Summary
We previously found that conditional deletion of integrin β1 in intestinal epithelium of mice caused early postnatal lethality and intestinal phenotypic changes including excessive proliferation of epithelial cells and defective epithelial differentiation. Here, we link these defects to the Hedgehog (Hh) signaling pathway and show that loss of integrin β1 also leads to excessive phosphorylation of MEK-1 and increased expression of ErbB receptors, including the epidermal growth factor receptor (EGFR). We show that EGFR signaling attenuates Hh abundance and that an EGFR inhibitor rescues conditional β1 integrin null pups from postnatal lethality. These studies link the loss of Hh expression in the intestinal epithelium of integrin β1-deficient mice to EGFR/MAPK signaling, and, identify a unique mechanism for crosstalk between stromal and epithelial signaling pathways that is critical for intestinal epithelial differentiation and function.
Keywords: Hedgehog, integrin, epidermal growth factor receptor, ErbB, intestinal epithelium, differentiation
Introduction
The small and large intestines are lined by one of the most rapidly renewing epithelia in mammals. This requires frequent turnover of the epithelial cells, which is promoted predominantly by the Wnt and EGFR pathways (1,2). Because of their high turnover rate, the epithelial cells must rapidly differentiate once they migrate out of the proliferative intestinal crypts and onto the villi so that nutrient absorption and transport are fully developed within their relatively short lifespan (∼4 days in mice) (3). The intestinal epithelium is strictly compartmentalized into proliferative crypts and differentiated villi with multiple signaling pathways maintaining a vital balance between proliferation and differentiation.
The extracellular matrix proteins, such as fibronectin, collagens, and laminins, upon which the intestinal epithelium rests are differentially expressed along the crypt-villous axis and thought to play a significant role in intestinal epithelial function (4,5). The extracellular matrix communicates with the epithelium through adhesion proteins such as integrins, which are the most abundant cell surface adhesion receptors expressed by intestinal epithelial cells. Functionally, integrins mediate cell survival, proliferation, differentiation and migration through their cytoplasmic tails, which are devoid of catalytic activity, but are linked to the cytoskeleton and signaling complexes through adaptor proteins (6).
Little was known of the role of integrins in intestinal epithelial proliferation and differentiation until we generated mice with conditional deletion in the intestinal epithelium of integrin β1, which heterodimerizes with several α integrin subunits (5). These mice had intestinal epithelial cell hyper-proliferation, loss of stromal expansion, polyp formation, lipid trafficking defects, fat malabsorption and epithelial cell hypo-differentiation. These changes were strikingly similar to mice with defective Hedgehog (Hh) signaling (7-9).
Vertebrates express three Hh homologues: Desert hedgehog (Dhh), Indian hedgehog (Ihh) and Sonic hedgehog (Shh), of which Shh and Ihh are expressed by the intestinal epithelium (10). Hedgehog signaling is essential during development of invertebrate and vertebrate species by virtue of its ability to modulate patterning, growth, and cell-type specification in a wide range of tissues (11). We found marked reductions in Shh protein and mRNA expression in intestinal epithelial cells from conditional β1 integrin null mice (5), which linked integrin signaling to Hh signaling in the intestine. However, we did not define how β1 integrins mediated Hh expression and signaling. Here, we show our novel discovery that Shh expression is regulated by β1 integrins through the EGFR-MAPK signaling pathway.
Experimental Procedures
Mice
The villin-Cre and Itgb1flox mice were previously described (5,12,13). Villin-Cre and Itgb1flox mice were crossed and the offspring backcrossed to generate villin-Cre/Itgb1flox/flox (Itgb1Δ) mice. Genotyping was performed on genomic DNA isolated from tail snips or whole intestine as previously described (5). Erlotinib (500μg/mouse) was administered every other day by subcutaneous injection into pregnant dams bearing Itgb1Δ fetuses from a week before birth to weaning. All animal studies were approved by the Institutional Animal Care and Use Committees at the University of Utah and Salt Lake City Veterans Affairs Health Care System.
Intestinal epithelial cell (IEC) isolation
Mouse IECs (crypts) were isolated from small intestine mucosa by using a non-enzymatic technique (14). Briefly, after opening the intestines longitudinally and washing in PBS, the tissue was incubated in a solution containing 3 mM EDTA plus 0.5 dithiothreitol in PBS for 90minutes at room temperature. Then the tissue was resuspended in PBS, and the crypts were detached by vigorous shaking. Crypts were collected by centrifugation at 50 × g for 5min and then lysed in lysis buffer (50 mM Hepes, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM Na2PO4, 1 mM Na3VO4, 10% glycerol, 1% Triton X-100, and 1 μg/ml each of aprotinin, leupeptin, chymostatin, and pepstatin). Protein concentrations of the lysates were determined by the Bradford protein assay (Pierce).
Cell culture and transfection
The rat intestinal epithelial (RIE) cell line was obtained from the ATCC and cultured on poly-L-lysine-coated dishes in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, glutamine, penicillin, and streptomycin. For cell transfection, RIE cells were cultured in DMEM with supplements on poly-L-lysine-coated dishes. Transfections were performed in 6-well plate using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen).
Antibodies
Ki67 mAb (Santa Cruz), β1 integrin mAb (Cell Signaling), phosphor-Mek1/2 mAb (Cell Signaling), phosphor-Akt mAb (Cell Signaling), Shh mAb (Santa Cruz), phosphor-Erk mAb (Cell Signaling), c-Cbl mAb (Santa Cruz), phosphor-Egfr (Tyr1173) mAb (Santa Cruz) and EGFR mAb (Santa Cruz), Gli-1 mAb (Cell Signaling), Patched mAb (Abcam).
Immunohistochemistry
Fixed tissues were embedded in paraffin as described previously 24. The samples were deparaffinized in xylene and rehydrated in a 30–100% ethanol series and ddH2O. Antigen retrieval was performed by boiling the samples in 10 mM Citrate Buffer, pH 6.0, in a microwave oven. The slides were then washed with 1× PBS for 5min at RT. The samples were blocked in 3% horse serum, 3% bovine calf serum, or 3% goat serum in 0.1% Triton X-100/1% BSA in PBS for 30 min at RT in a humidity chamber. Primary antibody dilutions in the blocking buffer were incubated with the samples overnight in a humidity chamber at 4°C. The slides were washed in PBS and a secondary antibody conjugated to Alexa 488 (diluted in blocking buffer) was added to the samples for 30min at RT. The slides were washed in PBS and then mounted with Prolong-Gold (Invitrogen) and coverslips. All pairs of slides were processed simultaneously, and all pairs of photomicrographs were performed with identical camera settings and exposure times to insure uniformity.
Quantitative RT-PCR
Total RNA was isolated using an RNeasy kit (Qiagen). First-strand cDNA was synthesized from 1μg of total RNA using M-MLV reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed using SybrGreen (Applied biosystem) incorporation on a Sequence Detection System (ABI PRISM 7900HT; Applied Biosystems). Threshold cycles were normalized to glyceraldehyde-3-phosphate dehydrogenase (G3PDH). The primers were designed to span intron–exon boundaries.
Primers for mouse
Shh, 5′ CCAATTACAACCCCGACATC 3′ and 5′ CCACGGAGTTCTCTGCTTTC 3′; G3PDH, 5′ CAGTGCTGAGTATGTC GTGG 3′ and 5′ AGAACGGACGGAGATGATGACC 3′; Gli1, 5′ GAAGGAATTCGTGTGCCATT3′ and 5′GCAACCTTCTTGCTCACACA 3′; Ptch1, 5′CAGTTCTCAGACTCCAGC 3′ and 5′GAACAATGTCCGTGAGGTCC 3′.
Primers for rat
G3PDH, 5′GCACAGTCAAGGCTGAGAATGG3′ and 5′TAGACTCCACGACATACTCAGC3′; Shh, 5′CAATTACAACCCCGACATC3′ and 5′TCACTCGAAGCTTCACTCCA3′. Primers for human: G3PDH, 5′GACATCAAGAAGGTGGTGAAGC3′ and 5′CTTCCTCTTGTGCTCTTGCTGG3′; Gli1, 5′ AGCGTGAGCCTGAATCTGT3′ and 5′GATGTGCTCGCTGTTGATGT3
Immunoblotting
10-15μg of total protein were boiled in SDS sample buffer for 3min and then resolved on 10% Tris-HCl SDS polyacrylamide gels by electrophoresis. The gels were transferred to nitrocellulose membrane and blocked in blocking buffer (3% bovine serum albumin in TTBS (100 mM Tris-Cl, pH 7.4, 0.9% NaCl, 0.1% Tween). The blots were then incubated with primary antibodies at 4°C overnight. The blots were washed in TTBS and incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 1hr at RT. The blots were washed 3×10 min in TTBS, incubated with SuperSignalTM horseradish peroxidase substrate (Pierce) for 1min and then exposed to film. Densitometry was performed on the bands on the blots using National Institutes of Health Image 1.63.
Results
Integrin β1 deletion in intestinal epithelium reduces Hh signaling
We found that conditional deletion of integrin β1 in the intestinal epithelium of villin-Cre/Itgb1flox/flox (Itgb1Δ) mice resulted in post-natal lethality between postnatal day 7 (P7) and P14 due to severe malnutrition caused by dramatic intestinal epithelial hyper-proliferation and reduced differentiation (5). The intestinal phenotype of the Itgb1Δ mice was very similar to that observed in mice with defective Hh signaling (7-9), suggesting a link between β1 integrin and Hh signaling. In support of this, we found that Shh levels in the intestinal epithelium were severely reduced in the Itgb1Δ mice compared with control littermates (5).
Hh signaling is paracrine in the mammalian intestine because Shh and Ihh are expressed by the intestinal epithelial cells while their receptor, Patched, is expressed in the intestinal stromal cells (7,8). In the presence of Hh ligand, Patched activity is inhibited, resulting in increased Smoothened activity which in turn causes accumulation of Gli transcriptional proteins which transactivate gene targets (7) (8,15,16). Both Ptch1 and Gli1 are major transcriptional targets of the Hh signaling pathway (16). In order to assess the activation state of the Hh signaling pathway in Itgb1Δ mice, we determined the levels of intestinal Ptch1 and Gli1 mRNA by real-time PCR. Both Gli1 and Ptch1 mRNA (Figure 1A) as well as protein levels (Figure 1B) were significantly reduced in the intestinal stroma of Itgb1Δ mice compared with wild-type littermates, demonstrating decreased Hh signaling in Itgb1Δ mice.
Figure 1. Integrin β1 and the Hh signaling pathway are functionally linked.
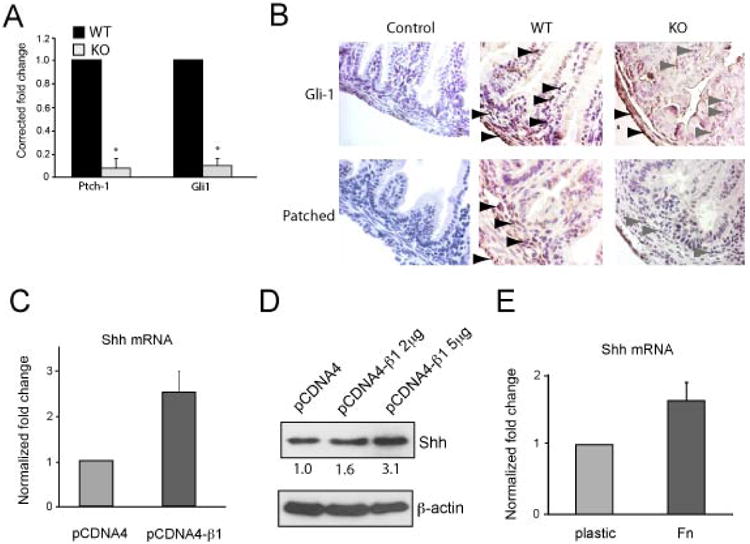
A, Quantitative RT-PCR of Ptch1 and Gli1 mRNA from the intestinal stroma of P6 wild-type (WT) and Itgb1Δ mice. Each result represents the average of three different mice. B, Immunohistochemical staining of Gli-1 and Patched proteins was performed on small intestinal sections from P6 Itgb1Δ pups (KO) and their wildtype littermates (WT). Controls were done using secondary antibodies only. The black and gray arrowheads show strong and weak staining (brown), respectively, for the proteins. C-D, Quantitative RT-PCR (C) and immunoblotting (D) results showing Shh mRNA and protein levels, respectively, in rat intestinal epithelial (RIE) cells that were transiently transfected with a human β1 integrin construct or vector. Relative densities of the protein bands are shown in D. The results shown are averages of triplicate samples. Error bars represent SEM. E, Average Shh mRNA levels in RIE cells cultured on plastic or fibronectin (Fn) – coated dishes for 8 hours. The results shown are the average of triplicate samples. Error bars represent SEM.
Next, we asked if we could promote Hh expression by over-expressing or activating integrin β1. We used a normal rat small intestinal epithelial (RIE) cell line and found induction of Shh mRNA and protein upon transient transfection with integrin β1 (Figure 1C-D) or following adhesion to fibronectin, an integrin β1 ligand (Figure 1E). Collectively, these data link integrin β1 to the Hh signaling pathway such that loss of epithelial integrin β1 blunts paracrine Hh signaling in the intestine.
Loss of integrin β1 leads to increased epithelial proliferation, abundant MAPK signaling, and elevated ErbB expression
We found hyperplastic intestinal crypts in Itgb1Δ mice (5), suggesting that loss of epithelial integrin β1 not only reduces Hh signaling, but promoted epithelial proliferation as well. In the small intestine, epithelial proliferation is normally restricted to crypt bases, but in Itgb1Δ mice (Figure 2A) we found that the zone of proliferating cells extended up in the normally non-proliferative villi compared to wild-type littermates (Figure 2B). These results suggested a link between integrin β1 loss and increased epithelial proliferation.
Figure 2. Evidence of increased proliferative signal transduction in intestinal epithelium from Itgb1Δ mice.
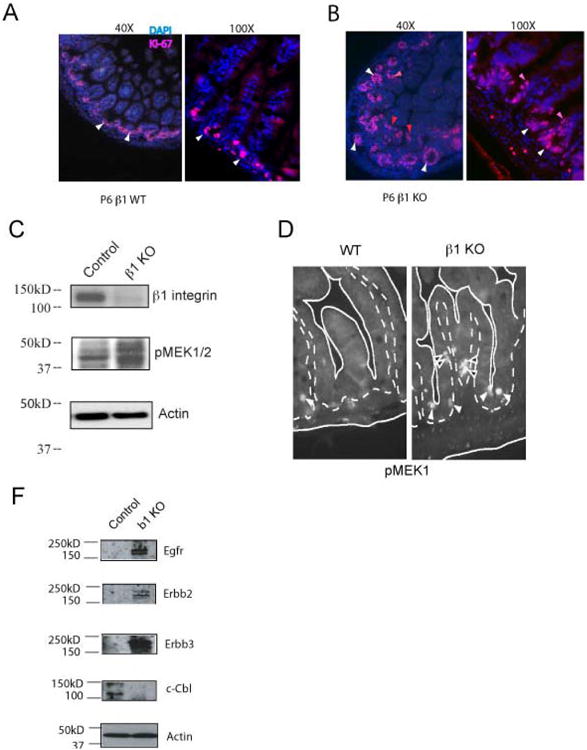
A-B, Ki-67 immunofluorescence micrographs of small intestinal sections of P6 WT (A) and P6 Itgb1Δ mice (B) The white and red arrows identify Ki-67-positive intestinal epithelial crypt and villous cells, respectively. *p < 0.05 compared with controls. C, Western blots showing expression of integrin β1 and levels of phospho-MEK-1/2 proteins in intestinal epithelial cells of P6 WT and Itgb1Δ mice. Actin loading controls are shown. The results shown are typical to two separate experiments. D, Immunofluorescence micrographs showing phospho-MEK1 in intestinal epithelial cells in the small intestinal crypts (white arrowheads) and villi (black arrowheads) of P6 WT and Itgb1Δ mice. Solid lines outline crypts and villi. Dotted lines outline the border of intestinal epithelial cells and stroma. E, Immunoblots showing EGFR, ErbB-2, ErbB-3, c-Cbl, and actin protein levels in small intestinal epithelial cell lysates obtained from WT and Itgb1Δ mice. The results shown are typical of two separate experiments.
In order to determine the mechanism leading to this effect in Itgb1Δ mice, we considered major signaling pathways that drive intestinal proliferation. Receptor tyrosine kinase (RTK) growth factor receptors are critical for intestinal epithelial proliferation and maturation (2), so we examined the canonical MAPK pathway to determine if it was hyperactive in Itgb1Δ mice. We found increased levels of phosphorylated MEK (pMEK) in the intestinal epithelium of Itgb1Δ mice compared to their wild-type littermates (Figure 2C). Moreover, in Itgb1Δ mice, pMEK1 was mis-localized to the villous epithelial cells (Figure 2D, right panel) whereas in wild-type mice pMEK1 was restricted to the crypts (Figure 2D, left panel), demonstrating loss of compartmentalized proliferation in Itgb1Δ mice.
Epidermal growth factor receptors are critical for intestinal epithelial proliferation and maturation in newborn mice (2) and lie upstream of MAPK enzymes. To investigate the potential role of EGFR in the integrin β1-deficient phenotype, we next examined the expression levels of EGFR in Itgb1Δ mice and control littermates. In the intestinal epithelium, expression of EGFR and related receptors, ErbB2 and ErbB3, were increased in Itgb1Δ mice compared with their wild-type littermates (Figure 2E). In contrast, c-Cbl protein, which regulates EGFR endocytosis and protein expression levels (17), was reduced in the intestinal epithelium of Itgb1Δ mice compared to their wild-type littermates (Figure 2E). Collectively, these data indicated that loss of integrin β1 augmented ErbB levels, which likely contributed to enhanced MAPK signaling in intestinal epithelium.
EGFR/MAPK signaling inhibits Shh expression
Since loss of integrin β1 led to increased MAPK signaling and ErbB levels, we hypothesized that ErbB/MAPK signaling might link integrin β1 to the Hh signaling pathway. To explore the relationship between the ErbB/MAPK and Hh signaling pathways, we first used a normal rat small intestinal epithelial (RIE) cell line. To determine if Shh expression was dependent on MAPK signaling, we treated RIE cells with the MEK inhibitors U0126 or PD98059 for 18 hours and found that this led to increased levels of Shh mRNA (Figure 3A) and protein (Figure 3B). Additionally, Shh mRNA and protein increased in a time-dependent manner following treatment with PD98059 (Figure 3C-D). Together, these data indicated that MEK signaling inhibited Shh expression.
Figure 3. MEK signaling pathway negatively regulates Shh expression in intestinal epithelial cells.
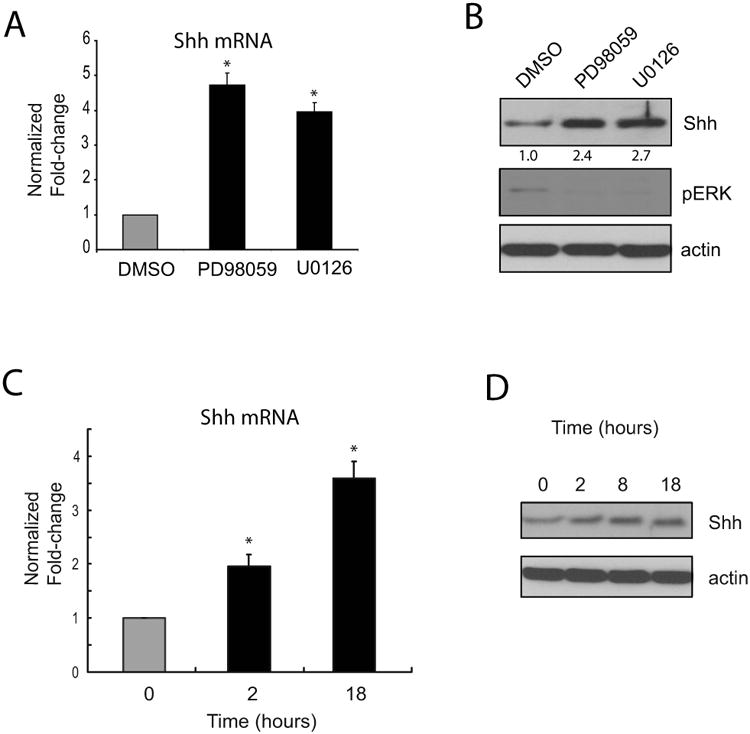
A-B, Quantitative RT-PCR (A) and immunoblot (B) results showing Shh mRNA and protein expression, respectively, in RIE cells treated with MEK inhibitors (10μM PD98059 or U0126) for 18 hours. Densitometry results (normalized for DMSO), and phospho-Erk1/2 and actin controls are shown in B. The results are typical for four separate experiments. Error bars represent SEM. C-D, Timecourse of induction of Shh mRNA (C) and protein (D) levels in RIE cells treated with 10μM PD98059 for the indicated times. The results are averages of triplicate samples and are typical of four separate experiments. Error bars represent SEM. * p < 0.05 compared with controls.
We then explored potential molecular links between ErbB signaling and Shh expression, focusing on EGFR because it is critical for normal intestinal development in mice (2). First, we treated RIE cells with EGF and found that this caused sustained phosphorylation of MEK (Figure 4A). Moreover, EGF treatment reduced Shh protein and mRNA levels in a time-dependent fashion (Figure 4A-B). Pre-treatment of RIE cells with the MEK inhibitor U0126 prior to adding EGF partially rescued Shh mRNA expression (Figure 4C), demonstrating that EGFR signaling inhibited Shh expression, in part, through MEK signaling. To further confirm a link between the EGFR/MEK signaling pathway and Shh expression, we tested the effects of inhibiting EGFR activity in RIE cells with the EGFR tyrosine kinase inhibitors PD153035 or erlotinib. Both PD153035 and erlotinib caused marked increases in Shh mRNA (Figure 4D) and protein (Figure 4E). Collectively, these results demonstrated that EGFR/MAPK signaling negatively regulates Shh expression in intestinal epithelial cells.
Figure 4. EGFR signaling negatively regulates Shh expression in intestinal epithelium of Itgb1Δ mice.
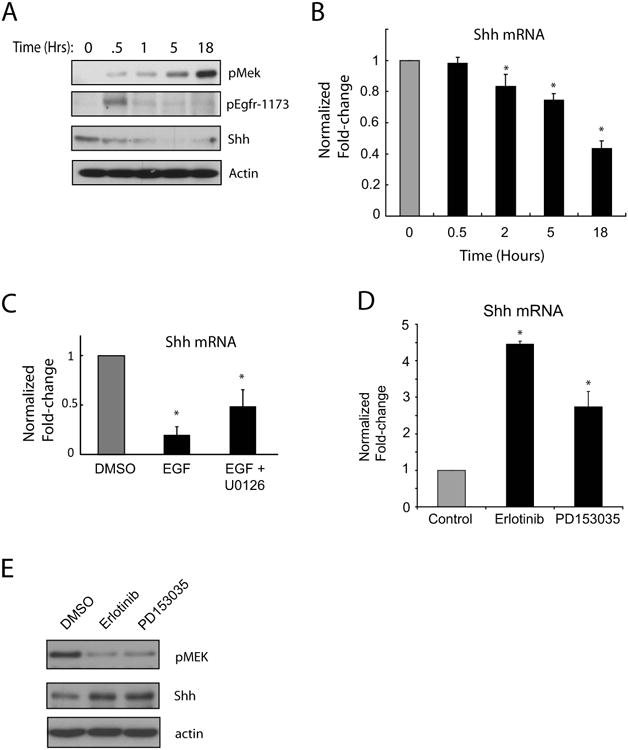
A, Immunoblots showing the levels of phospho-EGFR (phospho-tyrosine 1173), Shh, and phospho-MEK-1/-2 in RIE cells treated with 10ng/ml EGF for up to 18 hours. Actin loading controls are shown. The results shown are typical of two separate experiments. B, Quantitative RT-PCR of Shh mRNA from RIE cells treated with 10ng/ml EGF. The results shown are averages of triplicate samples and typical of three separate experiments. Error bars represent SEM. C, Quantitative RT-PCR of Shh mRNA from RIE cells treated with 10ng/ml EGF with or without 10μM U0126 for 16 hours. The results are averages of triplicate samples and typical of two separate experiments. Error bars represent SEM. D, Quantitative RT-PCR determinations of Shh mRNA in RIE cells treated with the EGFR inhibitors erlotinib (4μM) and PD153035 (8μM) for 18 hours. The results shown are averages of triplicate samples and typical of four separate experiments. Error bars represent SEM. E, Immunblots showing phospho-MEK1/2 and Shh protein levels in RIE cells treated with 4μM erlotinib or 8μM PD153035 for 18 hours. The results shown are typical of three separate experiments. * p < 0.05 compared with controls* p < 0.05 compared with controls
EGFR inhibition rescues early postnatal lethality following integrin β1 deletion in the intestinal epithelium
We next sought to link EGFR signaling to the β1 integrin null phenotype and to Hh signaling in vivo. We first treated murine small intestinal explants with erlotinib for 18 hours and found that this increased Shh mRNA levels in intestinal epithelial cells isolated from the explants (Figure 5A). Next, we tested the in vivo effects of erlotinib by treating pregnant dams bearing Itgb1Δ fetuses with subcutaneous erlotinib from E17 to P21, a period which we determined is critical for Hh-mediated intestinal development (2). All eight Itgb1Δ pups born to dams treated with erlotinib from 3 separate litters survived beyond weaning (approximately P21) and were normal in appearance and activity but proportionately smaller than their wild type littermates (average weight of Itgb1Δ = 7.3gm and average weight of wild-type = 16.2gm at 5 weeks) (Figure 5B). Evaluation of small intestinal epithelial cells from P6 Itgb1Δ mice revealed marked reductions in phosphorylated MEK (Figure 5C) and increased levels of Shh mRNA (Figure 5D) in pups exposed to erlotinib compared with control mice. Collectively, our data indicate that in Itgb1Δ mice, excessive EGFR signaling inhibits Shh expression leading to disruption of normal intestinal development.
Figure 5. Rescue of conditional Itgb1Δ mice from post-natal lethality with erlotinib.
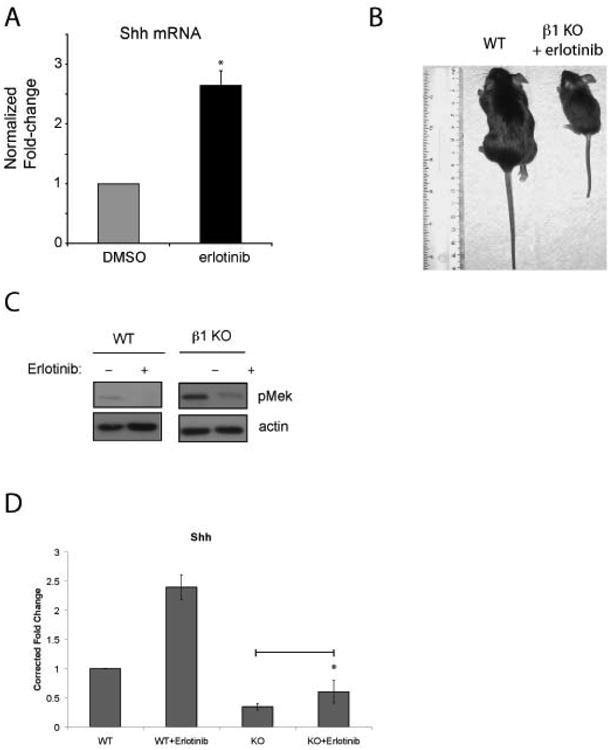
A, Small intestinal explants from wild-type P9 mice were cultured ex vivo in the presence of vehicle or erlotinib (final concentration of 8 μM) for 8 hours. mRNA was extracted from isolated intestinal epithelium after 8 hours of treatment. Quantitative RT-PCR determinations of Shh mRNA levels in the samples are shown. The results are averages of explants obtained from intestinal sections from three control mice and corrected for control Shh mRNA levels (explants from three mice treated with DMSO). Error bars represent SEM. B, Photograph of control littermate and Itgb1Δ mouse born and suckled from a mare that received erlotinib between 4 days pre- and 21 days post- partum. C, Immunoblots showing levels of phospho-MEK1 in WT and Itgb1Δ littermates that received erlotinib or DMSO. D, Quantitative RT-PCR results of Shh mRNA levels in the intestinal epithelial cells of P6 Itgb1Δ littermates born to dams that received DMSO or erlotinib, respectively. The results shown are the averages of three mice each. The results are normalized to the control mouse values. Error bars represent SEM. * p < 0.05 compared with controls.
Discussion
We previously showed that conditional deletion of integrin β1 in intestinal epithelium of mice led to reduced Hh levels, intestinal stem cell expansion, and an enlarged zone of proliferation, which is normally limited to crypt bases (5). Our new data link the reduced Hh levels to excessive EGFR signaling and identify a novel regulatory mechanism linking key stromal and epithelial signaling pathways. Our results suggest that integrin β1 loss leads to overexpression of EGFR, ErbB-2 and ErbB-3 protein, which in turn inhibit Shh expression. Ihh was shown previously to inhibit Wnt signaling, a major driver of intestinal proliferation (18) and loss of Ihh or Shh expression results in intestinal epithelial hyperproliferation (5,18) which inhibits intestinal epithelial differentiation leading to severe malabsorption (7-9).
The importance of EGFR signaling in the intestine has been demonstrated in Egfr knockout mice, which have severely abnormal intestinal epithelial development and maintenance (2). Conversely, over-expression of the EGFR ligand, TGFα, was found to cause intestinal epithelial hyperplasia (19), similar to what we observed in Itgb1Δ mice. We found abundant expression of EGFR and excessive phosphorylation of MEK in the intestinal epithelial cells of the Itgb1Δ mice indicating that integrin β1 loss promotes excessive EGFR-MAPK signaling. And our data in normal rat intestinal epithelial cells showing that manipulating EGFR signaling altered Shh expression in an inverse fashion provide novel evidence linking EGFR to Hh signaling.
Collectively, our data show interactions between integrin β1, EGFR, and Hh signaling pathways. We confirmed these links in mouse tissue by treating small intestinal explants from Itgb1Δ mice with EGFR inhibitors and showed that this restored Shh expression. Further evidence of this link is provided by the restoration of intestinal Shh levels and rescue of Itgb1Δ mice exposed to perinatal treatment of pregnant dams bearing them with an EGFR inhibitor.
Multiple studies showed cooperation of EGFR and Hh signaling in the regulation of stem cells (20,21) and promotion of tumorigenicity (22-38) but ours is the first study to show feedback inhibition of Hh expression by EGFR signaling. The disparity between our results and previous studies that showed cooperative Hh and EGFR signaling are likely due to tissue specific differences in Hh signaling. In the mammalian intestine, canonical Hh signaling occurs only in a paracrine fashion since the Hh receptor, Patched, and Hh signaling pathway effectors, Smoothened and Gli 1-3, are expressed by the mesenchymal cells in the lamina propria, while the Hh ligands are expressed by the intestinal epithelial cells (15) (Figure 6). This is in contradistinction to Hh signaling in the brain, where subventricular zone stem cells express Hh, Patched and Gli (20,21) and are thus capable of autocrine Hh signaling. The subventricular zone stem cells are capable of autocrine EGFR signaling as well, thus allowing crosstalk between Hh and EGFR signaling in the same cells.
Figure 6. Model of β1 integrin-mediated regulation of Egfr and Hh expression in intestinal epithelial cells.
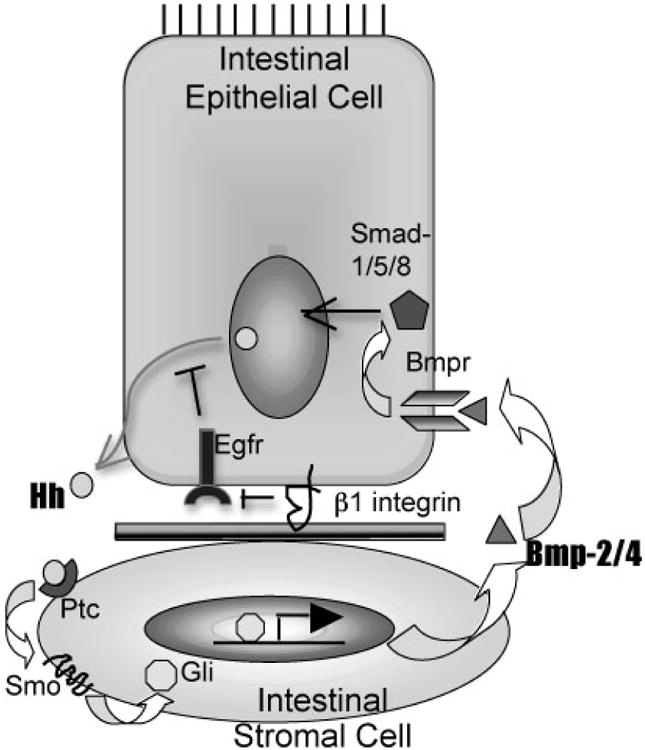
The model shows that β1 integrins enhance downregulation of Egfr signaling through endocytosis. Egfr signaling inhibits Hh expression. Hh signaling is paracrine in the intestinal with Shh and Ihh secreted by the intestinal epithelial cells which bind to their cognate receptor Patched (Ptc) expressed on intestinal stromal cells. Hh signaling in stromal cells is mediated by Smoothened (Smo) and Gli transcription factors, and, culminates in the induction of Bmp-2/-4 expression. Bmp signaling is paracrine as well since Bmp receptors are expressed by intestinal epithelial cells. Bmp signaling inhibits proliferative signaling, especially the Wnt pathway, and inhibits Shh transcription in intestinal epithelial cells.
In the intestines, Hh signaling in the mesenchymal (stromal) cells feeds back to the intestinal epithelial cells through the expression of the Decapentaplegic homologues, BMP2 and BMP4, also members of the TGF-β superfamily (15). The BMP receptors are expressed on the intestinal epithelial cells and mediate signaling through SMADs (39). BMP signaling has been shown to be important in regulating Wnt signaling in the intestinal epithelium (40) and thus offers one mechanism by which Hh signaling feeds back to the intestinal epithelium. In general, BMP and EGFR signaling exert opposing effects on epithelial cell proliferation through differential phosphorylation of SMAD-1, a principal effector of BMP signaling (41). Thus, EGFR signaling increases while Hh and BMP signaling inhibit intestinal epithelial cell proliferation. Intricate mechanisms are required for the regulation of intestinal epithelial proliferation so that proper intestinal epithelial cell differentiation and function can occur.
A system by which β1 integrins control EGFR expression and signaling, which in turn, negatively regulates Hh signaling in the intestinal epithelium, could be a useful mechanism to promote mucosal repair. An injury to the intestinal mucosa would disrupt epithelial-extracellular matrix interactions, resulting in increased EGFR expression and signaling. The increased EGFR signaling would in turn promote wound healing by driving epithelial proliferation and migration, and, inhibit Hh signaling, which would further enhance epithelial proliferation. Once the mucosal defect was repaired and β1 integrin-extracellular matrix interactions were re-established, EGFR signaling would return to basal levels leading to increased Shh expression that would then help re-establish intestinal homeostasis by balancing epithelial proliferation and differentiation along the crypt-villus axis.
We have begun to investigate the cause of EGFR abundance in integrin β1 deficient mice. Since c-Cbl is an E3 ligase that helps modulate ErbB receptor levels by promoting their degradation (42), its reduced levels might represent a clue to the mechanism causing over-expression of ErbB-1, -2, and -3 in Itgb1Δ mice. c-Cbl mediated ubiquitination is required for proper endocytosis and trafficking of ligand-bound EGFR to the lysosomes where they are degraded (43), so its lack of expression suggested a defect in c-erbB endocytosis. Electron photomicrographs of the intestinal epithelium from mice found greatly reduced endocytic vesicles in the subcortical region of cells in the Itgb1Δ mice compared to their wild-type littermates (Supplemental Figure 1A-B). These data suggested a defect in endocytosis and trafficking in the intestinal epithelial cells of Itgb1Δ mice. In addition, a functional assay of receptor-mediated endocytosis using fluorescently-labeled immunoglobulin G was performed. Suckling mammals express Fc receptors in the intestinal epithelium which mediate endocytosis of maternal immunoglobulins in colostrum (44), a process essential for passive immunity in mammalian neonates. We found markedly reduced uptake of the labeled IgG by the absorptive intestinal epithelial cells in Itgb1Δ mice compared to their wild-type littermates (Supplemental Figure 1C-D) demonstrating reduced IgG epithelial internalization in the former. Actin immunolocalization in wild-type P6 mice was characterized by a sharp subcortical band in the small intestinal epithelium (Supplemental Figure 1E), but this band was absent in Itgb1Δ mice. The actin in the intestinal epithelial cells of Itgb1Δ mice was clearly disorganized and diffusely distributed in the cytoplasm (Supplemental Figure 1F). Endocytosis in intestinal epithelial cells is dependent on the actin cytoskeleton (45). These data suggest that β1 integrins are required for proper actin cytoskeletal organization, which in turn may be necessary for the establishment and/or proper functioning of endocytosis during intestinal development. Further experiments are underway to functionally link these correlative findings, but we hypothesize that the increased EGFR, ErbB2, and ErbB3 expression in the Itgb1Δ mice appears to be a manifestation of impaired receptor endocytosis which in turn is due to defective actin cytoskeletal organization. Integrins are functionally linked to the actin cytoskeleton, which in turn is essential for Egfr endocytosis, a major mechanism for attenuating Egfr signaling (43).
Figure 6 presents a model of Shh regulation and signaling in the mammalian gut. In the intestinal epithelial cells, β1 integrins promote cell differentiation and actin cytoskeletal network organization, which is required for endocytosis. In epithelial cells, the major mechanism for down-regulation of EGFR signaling is endocytosis and lysosomal targeting (46). EGFR/MAPK signaling inhibits Shh expression at the level of transcription according to our studies. Since the EGFR/MAPK signal transduction pathway is a canonical proliferative pathway, its effects on the cell are counter to the inhibitory effect of Hh signaling, which inhibits epithelial cell proliferation through Bmp-2/-4 signaling. Although Hh signaling in the gut is paracrine, the effects of EGFR signaling on Hh expression we observed occur only in the intestinal epithelial cells (cell autonomous).
In summary, the reliance of intestinal epithelial growth factor signaling on β1 integrins suggests strong regulation of intestinal epithelial proliferation by the extracellular matrix. β1 integrins are uniquely poised at the epithelial-stromal interface to mediate communications between these two compartments, both of which contribute to the various glycoproteins that constitute the extracellular matrix that lies between them.
Supplementary Material
Supplemental Figure 1. Intestinal epithelial trafficking defects in villin-Cre/Itgb1flox/flox mice. A-B, Electron micrographs showing a paucity of endocytic vesicles (white arrows) in the subcortical regions of intestinal epithelial cells in the villi of P7 villin-Cre/Itgb1flox/flox mice (B) compared with their control littermates (A). C-D, Small intestinal explants from P7 villin-Cre/Itgb1flox/flox (D) and wild-type (C) littermates were incubated with Alexa-fluor-labeled IgG for various times. The explants were then fixed in paraformaldehyde, sectioned, and examined simultaneously with an immunofluoresence microscope using fixed settings. The white arrowheads show the presence of intraepithelial IgG in intestinal villi. The dotted lines denote the epithelial-stromal border. E-F, Small intestinal epithelia from P7 villin-Cre/Itgb1flox/flox mice (F) and their wild-type littermates (E) were stained with FITC-phalloidin. Cross-sections of the villi are shown. The white arrowheads in the insets denote actin (green) and the dotted lines demarcate the epithelial-stromal border. Insets are higher magnification photos of the boxed areas.
Acknowledgments
This work was funded by grants from the NIDDK (DK02531 to SK), the Huntsman Cancer Foundation (SK, MKT), Veterans Affairs (SK) and the NCI (P01CA073992 to MKT). The work was also supported by access to technical cores supported by a Cancer Center Support Grant (P30CA042014) to the Huntsman Cancer Institute.
References
- 1.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 3.Li YQ, Roberts SA, Paulus U, Loeffler M, Potten CS. The crypt cycle in mouse small intestinal epithelium. J Cell Sci. 1994;107(Pt 12):3271–3279. doi: 10.1242/jcs.107.12.3271. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183:363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- 5.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 8.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 9.Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–482. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 10.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiological reviews. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 12.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. Embo J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehead RH, Brown A, Bhathal PS. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol. 1987;23:436–442. doi: 10.1007/BF02623860. [DOI] [PubMed] [Google Scholar]
- 15.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 16.Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. American journal of human genetics. 2000;67:1047–1054. doi: 10.1016/s0002-9297(07)62934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- 18.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, Nielsen C, Gaffield W, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 19.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 20.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- 22.Reinchisi G, Parada M, Lois P, Oyanadel C, Shaughnessy R, Gonzalez A, Palma V. Sonic Hedgehog modulates EGFR dependent proliferation of neural stem cells during late mouse embryogenesis through EGFR transactivation. Frontiers in cellular neuroscience. 2013;7:166. doi: 10.3389/fncel.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotschel F, Berg D, Gruber W, Bender C, Eberl M, Friedel M, Sonntag J, Rungeler E, Hache H, Wierling C, Nietfeld W, Lehrach H, Frischauf A, Schwartz-Albiez R, Aberger F, Korf U. Synergism between Hedgehog-GLI and EGFR signaling in Hedgehog-responsive human medulloblastoma cells induces downregulation of canonical Hedgehog-target genes and stabilized expression of GLI1. PloS one. 2013;8:e65403. doi: 10.1371/journal.pone.0065403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu G, Zhou J, Song W, Wu D, Dang Q, Zhang L, Li L, Wang X, He D. Role of GLI-1 in epidermal growth factor-induced invasiveness of ARCaPE prostate cancer cells. Oncology reports. 2013;30:904–910. doi: 10.3892/or.2013.2534. [DOI] [PubMed] [Google Scholar]
- 25.Keysar SB, Le PN, Anderson RT, Morton JJ, Bowles DW, Paylor JJ, Vogler BW, Thorburn J, Fernandez P, Glogowska MJ, Takimoto SM, Sehrt DB, Gan GN, Eagles-Soukup JR, Serracino H, Hirsch FR, Lucia MS, Thorburn A, Song JI, Wang XJ, Jimeno A. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res. 2013;73:3381–3392. doi: 10.1158/0008-5472.CAN-12-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keng VW, Watson AL, Rahrmann EP, Li H, Tschida BR, Moriarity BS, Choi K, Rizvi TA, Collins MH, Wallace MR, Ratner N, Largaespada DA. Conditional Inactivation of Pten with EGFR Overexpression in Schwann Cells Models Sporadic MPNST. Sarcoma. 2012;2012:620834. doi: 10.1155/2012/620834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eimer S, Dugay F, Airiau K, Avril T, Quillien V, Belaud-Rotureau MA, Belloc F. Cyclopamine cooperates with EGFR inhibition to deplete stem-like cancer cells in glioblastoma-derived spheroid cultures. Neuro-oncology. 2012;14:1441–1451. doi: 10.1093/neuonc/nos266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitkara D, Singh S, Kumar V, Danquah M, Behrman SW, Kumar N, Mahato RI. Micellar Delivery of Cyclopamine and Gefitinib for Treating Pancreatic Cancer. Molecular pharmaceutics. 2012 doi: 10.1021/mp3002792. [DOI] [PubMed] [Google Scholar]
- 29.Qin CF, Hao K, Tian XD, Xie XH, Yang YM. Combined effects of EGFR and Hedgehog signaling pathway inhibition on the proliferation and apoptosis of pancreatic cancer cells. Oncology reports. 2012;28:519–526. doi: 10.3892/or.2012.1808. [DOI] [PubMed] [Google Scholar]
- 30.Eberl M, Klingler S, Mangelberger D, Loipetzberger A, Damhofer H, Zoidl K, Schnidar H, Hache H, Bauer HC, Solca F, Hauser-Kronberger C, Ermilov AN, Verhaegen ME, Bichakjian CK, Dlugosz AA, Nietfeld W, Sibilia M, Lehrach H, Wierling C, Aberger F. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO molecular medicine. 2012;4:218–233. doi: 10.1002/emmm.201100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mimeault M, Batra SK. Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacological reviews. 2010;62:497–524. doi: 10.1124/pr.109.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M, Aberger F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, Kanai F, Kawabe T, Omata M. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Molecular carcinogenesis. 2009;48:703–712. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- 34.Francis P, Namlos HM, Muller C, Eden P, Fernebro J, Berner JM, Bjerkehagen B, Akerman M, Bendahl PO, Isinger A, Rydholm A, Myklebost O, Nilbert M. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC genomics. 2007;8:73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. Journal of cellular and molecular medicine. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G, Philpott MP, Aberger F. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Molecular and cellular biology. 2006;26:6283–6298. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 38.Bigelow RL, Jen EY, Delehedde M, Chari NS, McDonnell TJ. Sonic hedgehog induces epidermal growth factor dependent matrix infiltration in HaCaT keratinocytes. The Journal of investigative dermatology. 2005;124:457–465. doi: 10.1111/j.0022-202X.2004.23590.x. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto M, Hoffenberg EJ, Carethers JM, Doctolero R, Tajima A, Sugano K, Franklin WA, Ahnen DJ. Nuclear accumulation of beta-catenin occurs commonly in the epithelial cells of juvenile polyps. Pediatr Res. 2005;57:4–9. doi: 10.1203/01.PDR.0000148062.57051.8F. discussion 1-3. [DOI] [PubMed] [Google Scholar]
- 40.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 41.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 42.Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 43.Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- 44.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 45.Mays RW, Beck KA, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 46.Nesterov A, Wiley HS, Gill GN. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8719–8723. doi: 10.1073/pnas.92.19.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Intestinal epithelial trafficking defects in villin-Cre/Itgb1flox/flox mice. A-B, Electron micrographs showing a paucity of endocytic vesicles (white arrows) in the subcortical regions of intestinal epithelial cells in the villi of P7 villin-Cre/Itgb1flox/flox mice (B) compared with their control littermates (A). C-D, Small intestinal explants from P7 villin-Cre/Itgb1flox/flox (D) and wild-type (C) littermates were incubated with Alexa-fluor-labeled IgG for various times. The explants were then fixed in paraformaldehyde, sectioned, and examined simultaneously with an immunofluoresence microscope using fixed settings. The white arrowheads show the presence of intraepithelial IgG in intestinal villi. The dotted lines denote the epithelial-stromal border. E-F, Small intestinal epithelia from P7 villin-Cre/Itgb1flox/flox mice (F) and their wild-type littermates (E) were stained with FITC-phalloidin. Cross-sections of the villi are shown. The white arrowheads in the insets denote actin (green) and the dotted lines demarcate the epithelial-stromal border. Insets are higher magnification photos of the boxed areas.


