Abstract
Objective
A shift toward later initiation of cervical cancer screening for women began in 2002. We generated national estimates of screening prevalence rates and guideline-consistent screening among U.S. women ages 15–29 before and after the first evidence-based recommendations for reduced cervical cancer screening.
Method
We used National Survey of Family Growth data to compare self-reported cervical cancer screening in 2002 and 2006–2008, stratified by age (15–17, 18–20, 21–29) and sexual activity. We also assessed receipt of guideline-consistent screening by selected demographic variables.
Results
Among females ages 15–17, the proportion screened decreased from 23% to 12%, and screening was significantly more likely to be guideline-consistent. Among females ages 18–20, 24% were screened too early in 2006–2008, but among those not yet sexually active, screening declined to 8%, appropriately reflecting new guidelines. In multivariable analysis, private health insurance, pregnancy, and hormonal contraceptive use were associated with guideline-consistent screening among sexually-active women.
Conclusion
Fewer adolescents were being screened before sexual initiation, representing newer guidelines. However, sexually-active young adult women also should have later screening initiation. Factors related to health care access contribute to receipt of screening. Monitoring and provider education are needed to improve guideline-consistent screening, as newer guidelines call for less screening.
Keywords: cervical cancer screening, clinical recommendations, guideline-consistent, adolescents, young adults, national surveillance
1. Introduction
Until 2002, most U.S. cervical cancer screening guidelines recommended women at average risk for cervical cancer begin annual screening at the onset of sexual intercourse or by age 18 (ACOG 1995; Smith et al. 2000; USPSTF 1996). These guidelines began to change in 2002 due to evidence concerning the transient nature of human papillomavirus (HPV) infections in adolescents and young adults, low incidence of cervical cancer among women less than 25 years old, and harms associated with overtreatment of precancerous lesions that would not necessarily progress (Arbyn 2008; Kyrgiou 2006; Watson 2008). In 2003, the United States Preventive Services Task Force issued a recommendation that women begin annual cervical cancer screening within 3 years of sexual initiation, or by age 21 (USPSTF 2003). Other professional and international organizations, including the American Cancer Society (ACS) (Saslow et al. 2002), American College of Obstetricians and Gynecologists (ACOG 2003), and the International Agency for Research on Cancer (IARC 2005) similarly updated their evidence and clinical recommendations.
These changes in screening guidelines have significant implications for women’s health care. Monitoring progress in the implementation of the revised clinical guidelines may help in identifying women least likely to receive recommended screening and to identify others who are likely to be over-screened for whom different preventive services should be prioritized. We therefore generated nationally representative estimates of the percentage of U.S. women aged 15–29 who were screened in accordance with screening guidelines in 2002 (before the guideline revisions) and in 2006–2008 (after the revisions), and identify factors associated with guideline-consistent screening.
2. Materials and Methods
We analyzed data from the 2002 and 2006–2008 National Survey of Family Growth (NSFG), when initial transitions toward later initiation of screening for cervical cancer occurred.
The NSFG is an in-person, population-based survey of U.S. women and men of reproductive age (15–44 years). The 2006–2008 NSFG data are comparable with data collected during earlier NSFG cycles, although the 2006–2008 survey was based on a continuous interviewing design (Groves et al. 2009). For both, interviews were conducted with computer-assisted personal interview technology, with more sensitive survey items administered via an audio-assisted computer self-interview. Blacks, Hispanics, and women aged 15–19 were oversampled for more reliable estimation. The 2002 NSFG collected data from 7,643 women and had a response rate of 80% (11, 12). The 2006–2008 NSFG collected data from 7,356 women and had a response rate of 76% (Lepkowski et al. 2006). The NSFG includes items asking women whether they had had a Papanicolaou (Pap) test in the previous 12 months, whether they have had vaginal heterosexual intercourse, and, if so, their age at the time of first sex. With this information, we constructed screening categories reflecting consistency with the dominant clinical guidelines in effect for 2002 and 2006–2008 for women aged 15–29. Women older than age 29 were not included because guidelines shifted to longer intervals after three normal tests, and the NSFG asked only about Pap testing in the prior year.
Measures
Our main outcome measure, whether women had had a Pap test in the previous year, was based on responses to the question, “In the past 12 months, have you received a Pap smear?” Other relevant measures included age at first vaginal intercourse (first sex), coded as never had sex, initiated sex in the past 36 months, initiated sex over 36 months ago.
We constructed a variable indicating whether women received a Pap test in accordance with screening guidelines, received a Pap test too early, or did not receive a recommended Pap test (Table 1). Survey respondents were classified as having received guideline-consistent care in 2002 if they 1) were aged 15–17, had not had sex, and had not received a Pap test or 2) were either sexually active or aged 18–29 and had received a Pap test. Respondents to the 2006–2008 NSFG were classified as having received guideline-consistent care if they 1) were aged 15–20, had either never had sex or first had sex less than 3 years earlier, and had not had a Pap test or 2) first had sex more than 3 years earlier or were aged 21–29 and had received a Pap test.
Table 1.
Classification of cervical cancer screening guideline-consistency for 2002 and 2006–2008, United States National Survey of Family Growth data by age and sexual activity
| Guideline-consistent | Too early | Missed | ||||
|---|---|---|---|---|---|---|
|
Based on clinical recommendations from 1995–20001 (2002 NSFG) |
Had Pap test | Had Pap test | No Pap test | |||
| • | Ages 15–17 and sexually active |
• | Ages 15–17 and not sexually active |
• | Ages 15–17 and sexually active |
|
| • | Ages 18–29, regardless of sexual activity status |
• | Ages 18–29 | |||
| No Pap test | ||||||
| 15–17 and not sexually active |
||||||
|
Based on clinical recommendations from 2002–2003 2 (2006–2008 NSFG) |
Had Pap test | Had Pap test | No Pap test | |||
| • | Ages 15–20 and ≥ 3 years from first intercourse |
• | Ages 15–20 and <3 years from first intercourse or never had sex |
• | Ages 15–20 and ≥ 3 years from first intercourse |
|
| • | Ages 21–29 | • | Ages 21–293 | |||
|
No Pap test |
||||||
| • | Ages15–20 and < 3 years from first intercourse or never had sex |
|||||
ACOG: Recommendations on frequency of Pap test screening. American College of Obstetrics & Gynecology Opinion Committee on Gynecologic Practice; 1995. USPSTF: U.S. Preventive services task force guide to clinical preventive services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996. ACS: American cancer society guidelines the early detection of cancer; 2000.
ACOG: Practice Bulletin No. 45: Cervical cytology screening; 2003. USPSTF: Screening cervical cancer, recommendations and rationale; 2003. http://www.uspreventiveservicestaskforce.org/3rduspstf/cervcan/cervcanrr.htm ACS: American cancer society guideline for the early detection of cervical neoplasia and cancer; 2002.
Missed screening for 21–29 year olds in 2006–2008 was defined according to ACOG and USPSTF recommendations for conventional cytology. Some women in this category could have received guideline-consistent screening based on ACS guidelines for liquid-based cytology every other year.
We used the Behavioral Model of Health Services Use (Andersen, 1995, 2008) to guide analysis of factors associated with receipt of guideline-consistent screening. The model relates predisposing, enabling, need, and contextual variables to the use of health services. The predisposing variables included race/ethnicity (white, black, Hispanic, other), maternal education level as a marker of socioeconomic status (the adolescents in our analysis have not yet completed their educations) (Trotter 2010), and U.S. nativity. Enabling measures of health care access were health insurance coverage (private or public, coverage gaps in the past year). Reproductive need and context variables were age at first sex, pregnancy in the previous 12 months, number of sexual partners in the previous 12 months, and use of a provider-dependent birth control method (a marker for reproductive health services use) (Schwarz 2005).
Statistical analysis
Data from 2002 were compared to data from 2006–2008 since these surveys occurred soon after changes to cervical cancer screening guidelines. Our analysis is limited to woman ages 15–29 to capture key time periods referenced in screening recommendations, including the age of sexual initiation. In the analytic subsamples, in 2002 there were 3,809 women and in 2006–2006 there were 4,047 women. Pap test among women who have not had sex and women who have had sex were compared and the percentage of women reporting a Pap test according to age and the timing of first sex was calculated.
The proportion of women having early, on time, or missed Pap testing for the two cycles of data was compared across the covariates described above. Design-based Pearson chi-squared tests for independence were used to test the significance of differences within data crosssections. Differences from 2002 to 2006–2008 were tested with standard two-tailed t-tests using design-based point estimates and standard errors. Multivariable logistic regression models were constructed to examine adjusted associations of sociodemographic, health care access, and reproductive characteristics on the receipt of guideline-consistent Pap testing. Sexually active women were included in the multivariable analysis so that adjusted relationships with reproductive health context factors such as recent pregnancy and contraceptive use could be considered.
Data were weighted to be representative of the U.S. population 15 to 29 years of age. To take into account the complex survey design of the NSFG, producing standard errors that account for clustering, stratification, and weighting of the data, we used SAS (version 9.2, SAS Institute, Cary, NC) and Sudaan (version 10, RTI International, Research Triangle Park, NC). The National Center for Health Statistics IRB has reviewed and approved the National Survey of Family Growth protocol.
3. Results
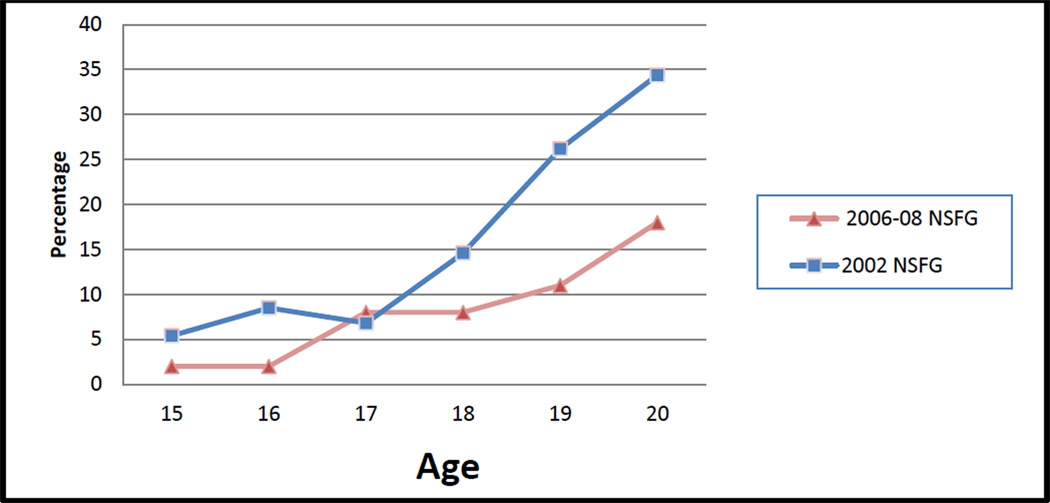
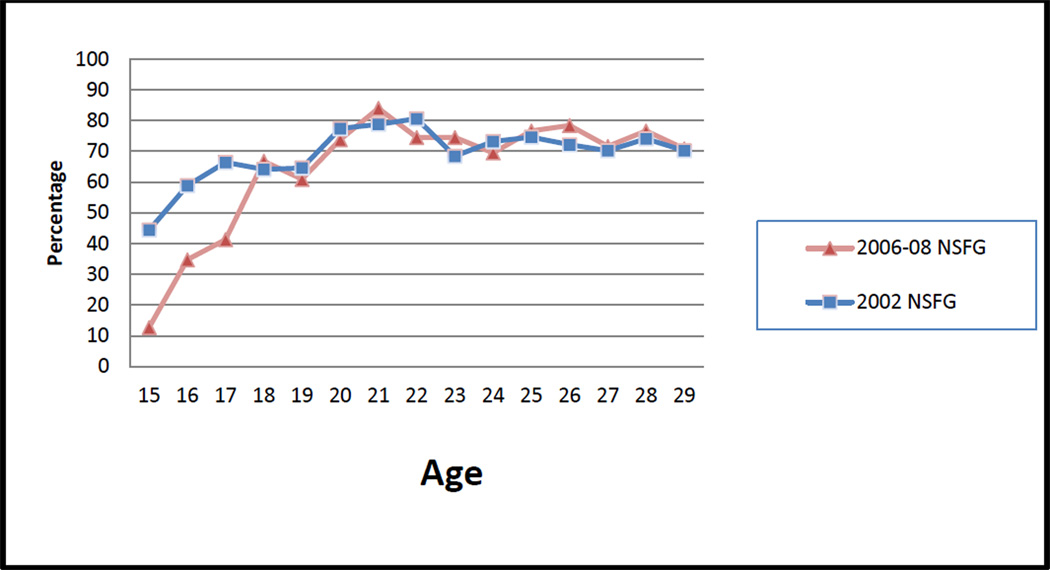
Overall, the percentage of women aged 15–29 who reported receiving a Pap test in the previous year changed little between 2002 (58%) and 2006–2008 (55%) (Table 2). However, the percentage receiving a Pap test was significantly lower in 2006–2008 among women aged 15–17, regardless of sexual activity status, and among those aged 18–20 who had never had sex. Among women who had never had sex, the proportion who received a Pap test at age 20 declined from 34% in 2002 to 18% in 2006–2008 (Figure 1). Among sexually active women there were no significant difference in the percentage who reporting receiving a Pap test at age 18 or older (Figure 2).
Table 2.
Pap test within past 12 months according to age and time since first sex, females ages 15–29, United States National Survey of Family Growth, 2002, 2006–20081
| Reported Pap test in past 12 months, % (weighted N) | |||||
|---|---|---|---|---|---|
| Year | Total | Ages 15–17 | Ages 18–20 | Ages 21–29 | |
| Total sample | 2002 | 58.1 (16,083) | 23.2 (1,350)*** | 56.6 (3,381) | 70.5 (12,072) |
| 2006–08 | 54.8 (16,825) | 11.8 (687)*** | 47.2 (3,125) | 71.3 (13,013) | |
| Never had sex | 2002 | 14.5 (991)* | 6.8 (277) | 23.6 (382)** | 28.1 (332) |
| 2006–08 | 10.3 (833)* | 3.7 (156) | 8.0 (180)** | 29.7 (497) | |
| 0–36 months since first sex |
2002 | 60.3 (3,321) | 57.6 (838)*** | 59.0 (1,255) | 64.0 (1,139) |
| 2006–08 | 54.7 (3,145) | 28.0 (383)*** | 60.7 (1,483) | 66.2 (1,279) | |
| > 36 months since first sex |
2002 | 75.3 (12,580) | 75.5 (235) | 78.3 (1,745) | 74.8 (10,600) |
| 2006–08 | 76.3 (12,847) | 56.2 (147) | 75.5 (1,462) | 76.8 (11,237) | |
Asterisks indicate significant differences between 2002 and 2006–2008 based on the standard two-tailed t-test on design-based point estimates and standard errors (* p< .05, ** p<.01, *** p<.001).
Figure 1.
Percentage of young women, ages 15–20, who have never had sex and received a Pap test in the past 12 months by age at interview, United States National Survey of Family Growth, 2002, 2006–2008
Figure 2.
Percentage of young women, ages 15 to 29, who have had sex and received a Pap test in the past 12 months by age at interview, United States National Survey of Family Growth, 2002, 2006–2008
Characteristics associated with receiving guideline-consistent screening in 2002 were similar to those associated with receiving guideline-consistent screening in 2006–2008 (Table 3), though the change in screening guidelines increased the number of women at risk of early screening and decreased the number at risk of missed screening.
Table 3.
Percentage of women receiving guideline-consistent, early, or missed cervical cancer screening by sociodemographic, health care access, and reproductive health characteristics, females ages 15–29, United States National Survey of Family Growth, 2002, 2006–20081
| Guideline-consistent | Early | Missed | ||||
|---|---|---|---|---|---|---|
| 2002 | 2006–08 | 20022 | 2006–082 | 2002 | 2006–08 | |
| Total % (weighted N) | 70.2 (20,303) | 74.1 (22,750) | 1.0 (277) | 6.9 (2,128) | 28.9 (8,343) | 19.0 (5,821) |
| Sociodemographic characteristics (%) | ||||||
| Age | ||||||
| 15 – 17 | 83.4 | 88.9* | 4.8 | 9.1** | 11.9 | 2.0*** |
| 18 – 20 | 56.6 | 68.8** | --- | 24.1 | 43.4 | 7.2*** |
| 21 – 24 | 70.2 | 68.8 | --- | --- | 29.8 | 31.2 |
| 25 – 29 | 70.7 | 73.3 | --- | --- | 29.3 | 26.7 |
| Race/ethnicity | ||||||
| Black (non-Hispanic) | 73.8 | 75.8 | [1.4] | 10.0*** | 24.8 | 14.2*** |
| White (non-Hispanic) | 72.2 | 75.3 | 1.0 | 6.5*** | 26.8 | 18.3*** |
| Hispanic | 63.2 | 71.5** | --- | 6.3 | 36.6 | 22.2*** |
| Other | 60.4 | 65.7 | --- | 5.6 | 38.3 | 28.7 |
| Born outside of U.S. | 58.6 | 66.2 | --- | [3.7] | 40.9 | 30.1** |
| Born in U.S. | 72.0 | 75.2 | 1.0 | 7.4*** | 26.9 | 17.5*** |
| Mother’s education, less than college | 67.6 | 74.6*** | 0.3 | 5.8*** | 31.3 | 19.6*** |
| Mother’s education, some college or more | 73.1 | 73.7 | [0.8] | 8.0*** | 26.1 | 18.4*** |
| Urban residence | 70.8 | 74.5* | 0.7 | 6.2*** | 28.5 | 19.3*** |
| Non-urban residence | 67.6 | 72.7 | [1.9] | 9.4** | 30.5 | 17.9*** |
| Health care access and use (%) | ||||||
| Any gap in health insurance, past 12 months | 61.6 | 68.5** | --- | 2.7 | 38.2 | 28.8*** |
| Continuous health insurance, past 12 months | 73.0 | 76.2 | 1.2 | 8.7*** | 25.7 | 15.1*** |
| Currently have no health insurance | 53.4 | 63.6** | --- | 1.5 | 46.3 | 34.9*** |
| Currently have private health insurance | 73.4 | 77.2 | 1.1 | 7.7*** | 25.5 | 15.1*** |
| Currently have public health insurance | 74.1 | 76.0 | [1.2] | 10.0*** | 24.8 | 14.0*** |
| Currently using hormonal contraception | 87.0 | 79.2*** | [1.1] | 11.7*** | 11.9 | 9.1 |
| Not currently using hormonal contraception | 59.0 | 66.4 | 0.0 | 3.6*** | 41.0 | 30.1** |
| Received HPV vaccine3 | na | 74.9 | na | 17.5 | na | 7.6 |
| Did not receive HPV vaccine | na | 75.5 | na | 8.9 | na | 15.6 |
| Reproductive health factors (%) | ||||||
| Multiple sexual partners in the past year | 70.4 | 71.2 | 0.0 | 11.3*** | 28.1 | 17.5*** |
| One or no sexual partners in the past year | 71.9 | 71.6 | 0.0 | 7.08*** | 29.6 | 21.3*** |
| Never had sex | 65.5 | 81.3*** | 4.0 | 4.1 | 30.5 | 14.5*** |
| Became sexually active in past 36 months | 60.3 | 57.4 | 0.0 | 31.2*** | 39.7 | 11.4*** |
| Became sexually active >36 months ago | 75.3 | 76.3 | 0.0 | --- | 24.7 | 23.7 |
| Pregnant in the past 12 months | 84.7 | 80.6 | 0.0 | 9.3*** | 15.4 | 10.1 |
| Not pregnant in the past 12 months | 68.7 | 73.5** | 1.1 | 6.7*** | 30.3 | 19.8*** |
Variables in bold-face type were significantly different within survey year, design-based Pearson chi-squared test (p<.05). Asterisks indicate differences between 2002 and 2006–08, standard two-tailed t-test on design-based point estimates and standard errors (* p< .05, ** p<.01, *** p<.001).
Estimates of early screening were based on fewer respondents. Estimates with relative standard errors >50% are not reported and those with relative standard error >30% are in brackets.
The HPV vaccine item was asked beginning in 2007 for those under the age of 25 (unweighted N = 1,244). Those who received the vaccine were significantly different from those not receiving the vaccine based on the overall design-based Pearson chi-squared test.
Age
The percentage of women receiving guideline-consistent screening increased from 83% to 89% among those aged 15–17 and from 57% to 69% among those aged 18–20. According to the revised guidelines, however, 24% of 18–20 year-olds were screened too early in 2006–2008.
Race/ethnicity and nativity
During 2006–2008, the overall percentage of black women screened too early (10%) was higher than the percentage among women in other racial/ethnic groups. For both survey periods, missed screening was highest among Hispanic women. Foreign-born women also missed screening at higher rates compared to women born in the United States. In each survey period, a majority of foreign-born women were Hispanic (53% in 2002 and 58% in 2006–2008).
Health care access and use
Women with a gap in or no health insurance coverage in the previous 12 months were less likely to report guideline-consistent screening during both periods. In 2006–2008, women using hormonal contraception were more likely to be screened too early than those who were not (12% vs. 4%), whereas those not using hormonal contraception were more likely to have missed screening (30% vs. 9%). Among women aged 24 or younger, 18% of those who received the HPV vaccine were screened earlier than recommended, whereas 16% of those who did not receive the HPV vaccine missed recommended cervical cancer screening.
Among sexually active women, multivariable logistic regression results (Table 4) showed that in 2002 the prevalence of guideline-consistent screening was positively associated with use of hormonal contraception (OR 6.0, 95% CI 4.2, 8.5), and a history of a pregnancy in the previous year (OR 3.4, 95% CI 1.7, 6.8) and negatively associated with being foreign-born (OR 0.6, 95% CI 0.4, 0.9) and not having health insurance (OR 0.5, 95% CI 0.3, 0.7). In 2006–2008, guideline-consistent screening was also negatively associated with not having health insurance (OR 0.5, 95% CI 0.4, 0.7), as well as with having public health insurance rather than private health insurance (OR 0.6, 95% CI 0.4, 0.9). Use of hormonal contraception (OR 2.1, 95% CI 1.5, 3.1) a history of pregnancy in the previous year (OR 2.0, 95% CI 1.2, 3.4) were associated with guideline-consistent screening.
Table 4.
Predictors of guideline-consistent Papanicolaou testing among sexually active women ages 15–29, Multivariable logistic regression, United States National Survey of Family Growth, 2002, 2006–2008
|
Sociodemographic, Health Care, and Reproductive Health Factors |
2002 NSFG unweighted n = 1,967 adjusted OR (95% CI) |
2006–2008 NSFG unweighted n = 1,966 adjusted OR (95% CI) |
|---|---|---|
| Age, years | 1.1 (1.0, 1.1)** | 1.1 (1.1, 1.1)*** |
| Race/ethnicity: | ||
| White, non-Hispanic | Reference | Reference |
| Black, non-Hispanic | 1.8 (1.3, 2.5)** | 1.2 (0.8, 1.9) |
| Hispanic | 1.0 (0.7, 1.5) | 0.7 (0.4, 1.2) |
| Other | 0.6 (0.3, 1.2) | 0.7 (0.4, 1.2) |
| Born outside of the U.S. | 0.6 (0.4, 0.9)* | 0.8 (0.4, 1.4) |
| Urban/suburban area | 0.9 (0.6, 1.3) | 1.1 (0.7, 1.6) |
| Mother’s education high school or less | 0.8 (0.6, 1.1) | 1.2 (0.9, 1.7) |
| Type of health insurance: | ||
| Private | Reference | Reference |
| None | 0.5 (0.3, 0.7)*** | 0.5 (0.4, 0.7)*** |
| Public | 0.8 (0.6, 1.2) | 0.6 (0.4, 0.9)** |
| Currently using hormonal contraception | 6.0 (4.2, 8.5)*** | 2.1 (1.5, 3.1)*** |
| Pregnant in previous 12 months | 3.4 (1.7, 6.8)*** | 2.0 (1.2, 3.4)** |
| 2 or more sexual partners in past 12 months |
1.1 (0.8, 1.5) | 1.3 (0.9, 1.9) |
p<.05,
<.01,
p<.001
4. Discussion
The percentage of sexually inactive women ages 15–20 who reported having had a Pap test decreased substantially between 2002 and 2006–2008, yet among sexually active adolescents aged 15–17, more than a quarter were tested earlier than guidelines recommended at the time of the survey in 2006–2008. Overall, however, cervical cancer screening among U.S. women aged 15–29 was more consistent with guidelines in 2006–2008 than in 2002. Women without health insurance and those with public health insurance were both significantly less likely than privately insured women to receive guideline-consistent screening in 2006–2008. Among sexually active women, these health care access factors were significant in adjusted analyses, whereas racial/ethnic differences were not. Other studies have identified health care access measures, including health insurance, usual source of care, and clinician supply, as important predictors of cervical cancer screening (Coughlin, 2002; Selvin and Brett, 2003; CDC, 2012).
Rates of guideline-consistent screening were higher among women using hormonal contraception, supporting the argument that medical visits associated with obtaining hormonal contraception are important opportunities for preventive health care, as has been previously documented (Saraiya, 2009). However, more than 10% of women who used hormonal contraception during 2006–2008 were screened for cervical cancer earlier than recommended. Many U.S. providers continue to require patients to have a pelvic examination when prescribing hormonal contraception, despite longstanding recommendations to the contrary, and may perform Pap tests as well in these visits (Henderson et al., 2010). For some young women, concerns about pelvic examination and Pap tests might inhibit health care use, and access to effective contraception. Health care visits for young, recently sexually active women could focus on other preventive care and services, including contraceptive counseling (Martinez et al., 2011; Hoover and Tao, 2008). Improvements in access to health insurance for young adult women due to recent health care reforms may increase access to effective hormonal contraceptive methods and to cervical cancer screening, potentially reducing missed screening for women previously having limited access to health care. Outreach to clinicians to prevent over-screening in this population also may be needed, as young women seeking contraceptive care at the onset of sexual activity may receive unnecessary Pap tests.
In 2009, ACOG released new guidelines for cervical cancer screening, recommending that women first be screened for cervical cancer at age 21, regardless of sexual activity status. In the current study, we found reduced levels of screening only among women who had never had sex. Ongoing monitoring is needed to assess whether clinicians continue to provide Pap tests at the onset of sexual activity, or adopt the new age-based recommendations. Results of a study based on data from the National Ambulatory Medical Care Survey showed that 3% of physician visits made by U.S. females in 2009 included an inappropriate Pap test and these inappropriate tests cost 48 million dollars (Kale et al., 2009).
Recent introduction of the HPV vaccine for girls increases the need to communicate the importance of on-time cervical cancer screening even for women who have been vaccinated. However, young women without health insurance are less likely to be vaccinated for HPV (Liddon et al., 2012), and in our study also less likely to receive recommended cervical cancer screening.
Study limitations
Study limitations include our use of data from self-reports of survey participants, some of whom may not recall or know whether they received a Pap test during a speculum examination to test for sexually transmitted infections or as part of a pelvic examination (Blake et al., 2004). Studies have shown that Pap testing may be over-reported, particularly among low-income and minority women (Pizarro et al., 2002; Suarez et al., 1995). Thus, estimates of the prevalence of screening could be elevated. Recall bias in Pap testing reports, however, was unlikely to have changed appreciably between the two survey periods and is unlikely to affect comparisons of screening over time.
Missed cervical cancer screening for women aged 21–29 in the 2006–2008 survey was defined on the basis of 2003 clinical guidelines from USPSTF and ACOG for conventional cytology (i.e., that women in this age group be screened annually). Some providers may adhere to ACS guidelines for liquid-based cytological testing (women aged 21 or older tested every 2 years) which would result in overestimation of the percentage of women who missed screening in 2006–2008. In future years, the NSFG will additionally gather data on the timing of first and last Pap tests and on prior abnormal results.
Conclusion
Unnecessary screening of young, sexually active women may interfere with provision of other important services, including contraception (Schwarz et al., 2005); many young women visiting health care providers for Pap tests currently do not receive other recommended screening and counseling (Hoover et al., 2009, 2010; Leyden, 2005; Vesco 2011). Effective implementation of new cervical cancer screening guidelines is needed to allow resources from unnecessary screening of young women at low risk for cervical cancer to be directed to screening of women in demographic groups that have historically faced challenges obtaining timely screening and those at greatest risk of cervical cancer.
Highlights.
Fewer adolescents were screened for cervical cancer following guideline changes.
Many sexually active young women were screened too early for cervical cancer.
Lack of health insurance was associated with missed cervical cancer screening.
Interventions are needed to reduce over-and under-screening for cervical cancer.
Acknowledgments
Support for Dr. Henderson’s effort on this project was provided by an NIH/NICHD Mentored Research Scientist Development Award in Population Research (K01HD054495) and resources from NIH/NCRR/OD UCSF-CTSI (KL2 RR024130). Dr. Sawaya was supported in part by an interagency personnel agreement with CDC. The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that there are no conflicts of interest.
Contributor Information
Jillian T. Henderson, Email: HendersonJ@obgyn.ucsf.edu.
Mona Saraiya, Email: MSaraiya@cdc.gov.
Gladys Martinez, Email: Gmm7@cdc.gov.
Cynthia C. Harper, Email: Harperc@obgyn.ucsf.edu.
George F. Sawaya, Email: Sawayag@obgyn.ucsf.edu.
References
- American College of Obstetrics & Gynecology. Practice Bulletin no. 152: Recommendations on frequency of Pap test screening. American College of Obstetrics & Gynecology Committee Opinion. Int J Gynaecol Obstet. 1995;49:210–211. [PubMed] [Google Scholar]
- American College of Obstetrics & Gynecology. Practice Bulletin No. 45: Cervical cytology screening. Obstet Gynecol. 2003;102:417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics & Gynecology. Practice bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- Andersen RM. National health surveys and the behavioral model of health services use. Med Care. 2008;46:647–653. doi: 10.1097/MLR.0b013e31817a835d. [DOI] [PubMed] [Google Scholar]
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DR, Weber BM, Fletcher KE. Adolescent and young adult women's misunderstanding of the term Pap smear. Arch Pediatr Adolesc Med. 2004;158:966–970. doi: 10.1001/archpedi.158.10.966. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Cancer screening - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–45. [PubMed] [Google Scholar]
- Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008;66:260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Groves R, Benson G, Mosher W, Rosenbaum J, Granda P, Axinn W, et al. Plan and operation of cycle 6 of the National Survey of Family Growth. Vital Health Stat. 2005;1(No. 42):1–86. [PubMed] [Google Scholar]
- Groves R, Mosher W, Lepkowski J, Kirgis N. Planning and development of the continuous National Survey of Family Growth. Vital Health Stat. 2009;1(No. 48) [PubMed] [Google Scholar]
- Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Sawaya GF, Blum M, Stratton L, Harper CC. Pelvic examinations and access to oral hormonal contraception. Obstet Gynecol. 2010;116:1257–1264. doi: 10.1097/AOG.0b013e3181fb540f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover K, Tao G. Missed opportunities for chlamydia screening of young women in the United States. Obstet Gynecol. 2008;111:1097–1102. doi: 10.1097/AOG.0b013e31816bbe9b. [DOI] [PubMed] [Google Scholar]
- Hoover K, Koumans EH, Montaño D, Kasprzyk D, Freeman C, Greek A, et al. Access of black, Hispanic, and nonprivately insured women to liquid-based cytology, human papillomavirus DNA testing, and on-site colposcopy in the United States. J Low Genit Tract Dis. 2009;13:17–27. doi: 10.1097/LGT.0b013e318194b87e. [DOI] [PubMed] [Google Scholar]
- Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolescent Health. 2010;46:324–330. doi: 10.1016/j.jadohealth.2009.09.002. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, World Health Organization. Cervix Cancer Screening. Lyon, France: IARC Press; 2005. IARC Handbooks of Cancer Prevention, Vol. 10. [Google Scholar]
- Kale MS, Bishop TF, Federman AD, Keyhani S. Prevalence of good stewardship working group “top 5” activities in US ambulatory care, 2009. Arch Intern Med. 20100 [Table published online October 1, 2011.] [Google Scholar]
- Lepkowski J, Mosher W, Davis K, Groves R, van Hoewyk J, Willem J. National Survey of Family Growth, cycle 6: sample design, weighting, imputation, and variance estimation. Vital Health Stat. 2006;1(No. 142):1–82. [PubMed] [Google Scholar]
- Lepkowski J, Mosher W, Davis K, Groves R, Van Hoewyk J. The 2006–2010 National Survey of Family Growth: sample design and analysis of a continuous survey. Vital Health Stat. 2010;2(No. 150) [PubMed] [Google Scholar]
- Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- Liddon NC, Leichliter JS, Markowitz LE. Human Papillomavirus vaccine and sexual behavior among adolescent and young women. Am J Prev Med. 2012;42:44–52. doi: 10.1016/j.amepre.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Martinez G, Copen CE, Abma JC. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2006–2010 National Survey of Family Growth. Vital Health Stat. 2011;23(No. 31) [PubMed] [Google Scholar]
- Pizarro J, Schneider T, Salovey P. A source of error in self-reports of Pap test utilization. J Commun Health. 2002;27:351–356. doi: 10.1023/a:1019888627113. [DOI] [PubMed] [Google Scholar]
- Saraiya M, Martinez G, Glaser K, Kulasingam S. Pap test and sexual activity among young women in the United States. Obstet Gynecol. 2009;114:1213–1219. doi: 10.1097/AOG.0b013e3181be3db4. [DOI] [PubMed] [Google Scholar]
- Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- Schwarz BE, Saint M, Gildengorin G, Weitz TA, Stewart FH, Sawaya GF. Cervical cancer screening continues to limit provision of contraception. Contraception. 2005;72:179–181. doi: 10.1016/j.contraception.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Selvin E, Brett KM. Breast and cervical cancer screening: sociodemographic predictors among white, black, and Hispanic women. Am J Public Health. 2003;93:618–623. doi: 10.2105/ajph.93.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2000;50:34–49. doi: 10.3322/canjclin.50.1.34. [DOI] [PubMed] [Google Scholar]
- Suarez L, Goldman D, Weiss N. Validity of pap smear and mammogram self-reports in a low-income Hispanic population. Am J Prev Med. 1995;11:94–98. [PubMed] [Google Scholar]
- Trotter LJ, Bowen DJ, Beresford SA. Testing for racial/ethnic differences in the association between childhood socioeconomic position and adult adiposity. Am J Public Health. 2010;100:1088–1094. doi: 10.2105/AJPH.2009.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. U.S. Preventive Services Task Force guide to clinical preventive services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996. [Google Scholar]
- U.S. Preventive Services Task Force. Screening for cervical cancer: recommendations and rationale. 2003 Available at: http://www.uspreventiveservicestaskforce.org/3rduspstf/cervcan/cervcanrr.htm.
- Vesco KK, Whitlock EP, Eder M, Burda BU, Senger CA, Lutz K. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:678–705. doi: 10.7326/0003-4819-155-10-201111150-00377. [DOI] [PubMed] [Google Scholar]
- Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States: 1998–2003. Cancer. 2008;113:2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]