced low levels of SHH-Np in the absence of induction, presumably due to the promiscuity of such inducible promoters, and these levels increased approximately twenty-fold in the presence of muristerone. We next compared the activity of SHH-Np from cell lysates obtained with or without muristerone treatment, measuring the ability of similar amounts of SHH-Np to activate an engineered SHH reporter cell line (Light-II cells) driving Firefly luciferase expression (Taipale et al., 2000) (Fig. 1B and Fig S1B). The normalized potency of SHH-Np produced under uninduced conditions was significantly higher than that produced when its expression was induced by muristerone. To compare the levels of SHH-Np produced by SHH-I cells to that produced in a physiologically relevant setting (Riddle et al., 1993), we compared the steady-state levels of SHH-Np from uninduced SHH-I cells to dissected posterior and anterior halves of chick limb buds (Fig. 1C). SHH-Np levels were similar for uninduced SHH-I cells and posterior chick limb bud tissue.
Figure 1. SHH-I cells produce endogenous-like levels of potent SHH-Np.
(A) An immunoblot showing SHH-Np abundance in SHH-I cells, under conditions in which its expression was induced (+) or uninduced (−) with muristerone. SHH-I parental cells, which are not engineered to express SHH, were used as a control (Ctrl). GAPDH was used to verify protein normalization. (B) An aliquot of SHH-I cellular lysate was tested for SHH-Np associated activity using the Light-II reporter cell line. SHH-Np activity measurements were carried out in the linear range of this assay (see Figure S1B), and these activity results were then normalized to overall SHH-Np levels to determine potency. Error bars represent the standard deviation (SD) in one representative experiment. (C) SHH-Np levels from uninduced SHH-I cells and chick embryo limb buds were compared by immunoblotting. During development SHH-Np is produced in the posterior portion of limb buds (Post). Here, the anterior (Ant) portion of limb buds serves as a negative control for SHH-Np. As only approximately 20% of the posterior tissue consists of SHH producing cells (Riddle et al., 1993), we mixed 20% of SHH-I cell lysate with 80% of lysate from anterior limb bud tissue for this comparison. GAPDH was used to verify normalization.
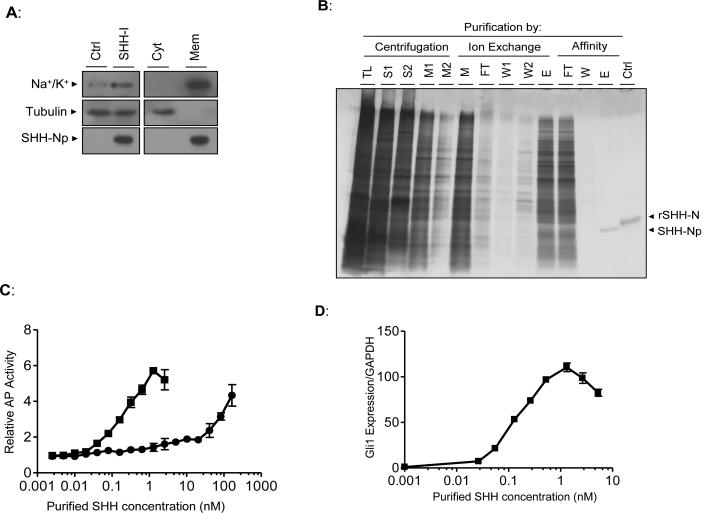
Using published purification protocols (Pepinsky et al., 1998; Taipale et al., 2000) we were unable to purify SHH-Np from uninduced SHH-I cells expressing such low-levels of SHH. Thus, we modified these procedures to maximize the yield of active SHH-Np from such cells. Uninduced SHH-I cells were dounce homogenized in an isotonic, detergent free buffer (Fig. 2A) to obtain a total lysate. This cellular lysate was further fractionated by centrifugation at 100,000 × g, producing cytoplasmic and membrane enriched fractions. Similarly prepared lysates of SHH-I parental cells, which do not express detectable amounts of SHH were used as a negative control. The bulk of SHH-Np was in the membrane fraction, consistent with previous reports (Taipale et al., 2000). Aliquots of these lysates, along with the cytoplasmic or membrane enriched fractions of these cells, were volume normalized then incubated with Light-II cells to estimate the levels of active SHH in each fraction (data not shown). The bulk of SHH activity was also found in the membrane enriched pellet.
Figure 2. SHH-Np purified from low-level SHH expressing cells is highly active.
(A) SHH-I cells, or the SHH-I parental cell line (Ctrl), were dounce homogenized under isotonic conditions and total lysate (left panel) separated by ultracentrifugation at 100,000 × g (right panel) to generate a cytosol-enriched fraction (Cyt) and a membrane-enriched fraction (Mem). These fractions were volume normalized to that of the original cellular lysate and immunoblotted as indicated. Tubulin serves as a cytosolic protein control while the Na+/K+ transporter serves as a membrane protein control. (B) Aliquots of the indicated fractions from various steps of SHH-Np purification were separated by SDS-PAGE, followed by visualization of proteins by silver staining (TL: total lysate, S: 100,000 × g supernatant, M: combined 100,000 × g pellet detergent extract, FT: non-bound material, W: column wash, E: column eluate). Recombinant, unmodified, SHH-N is shown as a control (rSHH-N). Electrophoretic retardation of rSHH-N, relative to cholesterol modified SHH-Np, has been previously noted (Lee et al., 1994). (C) The indicated amounts of purified SHH-Np were incubated with C3H10T1/2 fibroblasts, which differentiate into osteoblasts in response to SHH (squares: purified SHH-Np, circles: rSHH-N). Alkaline phosphatase activity, which is an indirect, quantitative measurement of this differentiation, was then measured. (D) The indicated amounts of purified SHH-Np were incubated with C3H10T1/2 fibroblasts, followed by RNA extraction. The levels of Gli1 and GAPDH were then determined by q-RT-PCR. Error bars represent the SD in one representative experiment.
Detergent extraction of the membrane fraction and purification by centrifugation, ion exchange chromatography and affinity chromatography (see Supplementary Experimental Procedures) resulted in 5 ng of purified SHH-Np per mg of total cellular lysate (Fig. 2B). We estimate the purity of this preparation to be greater than 95%, representing a 200,000-fold purification. The identity of the purified SHH-Np was confirmed by tandem mass spectrometry (data not shown). The vast majority of recovered peptides were derived from the amino-terminal domain of SHH, with a coverage against the predicted amino-acid sequence of SHH-N approaching 90%. To compare the potency of the SHH-Np isolated here to those previously described (Pathi et al., 2001; Pepinsky et al., 1998; Taipale et al., 2000) we assayed the differentiation of C3H10T1/2 embryonic fibroblasts into osteoblasts (Kinto et al., 1997). The EC50 of SHH-Np purified from uninduced SHH-I cells was 0.3 nM, while the EC50 of recombinant SHH-N was 60 nM (Fig. 2C). We also quantified the expression of the SHH target gene Gli1 as an indicator of activity (Ingram et al., 2002), treating C3H10T1/2 cells with purified SHH-Np (Fig. 2D). From this analysis we estimated the EC50 of purified SHH-Np to be 0.2 nM. In both of these assays the potency of SHH-Np was significantly greater than previously reported (Pathi et al., 2001; Pepinsky et al., 1998; Taipale et al., 2000). Purified SHH-Np was also able to stimulate the proliferation of primary cerebellar granular neuron precursor cells (GPC) (Dahmane and Ruiz i Altaba, 1999), confirming its activity (Fig. S2). Thus, our SHH-Np purification protocol isolates biologically active, potent SHH-Np, from cells expressing endogenous-like levels of SHH.
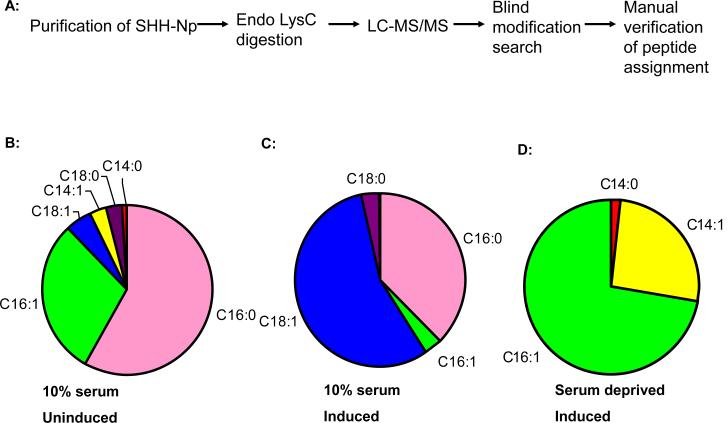
We speculated that the increased potency of SHH-Np purified from uninduced SHH-Np cells might result from differential amino-terminal fatty acid modifications, which can alter the activity of recombinant SHH-N in vitro (Pathi et al., 2001; Taylor et al., 2001). To investigate this possibility we analyzed Lys-C digested, purified SHH-Np by LC-MS/MS using a high-resolution LTQ Orbitrap mass spectrometer. The mass/charge ratios obtained during these analyses were cross-referenced against expected unmodified masses of individual peptides, and the MS/MS of modified forms validated manually (Table S1 and Fig. S3A-C). Contrary to previous reports (Pepinsky et al., 1998; Taipale et al., 2000), we identified a diverse assortment of saturated and unsaturated fatty acid modifications on SHH-Np. Based on extracted ion chromatogram (XIC) peak areas, the most abundant fatty acid-modified forms of SHH-Np were modified with palmitate (C16:0), a palmitoleoyl (C16:1), followed by a stearoleyl (C18:1), a myristoleyl (C14:1), and then to a lesser degree, a stearoyl (C18:0) and myristoyl (C14:0) groups. A number of amino-terminal peptides showed masses encompassing as yet undetermined modifications, consistent with SHH-Np being modified by a diversity of lipid species.
To extend these findings we also purified SHH-Np from SHH-I cells induced to express higher levels of SHH: 1) in the presence of 10% FBS, 2) in the presence serum free media, and 3) in the presence of serum free media supplemented with myristate (C14:0). The pattern of lipid speciation on SHH-Np changed significantly under all of these conditions, suggesting that the lipid speciation of SHH is very sensitive to cellular context (Fig. 3). We noted that unsaturated fatty acid modified SHH-Np is the dominant species regardless of the cellular context, whereas the abundance of SHH-Np forms modified with saturated fatty acids were significantly more variable. Interestingly, the fatty acid modifications on SHH-Np isolated from cells expressing high levels of SHH, grown in FBS, approximately mirrored the abundance of fatty acids found in the membranes of cells grown in FBS, whereas the lipid speciated forms of SHH-Np purified from cells grown under the other conditions did not. We further noted that SHH-Np isolated from cells grown under serum free conditions but supplemented with myristate (C14:0) showed an ~500% increase of myristate modified SHH-Np (data not shown), consistent with us being able to experimentally manipulate the fatty acid speciation of SHH-Np. Significantly, we did not detect an amino-terminal peptide lacking fatty acid modifications in these experiments. Although MS is not generally quantitative, the abundance of the same peptide in different samples may be quantitatively compared (Old et al., 2005). Therefore, the absence of an unmodified amino-terminal peptide in our extracted chromatograms suggests that SHH-Np is quantitatively modified by fatty acids in these cells.
Figure 3. Fatty acid speciation of SHH-Np is cell context dependent.
(A): A schematic showing the procedure used to identify the fatty acid modification on SHH-Np. (B-D): Pie charts showing the relative abundance of lipid species identified on SHH-Np purified isolated under three different cell contexts: 10% FBS without muristerone induction of SHH expression , 10% FBS and muristerone induction of SHH expression, and serum deprivation and muristerone induction of SHH expression.
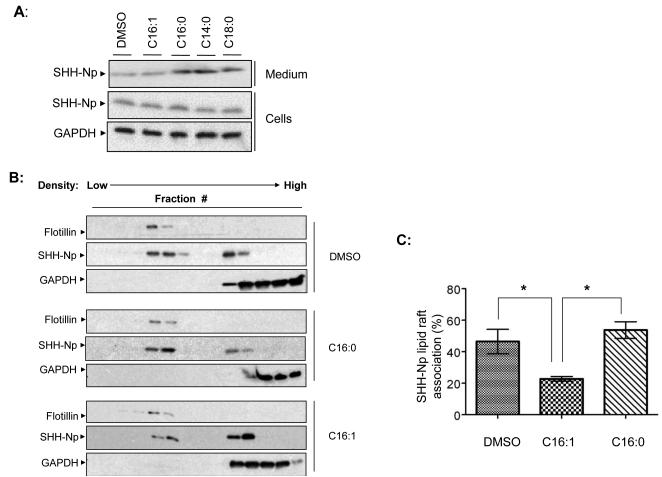
The hydrophobic properties of the various fatty acid modifications we observed on SHH-Np vary over a 500-fold range (Table S1), suggesting that they would alter the biological properties of SHH-Np. Further, unsaturated fatty acids, such as those found on SHH-Np, are known to segregate away from lipid rafts (Levental et al., 2010), where HH proteins are thought to enrich as part of its regulated intracellular movement (Callejo et al., 2011; Creanga et al., 2012; Mao et al., 2009; Rietveld et al., 1999; Taipale et al., 2000). To test this hypothesis, we altered the fatty acid composition of media used with cells or chick limb bud explants expressing SHH, and then measured various properties of the resulting SHH-Np. Such lipid doping experiments have previously been used to alter the covalent lipid modifications of numerous proteins (Hashimoto et al., 2004; Liang et al., 2001; Wolven et al., 1997), prior to determining their changes in biological function. We therefore incubated serum-deprived SHH-I cells with saturated C14:0, C16:0, C18:0, or unsaturated C16:1 fatty acids, and examined the levels of SHH-Np in both cell lysates and secreted from those cells into conditioned media. While we did not observe changes in the absolute levels of cell associated SHH-Np when cells were doped with different fatty acids, consistent with previous reports (Bumcrot et al., 1995), we did observe differences in the secretion of SHH-Np forms from cells incubated with different fatty acids (Fig. 4A).
Figure 4. Fatty acid speciation of SHH-Np alters its lipid raft enrichment.
(A) An immunoblot of cell lysates and conditioned media, from SHH-I cells incubated in the presence of indicated fatty acids. GAPDH served as a normalization control for cellular lysates. The same volume of conditioned medium was subjected to TCA precipitation prior to loading. (B) Lysates from SHH-I cells incubated with the indicated lipids, or DMSO control, were separated over an OptiPrep gradient to isolate a lipid raft enriched fraction. Fractions from these various OptiPrep density gradients were resolved by SDS-PAGE then analyzed by immunoblotting for SHH-Np, GAPDH as a cytoplasmic protein marker, or the lipid raft marker flotillin. Note that flotillin localization does not change with various lipid additions. Quantification of SHH-Np lipid raft enrichment from cells incubated with the indicated fatty acids is shown in (C). Error bars represent the standard error of the mean (SEM) of three independent experiments. p values ≤ 0.05 are considered statistically significant, and indicated by a asterisk.
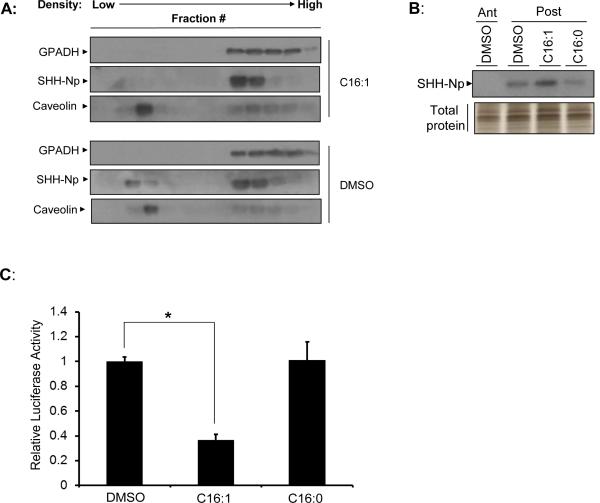
Because lipid raft localization is thought to be a prerequisite for the secretion of HH proteins (Callejo et al., 2011; Creanga et al., 2012; Rietveld et al., 1999), we asked whether the various fatty acid modified SHH-Np forms would differentially localize to lipid rafts. The cellular lysates of cells incubated with various fatty acids were therefore fractionated over an OptiPrep gradient to isolate lipid raft enriched fractions (Fig. 4B, and data not shown). Incubation of uninduced SHH-I cells with any of the tested saturated fatty acids (C14:0, C16:0 or C18:0) increased the percentage of SHH-Np enriched in lipid rafts (Fig. 4C). In contrast, incubation of SHH-I cells with palmitoleate (C16:1) reduced the percentage of SHH-Np enriched in lipid rafts. Similar experiments were performed on anterior or posterior chick limb bud explants. Consistent with our SHH-I cell based observations, treatment of the explants with unsaturated C16:1 resulted in decreased SHH-Np localization to lipid rafts in posterior tissue (Fig. 5A). While incubation of tissue explants with saturated fatty acids had no effect on steady-state SHH-Np levels, increased levels of tissue SHH-Np were observed upon palmitoleoyl (C16:1) incubation. The mRNA levels of SHH were unchanged by different fatty acid incubation (data not shown). These results are consistent with decreased secretion of palmitoleoyl (C16:1) modified SHH-Np, resulting in increased retention in posterior limb bud tissue. We further measured the activity of SHH-Np from these tissues and normalized this activity to their relative abundance (Fig. 5B and Fig. 5C). This analysis showed that incubation of posterior limb bud fragments with C16:1 reduced SHH-Np activity.
Figure 5. Modification of SHH-Np with distinct fatty acids alters its functionality.
(A) Lysates from embryonic limb bud explants exposed to different fatty acids were separated over an OptiPrep density gradient to isolate the lipid raft fraction. Various gradient fractions were resolved by SDS-PAGE than analyzed by immunoblotting for SHH-Np. Caveolin-1 was used as a lipid raft marker while GAPDH served to label non-lipid raft associated subcellular fractions. Treatment with saturated fatty acids did not change SHH-Np localization pattern over the DMSO control (data not shown). (B) An immunoblot (upper panel) of limb bud lysates showing effect of lipid modifications on SHH-Np levels. Lysates contain similar amount of total protein as indicated by total protein silver staining (lower panel). (C) The potency of SHH-Np was determined by incubating lysate from treated limb buds with Light-II cells, and then normalizing this activity to SHH-Np levels. Error bars represent the SEM of three independent experiments. p values ≤ 0.05 are considered statistically significant, and indicated by an asterisk.
DISCUSSION
We now demonstrate that SHH-Np is actually a family of distinct lipid speciated forms, which exhibit a variety of differential properties. Thus we favor the idea that modification of SHH-Np by a spectrum of fatty acids provides another biologically relevant layer of SHH-Np regulation. Although the spectrum of fatty acid modifications on SHH-Np was not initially described, a similar spectrum of modifications for a small percentage of the SHH mutant SHH-N, which is a non-physiologically relevant form of SHH that is not cholesterol modified, was described (Pepinsky et al., 1998). We speculate that differences in purification protocols or levels of expression resulted in the identification of only the most abundant, palmitoyl-modified form of SHH-Np in these previous reports. For example, one of the previous purification protocols for SHH-Np started with a lipid raft enriched fraction (Taipale et al., 2000) , which based on our data might exclude SHH-Np modified by unsaturated fatty acids. How such differential forms of SHH-Np might arise is also not yet clear. However, in vitro, Skinny Hedgehog is able to utilize a wide spectrum of fatty acids, many of which have a higher affinity for Skinny Hedgehog than palmitate (Buglino and Resh, 2008). Interestingly, the potency of recombinant forms of SHH-N is altered when it is modified by different fatty acids in vitro (Taylor et al., 2001). This observation is consistent with Skinny Hedgehog being sufficient to modify SHH with the diverse spectrum of fatty acids described here.
Our findings suggest that one consequence of SHH-Np's fatty acid speciation is the regulation of its intracellular trafficking, with decreased localization of SHH-Np modified with unsaturated fatty acids to lipid rafts. This decreased localization is likely the result of unsaturated fatty acids lacking the compactness required to enrich in lipid raft compartments of cellular membranes (Levental et al., 2010), although such fatty acid doping experiments likely result in the production of a number of different fatty acylated species. However, a simple differential localization of SHH-Np proteins to the lipid raft or non-lipid raft compartments of cellular membranes could arise solely by regulating the degree of fatty acid saturation on SHH-Np. One observed functional consequence of this differential localization is the secretion of lipid raft localized SHH-Np forms and retention of raft excluded forms. Such SHH-Np speciation might then contribute to the gradient of SHH-Np observed in vivo (Gritli-Linde et al., 2001), with SHH-Np modified with the least hydrophobic fatty acids moving further away from the SHH producing cells than SHH-Np family members modified with more hydrophobic fatty acids. In such a scenario, different fatty acid modifications might modulate SHH-Np's affinity for the various lipoprotein complexes suggested to regulate the movement of HH proteins (Callejo et al., 2008; Gradilla et al., 2014; Guerrero and Chiang, 2007; Matusek et al., 2014; Palm et al., 2013; Therond, 2012; Zeng et al., 2001). Alternatively, fatty acid speciation of SHH-Np might alter its targeting “barcode” (Kornberg, 2011), allowing it to associate with diverse types of lipid microdomains enriched on the cytonemes responsible for HH movement (Fifadara et al., 2010; Gupta and DeFranco, 2003; Kornberg, 2013; Sanders et al., 2013). In either model, the fatty acid speciation of SHH described here might be utilized to encode dramatic changes in cellular growth and metabolism, such as those occurring during early development or cancer, directly into the HH proteins regulating these biological processes.
Supplementary Material
List of Changes.
Editing Title into 3 lines of text at no more than 50 characters per line
Reduction of Abstract size to comply with the restriction (less than 150 words)
Moving Table 1 to supplemental materials as Table S1 to comply with the restriction of the number of Figures/tables for a Report (no more than 5)
Editing Experimental Procedures of SHH-Np purification and Mass Spectrometry
Reduction of manuscript length to comply with the general length restriction for a Report (less than 42,000 characters)
ACKNOWLEDGEMENTS
We thank Drs. Murray Deuscher, Nadia Dahmane, Tom Kornberg, Stacey Ogden and Karoline Brigel, for insightful discussions during the course of this work. We also thank the members of the Capobianco and Robbins laboratories for their insightful advice. This work was supported by NIH grants GM64011 (DJR), NIH UL1 TR000154 and CA155453 (WMO), and CA118972 (NGA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Jun Long performed the purification of SHH from different cellular contexts, and performed experiments to determine SHH's potency lipid raft association. Robert Tokhunts optimized the purification protocol, and performed additional experiments to determine SHH potency. Will Old performed LC-MS/MS on purified SHH and identified the various fatty acid modifications on SHH described here.
REFERENCES
- Bale AE. Hedgehog signaling and human disease. Annual review of genomics and human genetics. 2002;3:47–65. doi: 10.1146/annurev.genom.3.022502.103031. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Bruses JL, Chauvet N, Rutishauser U. Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:504–512. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. The Journal of biological chemistry. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Molecular and cellular biology. 1995;15:2294–2303. doi: 10.1128/mcb.15.4.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo A, Bilioni A, Mollica E, Gorfinkiel N, Andres G, Ibanez C, Torroja C, Doglio L, Sierra J, Guerrero I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo A, Culi J, Guerrero I. Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:912–917. doi: 10.1073/pnas.0705603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Chang DT, Lopez A, von Kessler DP, Chiang C, Simandl BK, Zhao R, Seldin MF, Fallon JF, Beachy PA. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes & development. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes & development. 2012;26:1312–1325. doi: 10.1101/gad.191866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development (Cambridge, England) 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Singh S, Schilling NS, Robbins DJ. The adventures of sonic hedgehog in development and repair. III. Hedgehog processing and biological activity. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G844–849. doi: 10.1152/ajpgi.00564.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifadara NH, Beer F, Ono S, Ono SJ. Interaction between activated chemokine receptor 1 and FcepsilonRI at membrane rafts promotes communication and F-actin-rich cytoneme extensions between mast cells. International immunology. 2010;22:113–128. doi: 10.1093/intimm/dxp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilla AC, Gonzalez E, Seijo I, Andres G, Bischoff M, Gonzalez-Mendez L, Sanchez V, Callejo A, Ibanez C, Guerra M, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nature communications. 2014;5:5649. doi: 10.1038/ncomms6649. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Developmental biology. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Guerrero I, Chiang C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol. 2007;17:1–5. doi: 10.1016/j.tcb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Molecular biology of the cell. 2003;14:432–444. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Matsuda T, Matsuura Y, Haga T, Fukada Y. Production of N-lauroylated G protein alpha-subunit in Sf9 insect cells: the type of N-acyl group of Galpha influences G protein-mediated signal transduction. Journal of biochemistry. 2004;135:319–329. doi: 10.1093/jb/mvh039. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes & development. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–8205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- Kinto N, Iwamoto M, Enomoto-Iwamoto M, Noji S, Ohuchi H, Yoshioka H, Kataoka H, Wada Y, Yuhao G, Takahashi HE, et al. Fibroblasts expressing Sonic hedgehog induce osteoblast differentiation and ectopic bone formation. FEBS letters. 1997;404:319–323. doi: 10.1016/s0014-5793(97)00014-8. [DOI] [PubMed] [Google Scholar]
- Kornberg TB. Barcoding Hedgehog for intracellular transport. Science signaling. 2011;4:pe44. doi: 10.1126/scisignal.2002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TB. Cytonemes extend their reach. The EMBO journal. 2013 doi: 10.1038/emboj.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lee JJ. Autoproteolysis in Hedgehog Protein Biogenesis (Vol 266, Pg 1528, 1994). Science. 1995;267:777–777. doi: 10.1126/science.7985023. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science. 1994;266:1528–1537. doi: 10.1126/science.7985023. [DOI] [PubMed] [Google Scholar]
- Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- Liang XQ, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. Journal of Biological Chemistry. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang ZL, Hermanowskivosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of Caveolin-Rich Membrane Domains Isolated from an Endothelial- Rich Source-Implications for Human-Disease. Journal of Cell Biology. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martinez A, Chang DT, Chiang C, Porter JA, Ros MA, Simandl BK, Beachy PA, Fallon JF. Limb-patterning activity and restricted posterior localization of the amino-terminal product of Sonic hedgehog cleavage. Current biology : CB. 1995;5:791–796. doi: 10.1016/s0960-9822(95)00156-4. [DOI] [PubMed] [Google Scholar]
- Mao H, Diehl AM, Li YX. Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Laboratory investigation; a journal of technical methods and pathology. 2009;89:290–300. doi: 10.1038/labinvest.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bumcrot DA, Takada R, McMahon AP. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- Matusek T, Wendler F, Poles S, Pizette S, D'Angelo G, Furthauer M, Therond PP. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516:99–103. doi: 10.1038/nature13847. [DOI] [PubMed] [Google Scholar]
- Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Molecular & cellular proteomics : MCP. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- Palm W, Swierczynska MM, Kumari V, Ehrhart-Bornstein M, Bornstein SR, Eaton S. Secretion and Signaling Activities of Lipoprotein-Associated Hedgehog and Non-Sterol-Modified Hedgehog in Flies and Mammals. PLoS biology. 2013;11 doi: 10.1371/journal.pbio.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, Bumcrot D, Tabin CJ, Pepinsky RB, Williams KP. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mechanisms of Development. 2001;106:107–117. doi: 10.1016/s0925-4773(01)00427-0. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. The Journal of biological chemistry. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Neutz S, Simons K, Eaton S. Association of sterol-and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. Journal of Biological Chemistry. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013 doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Tokhunts R, Baubet V, Goetz JA, Huang ZJ, Schilling NS, Black KE, MacKenzie TA, Dahmane N, Robbins DJ. Sonic hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner. Human Genetics. 2009;125:95–103. doi: 10.1007/s00439-008-0599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Tanner S, Pevzner PA, Bafna V. Unrestrictive identification of post-translational modifications through peptide mass spectrometry. Nature Protocols. 2006;1:67–72. doi: 10.1038/nprot.2006.10. [DOI] [PubMed] [Google Scholar]
- Taylor FR, Wen DY, Garber EA, Carmillo AN, Baker DP, Arduini RM, Williams KP, Weinreb PH, Rayhorn P, Hronowski XP, et al. Enhanced potency of human sonic hedgehog by hydrophobic modification. Biochemistry. 2001;40:4359–4371. doi: 10.1021/bi002487u. [DOI] [PubMed] [Google Scholar]
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochimica et biophysica acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Therond PP. Release and transportation of Hedgehog molecules. Current opinion in cell biology. 2012;24:173–180. doi: 10.1016/j.ceb.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Tokhunts R, Singh S, Chu T, D'Angelo G, Baubet V, Goetz JA, Huang Z, Yuan Z, Ascano M, Zavros Y, et al. The full-length unprocessed hedgehog protein is an active signaling molecule. The Journal of biological chemistry. 2010;285:2562–2568. doi: 10.1074/jbc.M109.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Molecular biology of the cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Goetz JA, Suber LM, Scott WJ, Jr., Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.