Summary
The blood-brain barrier (BBB) limits entry of blood-derived products, pathogens and cells into the brain that is essential for normal neuronal functioning and information processing. Post-mortem tissue analysis indicates BBB damage in Alzheimer’s disease (AD). The timing of BBB breakdown remains, however, elusive. Using an advanced dynamic contrast-enhanced magnetic resonance imaging protocol with high spatial and temporal resolutions to quantify regional BBB permeability in the living human brain, we show an age-dependent BBB breakdown in the hippocampus, a region critical for learning and memory that is affected early in AD. The BBB breakdown in the hippocampus and its CA1 and dentate gyrus subdivisions worsened with mild cognitive impairment that correlated with injury to BBB-associated pericytes, as shown by the cerebrospinal fluid analysis. Our data suggest that BBB breakdown is an early event in the aging human brain that begins in the hippocampus and may contribute to cognitive impairment.
Keywords: Blood-brain barrier breakdown, hippocampus, living human brain, high resolution magnetic resonance imaging, cognitive impairment
Introduction
Neuronal computation and normal functioning of the central nervous system (CNS) requires tight control of the chemical composition of the neuronal ‘milieu’ that is maintained by the blood-brain barrier (BBB) (Iadecola, 2004; Zlokovic, 2008, 2011; Iadecola, 2013). Brain endothelial cells and perivascular mural cells, pericytes, form the BBB which limits entry of neurotoxic plasma-derived proteins, circulating metals, pathogens, red blood cells and leucocytes into the brain. Studies in murine transgenic models have shown that a chronic BBB breakdown leads to accumulation of blood-derived neurotoxic proteins in the CNS including fibrin, thrombin, hemoglobin, iron-containing hemosiderin, free iron and/or plasmin (an extracellular matrix-degrading enzyme) causing progressive neurodegeneration with loss of neurons mediated by either direct neuronal toxicity, oxidant stress and/or detachment of neurons from their supporting extracellular matrix (Daneman et al., 2010; Armulik et al., 2010; Bell et al., 2010, 2012; Winkler et al., 2012, 2014).
Alzheimer’s disease (AD) is characterized by selective neuronal vulnerability resulting in progressive loss of memory (LaFerla, 2012). Post-mortem studies have shown BBB damage in AD including accumulation in the hippocampus and cortex of blood-derived proteins (e.g., immunoglobulins, albumin, fibrinogen, and thrombin) (Fiala et al., 2002; Salloway et al., 2002; Zipser et al., 2007; Ryu et al., 2009; Hultman et al., 2013; Sengillo et al., 2013) and degeneration of BBB-associated pericytes (Sengillo et al., 2013; Farkas and Luiten, 2001; Baloyannis and Baloyannis, 2012). Brain imaging studies have shown microbleeds and accumulation of iron in AD (Cullen et al., 2005; Goos et al., 2009; Zonneveld, 2014), particularly in the hippocampus (Raven et al., 2013). Some studies using the cerebrospinal fluid (CSF) to plasma ratio of blood-derived albumin, reported BBB damage in AD particularly associated with vascular risk factors (Blennow et al., 1990; Bowman et al., 2012) or in individuals at a genetic risk for AD (Halliday et al., 2013). At what stage BBB breakdown occurs in the living human brain and whether it contributes to cognitive impairment remains, however, controversial.
Here, we used an advanced dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and post-processing analysis with improved spatial and temporal resolutions to quantify the BBB regional permeability Ktrans constant in the living human brain in individuals with no cognitive impairment (NCI) and mild cognitive impairment (MCI). Compared to a previous approach limited to measurements of BBB permeability in the white matter (WM) (Taheri et al., 2011a, 2011b, 2013), the current method allows simultaneous measurements of BBB Ktrans permeability in different grey and WM regions. Additionally, we analyzed CSF biomarkers of BBB breakdown, injury to brain vascular cells including pericytes and endothelial cells, inflammatory response, neuronal injury (i.e., tau and pTau) and amyloid β-peptides (Aβ). We found an age-dependent BBB breakdown in the hippocampus, a region involved in learning and memory (Squire, 1992) that is damaged early in AD (Braak et al., 1993; Mu and Gage, 2011; Whitwell et al., 2012). The BBB breakdown in the hippocampus worsened with MCI that correlated with measures of injury to BBB-associated pericytes.
Results
BBB breakdown in the hippocampus during normal aging
We studied twelve CNS regions including hippocampus and its subdivisions CA1, CA3 and dentate gyrus (DG), different cortical regions, subcortical regions (e.g., thalamus, striatum, and caudate nucleus) and the WM regions including corpus callosum and internal capsule (Fig. 1A–B), and generated regional Ktrans BBB permeability maps for each individual using a modified Patlak linearized regression mathematical analysis (Patlak and Blasberg, 1985) (Fig. 1C). We determined in each individual the arterial input function (AIF) from the common carotid artery instead of using an average value from the superior sagittal venous sinus to determine tracer concentration in blood (Taheri et al., 2011a, 2011b, 2013; Larsson et al., 2009). Individual AIF measurements are important particularly if the studied population diverges by age as changes in blood volume and flow may affect AIF and the Ktrans measurements.
Fig. 1. Blood-brain barrier breakdown in the hippocampus in the living human brain during normal aging.
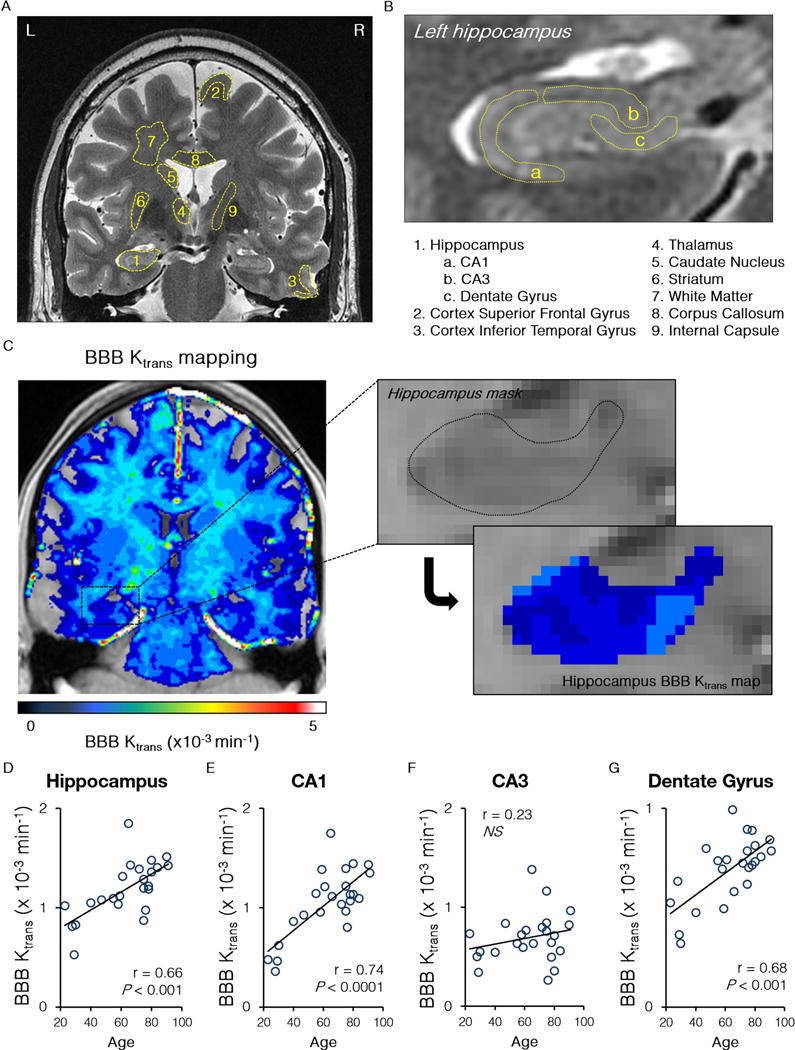
(A–B) A dynamic contrast enhanced magnetic resonance imaging was used to quantify the blood-brain barrier (BBB) regional permeability Ktrans constant in 12 regions-of-interest in the gray and white matter in the living human brain (A) including subdivisions of the hippocampus (B). (C) Representative BBB Ktrans maps of the whole brain (left) and the hippocampus (right) in a young participant with no cognitive impairment. (D–G) Age-dependent increase in the BBB permeability Ktrans constant in the entire hippocampus, its CA1 region and dentate gyrus, but not CA3 region. Single data points for the Ktrans constant from 24 individuals with no cognitive impairment (both genders, ages 23–91; see Table 1) were plotted against age; r = Pearson’s coefficient; P, significance; NS, non-significant. See also Supplementary Figure S1.
Unexpectedly, we found that NCI individuals (Table 1) have an age-dependent progressive loss of BBB integrity in the hippocampus, as shown by an age-dependent increase in the Ktrans values in the entire hippocampus, its CA1 region and DG, but not the CA3 region (Fig. 1D–G). No significant BBB changes during aging were found in cortical (e.g., frontal cortex, temporal cortex) or subcortical (e.g., thalamus, striatum) regions except for the caudate nucleus (Supplementary Fig. S1A–E). Surprisingly, we did not find significant age-dependent changes in the BBB in subcortical WM fibers, corpus callosum and internal capsule (Supplementary Fig. S1F–H), even though WM is believed to be affected by vascular changes early in AD (Iadecola, 2013; Yoshita et al., 2006). Collectively, our data suggest that early vascular leakage in the aging human brain begins in the hippocampus that normally shows the highest barrier properties (i.e., the lowest Ktrans values) compared to other brain regions.
Table 1.
Patients' Demographic Information
| NCI, young | NCI, older | MCI | MS | |
|---|---|---|---|---|
| Clinical Dementia Rating scale | 0 | 0 | 0.5 | 0 |
| Number of participants | 6 | 18 | 21 | 19 |
| Female, % | 50 | 55.6 | 52.4 | 63.2 |
| Age range | 23–47 | 55–91 | 55–85 | 26–53 |
| DCE-MRI | 6/6 | 18/18 | 20/21 | 19/19 |
| Lumbar puncture 0/6 15/18 17/21 | 0/6 | 15/18 | 17/21 | 0/19 |
| Age at lumbar puncture, Mean (SO) | N/A | 73.2 (10.6) | 72.0 (8.5) | N/A |
NCI, no cognitive impairment; MCI, mild cognitive impairment; MS, multiple sclerosis; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; SD, standard deviation; N/A, not applicable.
Accelerated BBB breakdown in the hippocampus in individuals with MCI
Next, we compared the BBB permeability in young NCI and older NCI (both CDR=0), and MCI (CDR=0.5) groups (Fig. 2; Table 1). We found a significant increase in the BBB permeability Ktrans values in the hippocampus (Fig. 2A) and its CA1 and DG regions, but not CA3, by 41%, 107% and 48% in the older compared to the young NCI group, and by 24%, 53% and 27% in MCI compared to age-matched older NCI controls, respectively (Fig. 2B–E). In contrast, there were no significant differences in the BBB permeability in cortical, subcortical and WM regions between young and older NCI, and/or MCI and age-matched older NCI participants (Supplementary Table 1), except for an increase in the caudate nucleus in older compared to young NCI group. We did not find significant changes in hippocampal volumes between the studied groups determined on coronal T2-weighted MR images (Fig. 3).
Fig. 2. Blood-brain barrier breakdown in the hippocampus during normal aging and aging associated with mild cognitive impairment.
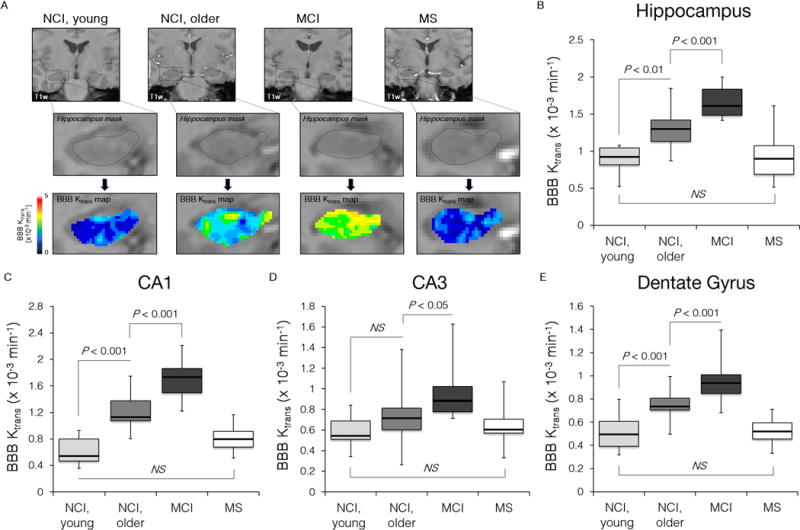
(A) Representative Ktrans maps within the hippocampus in young and older individuals with no cognitive impairment (NCI) and mild cognitive impairment (MCI). MS, a multiple sclerosis case with no cognitive impairment. (B–E) A progressive significant increase in the BBB permeability constant Ktrans in older compared young NCI group and MCI compared to older NCI group in the entire hippocampus, CA1 region and dentate gyrus. MS group was compared with age-matched young NCI group. Boxplots represent the median (dark horizontal line), with the box representing the 25th and 75th percentiles, the whiskers the 5th and 95th percentiles. P, significance by ANOVA followed by Tukey’s post hoc tests; NS, non-significant. NCI, young (n=6, ages 23–47, both genders); NCI, older (n=18, ages 55–91, both genders); MCI (n=20, ages 55–85, both genders); MS (n=19, ages 26–53, both genders). See Table 1 and Supplementary Figure S2.
Fig. 3. Hippocampus volume in the studied groups.
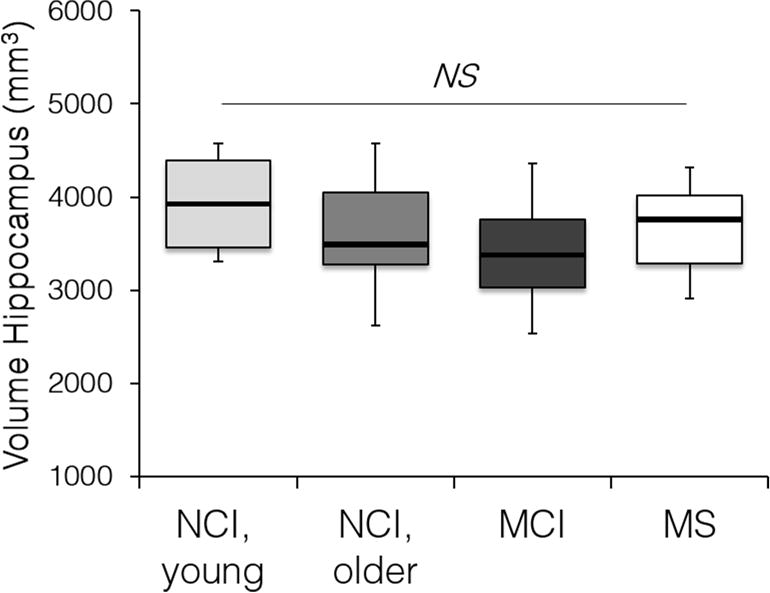
Hippocampus volume was determined on T2-weighted images in individuals with no cognitive impairment (NCI), with mild cognitive impairment (MCI), and multiple sclerosis (MS) cases with no cognitive impairment. NS, non-significant by ANOVA followed by Tukey’s post hoc tests. NCI, young (n=6, ages 23–47, both genders); NCI older (n=18, ages 55–91, both genders); MCI (n=20, ages 55–85, both genders); MS (n=19, ages 26–53, both genders). See Table 1.
To validate our method, we studied multiple sclerosis (MS) cases with established BBB breakdown in the WM, as reported (Taheri et al., 2011b), as an additional neurological control. MS cases had a diagnosis of the relapsing remitting MS and met McDonald Criteria (Polman et al., 2011). We selected younger MS cases without cognitive complaints that were age-matched to younger NCI controls (Table 1). The MS patients did not show changes in the Ktrans values in the hippocampus or hippocampal subregions (Fig. 2B–E), neither in other CNS grey matter regions (Supplementary Table 1). They had, however, increased BBB permeability in the total WM, corpus callosum and internal capsule compared to age-matched younger NCI group by 32%, 26% and 23% (p<0.001), respectively (Supplementary Table 1), consistent with the reported BBB alterations in the WM in MS (Taheri et al., 2011b). Our data extend importantly previous findings by showing no changes in the BBB integrity in the hippocampus and other studied grey matter regions in MS.
Molecular biomarker CSF analysis
A significant 30% increase in the CSF/plasma albumin ratio (Blennow et al., 1990; Bowman et al., 2012; Halliday et al., 2013) additionally confirmed BBB breakdown in MCI individuals compared to age-matched NCI controls (Supplementary Fig. S2A). The increase in the CSF/plasma albumin ratio correlated with an increase in the Ktrans values in the hippocampus and its CA1 and DG subregions (Supplementary Fig. S2B–D) that showed an increase in the BBB permeability in MCI compared to age-matched NCI controls (Fig. 2B, C and E).
Next, we studied correlations between Ktrans values and CSF levels of soluble platelet-derived growth factor receptor β (sPDGFRβ). PDGFRβ is an established marker of the BBB-associated pericytes (Armulik et al., 2010; Bell et al., 2010, 2012; Winkler et al., 2011) that play a key role in maintaining the BBB integrity (Zlokovic, 2008, 2011; Iadecola, 2013; Daneman et al., 2010; Armulik et al., 2010; Bell et al., 2010, 2012; Winkler et al., 2012). Pericytes degenerate in AD (Sengillo et al., 2013; Farkas and Luiten, 2001; Baloyannis and Baloyannis, 2012) and have a key role in BBB clearance of Alzheimer’s toxin amyloid β-peptide (Aβ) (Sagare et al., 2013). Pericytes die when Aβ intracellular accumulation overrides their Aβ clearance capability (Sagare et al., 2013) and when exposed to hypoxia. Both conditions are associated with shedding of the soluble form of the receptor (sPDGFRβ) from primary human cultured pericytes (Supplementary Fig. S3A–D). Furthermore, sPDGFRβ levels were increased by 115% in MCI compared to age-matched NCI controls (Fig. 4A). There was a positive correlation between sPDGFRβ CSF levels and the Ktrans values in the hippocampus including its CA1 and DG subdivisions (Fig. 4B–D) that showed increased BBB permeability in MCI compared to NCI individuals (Fig. 2B, C and E).
Fig. 4. Soluble platelet-derived growth factor receptor β in the cerebrospinal fluid in humans and mice.
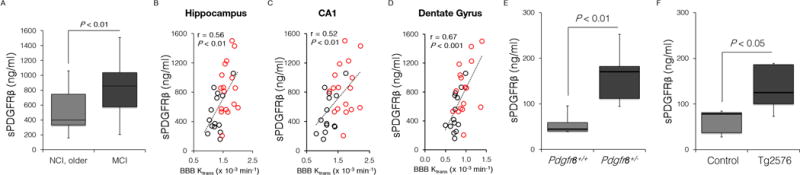
(A) Elevated sPDGFRβ levels in the CSF in individuals with mild cognitive impairment (MCI; n=17) compared to age-matched group with no cognitive impairment (NCI, older; n=14). (B–D) Single data points for sPDGFRβ CSF levels from 31 individuals with NCI (n=14, black) or MCI (n=17, red) plotted against the Ktrans constant in the hippocampus (B), its CA1 region (C), and dentate gyrus (D); r = Pearson’s coefficient; P, significance. (E–F) sPDGFRβ CSF levels in 16-month-old Pdgfrβ+/− mice and Pdgfrβ+/+ controls (C) and 16-month-old Tg2576 mice compared to age-matched littermate controls (D). In C and D, n=5 mice per group. P, significance by a Student’s t-test. See also Supplementary Figures S3 and S4.
To validate CSF sPDGFRβ as a marker of pericyte injury in vivo, we studied sPDGFRβ CSF levels in 16-month-old pericyte-deficient Pdgfrβ+/− mice which develop ~45–50% loss of brain pericytes (Bell et al., 2010) and 16-month-old Alzheimer’s Tg2576 mice which develop an age-dependent pericyte loss from 17% at 9 months of age (Sagare et al., 2013) to 35% at 18 months of age (Park et al., 2013). There was a significant 289% and 58% increase in sPDGFRβ CSF levels in Pdgfrβ+/− mice and Tg2576 mice, respectively, compared to their corresponding littermate controls (Fig. 4E–F), indicating that sPDGFRβ is a reliable CSF marker of pericytes injury in mice.
The CSF analysis revealed no injury to other cell types in the neurovascular unit (Iadecola, 2004; Zlokovic, 2008, 2011) in NCI or MCI including endothelial cells as shown by unaltered CSF levels of biomarkers of endothelial cell injury such as soluble intercellular adhesion molecule-1 (sICAM-1) and vascular cell adhesion molecule-1 (sVCAM-1) (Iadecola, 2004; Zlokovic, 2008); no change in the inflammatory response as shown by unaltered CSF levels of several studied cytokines (e.g., interleukins IL-2, IL-6, and IL-8, tumor necrosis factor-α, and interferon-γ); no change in neuronal injury (e.g., tau and pTau) and Aβ (e.g., Aβ38, Aβ40 and Aβ42); and no change in matrix metalloproteinase-9 that is involved in degradation of the BBB tight junction and the basement membrane proteins of the vessel wall (Bell et al., 2012; Halliday et al., 2013) (Supplementary Fig. S4).
Discussion
We developed an advanced DCE-MRI approach and post-processing analysis resulting in improved spatial resolution and signal-to-noise ratio (SNR) of the Ktrans BBB maps with the analysis of the arterial input function in each individual allowing for accurate measurements of the regional BBB permeability in the living human brain in different grey and WM regions. For example, our high resolution hippocampal imaging allows for characterization of the Ktrans BBB values not only in the hippocampus, but also in the hippocampal subfields. In comparison, studies on the blood-brain tumor barrier permeability (Larsson et al., 2009) or BBB in stroke (Aksoy et al., 2013) do not generally require spatial resolution or SNR as high as the present study, as changes in the barrier permeability in brain tumors or after stroke are typically one order of magnitude or more higher than the presently measured BBB changes during normal aging, aging associated with MCI and/or possibly other neurodegenerative conditions. The BBB permeability Ktrans values in the hippocampus and cortex and other brain regions in young NCI individuals were within a range of previously reported BBB Ktrans values to small inert polar molecules in mammals including rodents (Zlokovic, 2011; Bell et al., 2010; Deane et al., 2003).
We show that the BBB breakdown during normal aging occurs initially in the hippocampus, a region critical for learning and memory. The BBB breakdown was more pronounced in MCI compared to age-matched neurologically intact controls raising a possibility that it might contribute to early cognitive impairment. Interestingly, our data show that the BBB integrity in other brain regions including cortical and subcortical regions or the WM remains relatively unaffected during normal aging or aging associated with MCI. Although we did not find significant changes in hippocampal volumes between the young and older NCI and MCI individuals, it is possible that an early and progressive increase in the BBB permeability, as we show in the hippocampus in older NCI and MCI individuals, might precede hippocampal atrophy seen later in AD (Whitwell et al., 2012; Apostolova et al., 2010) particularly in MCI progressing to AD. This would be similar to findings in animal models with a chronic BBB disruption showing that vascular leakages over time lead to hippocampal and cortical atrophy, loss of neurons and progressive behavioral changes (Bell et al., 2010, 2012; Winkler et al., 2012, 2014).
Findings in murine models of a small vessel brain disease (Daneman et al., 2010; Armulik et al., 2010; Bell et al., 2010, 2012) and human post-mortem AD studies (Fiala et al., 2002; Salloway et al., 2002; Zipser et al., 2007; Ryu et al., 2009; Hultman et al., 2013; Sengillo et al., 2013) have shown that BBB breakdown leads to tissue accumulation of potentially neurotoxic blood-derived products that normally do not enter the brain, but can damage neurons when the vessels become leaky. We show that pericyte injury and possibly early degeneration correlates with increased BBB permeability within the hippocampus, a region known to be affected by pericyte loss and BBB breakdown on post-mortem tissue analysis in AD (Sengillo et al., 2013). Although, our CSF biomarkers analysis did not show endothelial cell injury, involvement of inflammatory cytokines and/or direct vasculotoxic effects of Aβ in MCI, it is possible that some of these factors could play a role in magnifying BBB damage at later disease stages during progression to dementia due to AD, as they all were shown to alter BBB permeability in experimental models (Zlokovic, 2011).
In summary, our data suggest loss of cerebrovascular integrity during normal aging and aging associated with MCI that begins in the hippocampus which may contribute to early stages of dementia associated with AD.
Supplementary Material
Acknowledgments
We would like to thank the National Institutes of Health for grants R37NS34467 (B.V.Z.), R37AG23084 (B.V.Z.), R01AG039452 (B.V.Z.), Zilkha Senior Scholar support (B.V.Z.), R21EB013456 (M.L.), UL1TR000130 (M.L.), L.K. Whittier Foundation (M.G.H), P50AG05142 (H.C.C.), 7P41EB015922 (A.W.T.) and EB000993 (R.E.J.).
Footnotes
Experimental procedures
Please see Supplemental Materials.
Author contributions
A.M. and B.V.Z. designed research and analyzed and interpreted data; A.M., S.R.B., M.D.S., M.R.H., A.P.S. and Z.Z. performed experiments and analyzed data; A.M., S.R.B. and R.E.J. contributed to a new analytic software; C.Y.L., H.C.C., M.L., L.A. and M.G.H. recruited participants and performed and provided imaging scans; S.R.B., A.W.T., R.E.J., H.C.C., M.L. and M.G.H. provided critical reading of the manuscript; A.M. contributed to manuscript writing; and B.V.Z. wrote the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW, Gupta SN, Narayana R, Fischbein N, Wijman CA. Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. J Am Heart Assoc. 2013;2:e000161. doi: 10.1161/JAHA.113.000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, Cummings JL, Toga AW, Trojanowski JQ, Shaw LM, et al. Alzheimer’s Disease Neuroimaging Initiative, 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: A study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare AP, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, et al. SRF and myocardin regulate LRP-mediated amyloid-β clearance in brain vascular cells. Nature Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol Scan. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res 2012. 2012 doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Kócsi Z, Stone J. Pericapillary haem-rich deposits: Evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nature Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM. Patients with Alzheimer disease with multiple microbleeds: Relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40:3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin A levels and matrix metalloproteinase-9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman K, Strickland S, Norris EH. The APOE e4/e4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Reviews Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Preclinical success against Alzheimer’s disease with an old drug. N Engl J Med. 2012;367:570–572. doi: 10.1056/NEJMcibr1204890. [DOI] [PubMed] [Google Scholar]
- Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med. 2009;62(5):1270–1281. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. PNAS. 2013;110:3089–3094. doi: 10.1073/pnas.1300021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimer’s Disease. 2013;37:127–136. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier: Fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nature Commun. 2013;4 doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salloway S, Gur T, Berzin T, Zipser B, Correia S, Hovanesian V, Fallon J, Kuo-Leblanc V, Glass D, Hulette C, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J Neurol Sci. 2002;203:183–187. doi: 10.1016/s0022-510x(02)00288-5. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011a;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Gasparovic C, Shah NJ, Rosenberg GA. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med. 2011b;65:1036–1042. doi: 10.1002/mrm.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Rosenberg GA, Ford C. Quantification of blood-to-brain transfer rate in multiple sclerosis. Mult Scler Relat Disord. 2013;2:124–132. doi: 10.1016/j.msard.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, et al. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Roberts RO, Boeve BF, Petersen RC, Jack CR., Jr Comparison of imaging biomarkers in the Alzheimer disease neuroimaging initiative and the Mayo Clinic Study of Aging. Arch Neurol. 2012;69:614–622. doi: 10.1001/archneurol.2011.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nature Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, et al. Blood-spinal cord barrier disruption contributes to early motor neuron degeneration in ALS model mice. PNAS. 2014;111:E1035–42. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare AP, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld HI. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014;82:698–704. doi: 10.1212/WNL.0000000000000150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


