Abstract
Preeclampsia (PE) is characterized by increased uterine artery resistance index (UARI), chronic immune activation and decreased circulating nitric oxide levels (NO). 17-alpha-hydroxyprogesterone caproate (17-OHPC) is a synthetic metabolite of progesterone used for the prevention of recurrent preterm birth. We hypothesized that 17-OHPC could reduce blood pressure (MAP) by decreasing inflammation while improving vasodilation by increasing NO bioavailability and UARI during late gestation in the Reduced Uterine Perfusion Pressure, RUPP, rat model of PE. 17-OHPC (3.32mg/kg) was intraperitoneally administered on gestation day 18 into RUPP rats, carotid catheters inserted and MAP, blood and tissues were collected on day 19. MAP in normal pregnant (NP) (n=13) was 92±2.0 and increased to123±2.0 in RUPP (n=18, P<0.0001), which was improved to 116 ±1.5 mmHg in RUPP+17-OHPC (n=10, P<0.05). Circulating CD4+ T cells were 1.19±1.0% of gated cells in NP (n=7), which increased to 8.52±2.4% in RUPP rats (n=10, P<0.05) but was reduced to 2.72±0.87% (n=14, P<0.05) in RUPP+17-OHPC. Circulating nitrate/nitrite was 26.34 ±3.5 µM in NP (n=12) but was reduced to14.58±3.1 in RUPP rats (n=8, P=0.03) and increased to 22.69±1.62 in RUPP+17-OHPC (n=7, P=0.05). eNOS expression was 0.65±0.11 A.U in NP (n=4), which decreased to 0.33±0.01 in RUPP rats (n=4, P=0.05) but increased to 0.57±0.01 in RUPP+17-OHPC (n=5, P=0.03). UARI was 0.54±0.02 in NP (n=3), 0.78±0.03 in RUPP (n=4) and 0.63±0.038 in RUPP+17-OHPC (n=8, both P<0.05). Our findings demonstrate that even though modest, lowering blood pressure with 17OHPC could be a viable treatment option for suppressing inflammation, uterine artery vasoconstriction while improving litter size.
Keywords: pregnancy, hypertension, progesterone, inflammation, nitric oxide
INTRODUCTION
Preeclampsia (PE) is a relatively common pregnancy disorder usually characterized by hypertension, abnormal amounts of protein in the urine, increased inflammatory cytokines, decreased vasodilators such as nitric oxide (NO) and other systemic disturbances1–7. This condition affects about 5–8% of pregnancies and despite being one of the leading causes of death in pregnant women, complete understanding of the mechanisms responsible for PE pathogenesis remains elusive2, 8, 9.
A major initiating event leading to the development of PE is thought to be reduced placental perfusion that leads to widespread dysfunction of the maternal vascular endothelium by mechanisms that remain to be determined2, 8, 10. In addition, mediators of endothelial dysfunction such as decreased production of the nitric oxide, increased production of the vasoconstrictor endothelin-1 (ET-1) and enhanced vascular reactivity to angiotensin II (ANG II) type 1 receptor autoantibodies (AT1-AA), play a role in the development of hypertension during pregnancy4, 6, 11–17.
Currently there is no effective treatment for very preterm PE except for early delivery of the fetus along with the placenta. Thus PE continues into the 21st century as the primary global cause of prematurity and perinatal morbidity/mortality. Progesterone supplementation in the form of 17-alpha-hydroxyprogesterone caproate (17-OHPC) is currently used obstetrically to prevent recurrent preterm birth in patients with pregnancies not complicated by PE18–20. We reported that patients with severe preeclampsia exhibit significantly lower serum progesterone concentrations than gestational age- and race-matched non-preeclamptics21. In addition, we have previously shown that supplementation of placental ischemic rats with 17-OHPC decreased blood pressure, inflammatory cytokines, and ET-1 within 24 hours of treatment21–23. In addition, we have shown that progesterone inhibits TNF alpha induced ET-1 secretion within 6 hours of exposure of human umbilical venous endothelial cells (HUVECs) to TNF-alpha in vitro22. Furthermore, HUVECs secreted significantly greater ET-1 following exposure to PE serum than when exposed to NP serum. This response was blunted within 6 hours of exposure to progesterone21.
Additionally, there is evidence that progesterone beyond the anti-inflammatory effects may have vasodilatory effects and can improve NO availability24, 25. Interestingly, our previous study has shown that administration of 17-OHPC increased placental NO and decreased AT1-AA, thus improving hypertension in the IL-6 induced hypertensive pregnant rats26. Our previous studies examining an effect of 17-OHPC on pregnancy outcome in the RUPP rat did not investigate the effect of 17-OHPC to decrease immune cells as potential source of lowered cytokines, nor did we examine the vasodilatory effects of 17-OHPC.
Although 17-OHPC is administered routinely for the prevention of recurrent of preterm labor, the addition of 17-OHPC for the management of preeclampsia has been debated but the benefit of 17 OHPC in response to placental ischemia is still unclear. While preeclampsia is associated with decreased circulating progesterone and increases in inflammatory cytokines, it remains unclear what role 17-OHPC may have in decreasing immune activation while improving vasodilation and hypertension in response to placental ischemia. Therefore, in the present study, we hypothesized that 17-OHPC could reduce blood pressure (MAP), pro-inflammatory cytokines and CD4+ T cells, while improving uterine artery resistance index (UARI) and increasing NO bioavailability in the hypertensive Reduced Uterine Perfusion Pressure (RUPP) rat model of PE.
MATERIALS AND METHODS
Animals and treatment
This study complied with guidelines of the University of Mississippi Medical Center, and the animals were handled according to the guiding principles published in the National Institutes of Health Guide for the Care of Animals and the Institutional Animal Care and Use Committee (IACUC). Sprague Dawley rats purchased Harlan Inc. (Indianapolis, IN) were used in the present study. Rats were housed in a temperature-controlled room (23°C) with a 12:12 hour light/dark cycle with free access to standard rat chow and water. Surgical procedures were carried out under appropriate anesthesia and analgesics were given post-operatively as needed. In general inhalant anesthetics are safer than injectable anesthetics. Then, pregnant rats at gestational day 14 and day 18 were either exposed to 2.0% isoflurane in a humidified 100% oxygen carrier gas. On day 14 of gestation, rat dams weighting approximately 200–250g were randomly assigned to either RUPP or normal pregnant (NP) control groups. Following a midline incision, pregnant rats in the RUPP group underwent a midline incision and the lower abdominal aorta was isolated. A silver clip (0.203 mm ID) was placed around the aorta above the iliac bifurcation. To prevent augmentation of blood flow to the uterus via the ovarian arteries, silver clips (0.100 mm ID) were also placed on the branches of both ovarian arteries that supply the uterus. When the clipping procedure resulted in total reabsorption of the fetuses, the rats were excluded from the study2 Previous studies indicate no physiological changes in blood pressure, pup weight nor inflammation in NP rats undergoing sham surgeries, therefore these surgeries are not a necessary use of pregnant rats and are not repeated with each RUPP study performed.
RUPP rats were injected with 17-alpha-hydroxyprogesterone caproate (17-OHPC) diluted in sterile normal saline at day 18 of gestation. Previous studies were performed with administration of 17 OHPC to Normal pregnant rats and no differences were noted in blood pressure, pup weights, nor inflammatory cytokines or ET-1, therefore this group was not repeated. The 17-OHPC (Marty’s Compounding Pharmacy, Jackson, MS) was diluted in normal saline and administered intraperitoneally as 0.5 cm3 solution of 3.32 mg/kg 17-OHPC to pregnant rats. We chose the one-time 17-OHPC dose to be the weight equivalent of a typical human dose for the prevention of preterm labor and what was previously shown to be effective in RUPP rats21.
Please see the online-only Data Supplement for detailed methods regarding mean arterial blood pressure, uterine artery resistance index, circulating CD4+ T cells, western blotting analysis of endothelial nitric oxide synthase (eNOS), determination of TNF-α levels, Circulating nitrate/nitrite bioavailability and determination of 8-isoprostane (8-isoPGF2α) concentrations.
Statistical Analysis
All data are expressed as mean ± standard error means (SEM). Comparisons between groups were assessed by two-way analysis of variance. A student t test was used for comparison of eNOS expression, nitrate/nitrate concentration and TNF-alpha measurements between the groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Administration of 17-OHPC blunted hypertension in RUPP rats
As in previous studies with RUPP, mean arterial pressure (MAP) in response to placental ischemia in RUPP rats was significantly increased compared with NP rats (P <0.0001, figure 1). Administration of 17-OHPC significantly attenuated this increase in response to placental ischemia. MAP in NP rats was 92±2.0 (n=13); 123±2.0 in RUPP (n=18), and 116 ±1.5 mmHg in RUPP+17-OHPC (n=10, P <0.05, figure 1).
Figure 1.
17-OHPC supplementation blunts hypertension in RUPP rats. Data are shown as means ± S.E.M. (n=13–18/group).
*P <0.05 versus NP group
# P<0.05 versus RUPP group
Administration of 17-OHPC blunted CD4+ T cells and TNF alpha in RUPP rats
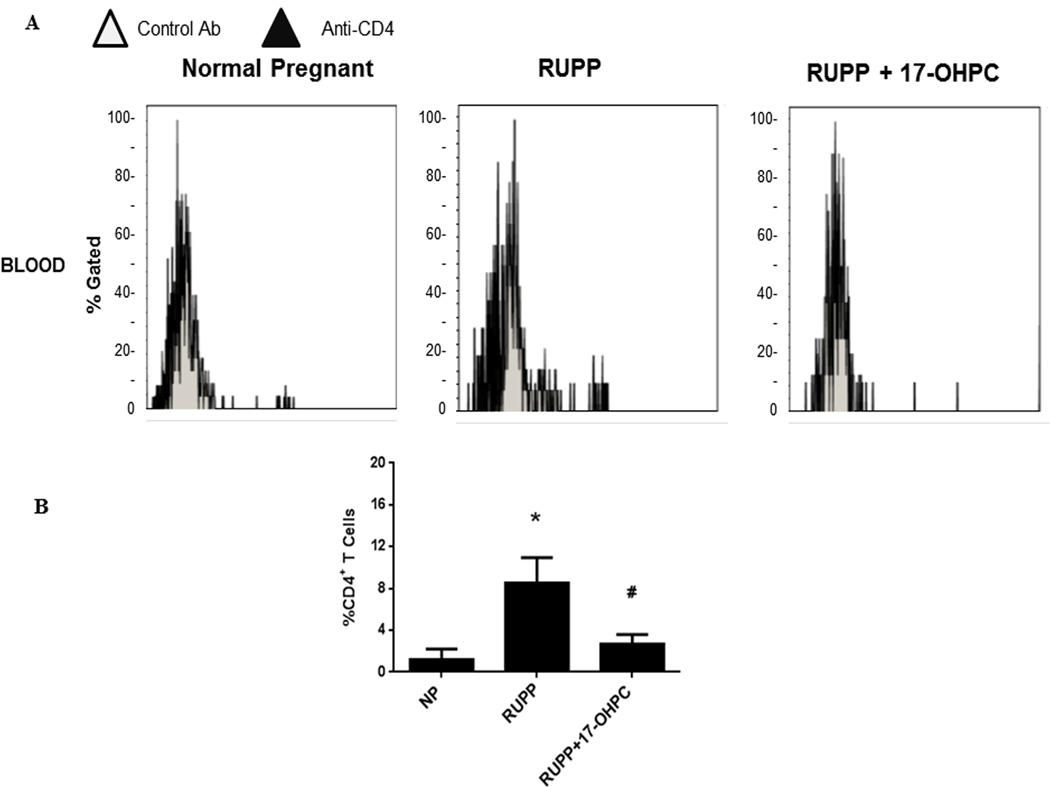
To determine whether 17-OHPC could be a viable treatment for preeclampsia by decreasing inflammation (CD4+ T cells) in response to placental ischemia, we analyzed circulating CD4+ T cells in plasma from the whole blood collected on day 19 of gestation. Circulating CD4+ T cells were 1.19±1.0% of gated cells in NP (n=7), which increased to 8.52±2.4% in RUPP rats (n=10) but was significantly reduced to 2.72±0.87% (n=14) in RUPP + 17- OHPC (P <0.05, figure 2). In addition, as we have previously shown, 17-OHPC decreases circulating inflammatory cytokines. TNF-alpha was 65.84±17.7 pg/ml in RUPP rats (n=5), but was blunted to 17.24±3.9 in RUPP+17-OHPC (n=8).
Figure 2.
17-OHPC supplementation blunts CD4+ T cells in RUPP rats. Panel (A) are representative flow cytometry plots of Normal pregnant (NP), RUPP and RUPP+17-OHPC rats stained with anti-CD4. Bar graphs (B) demonstrate the averaged %CD4+ cells. Data are shown as means ± S.E.M. (n=7–14/group).
*P <0.05 versus NP group
# P<0.05 versus RUPP group
Administration of 17-OHPC increased eNOS expression and total circulating nitrate/nitrite in RUPP rats
To determine whether 17-OHPC improved vasodilation, we analyzed endothelial nitric oxide synthase (eNOS) expression in protein isolated from the aorta collected on day 19 of gestation. Aortic eNOS expression was 0.65±0.11 A.U in NP, which decreased to 0.33±0.01 in RUPP rats but increased to 0.57±0.01 in RUPP+17-OHPC (p <0.05, figure 3A). Conduit vessels have a relative role in long term regulation of arterial pressure. However, it is reasonable to expect that the vascular alterations reported here may well be found in small, resistance vessels, which are more relevant to control arterial pressure. In addition, we have measured the expression of eNOS and phosphorylated eNOS in placentas and we have observed that 17-OHPC could increase eNOS expression compared with RUPP but difference between the groups was not significant. Total circulating nitrate/nitrite was 26.34 ±3.5 µM in NP (n=12); 14.58±3.1 in RUPP rats (n=8) and increased to 22.69±1.62 in RUPP+17-OHPC (n=7, P =0.05, figure 3B).
Figure 3.
17-OHPC supplementation increased aortic eNOS expression and circulating nitrate/nitrite in RUPP rats. NOS protein expression was determined by Western blot analysis and Nitrate/nitrite was determined via Colorimetric Assay Kit as described in Methods. (A) Representative Western blots showing eNOS expression in the aortas from rats and bar graph showing the densitometric data. β-Actin content was used for normalization. Data are shown as means ± S.E.M. (n= 4–5/group). (B) Bar graph showing the total circulating nitrate/nitrite in RUPP rats. Data are shown as means ± S.E.M (n= 7–12/group).
*P <0.05 versus NP group
# P<0.05 versus RUPP group
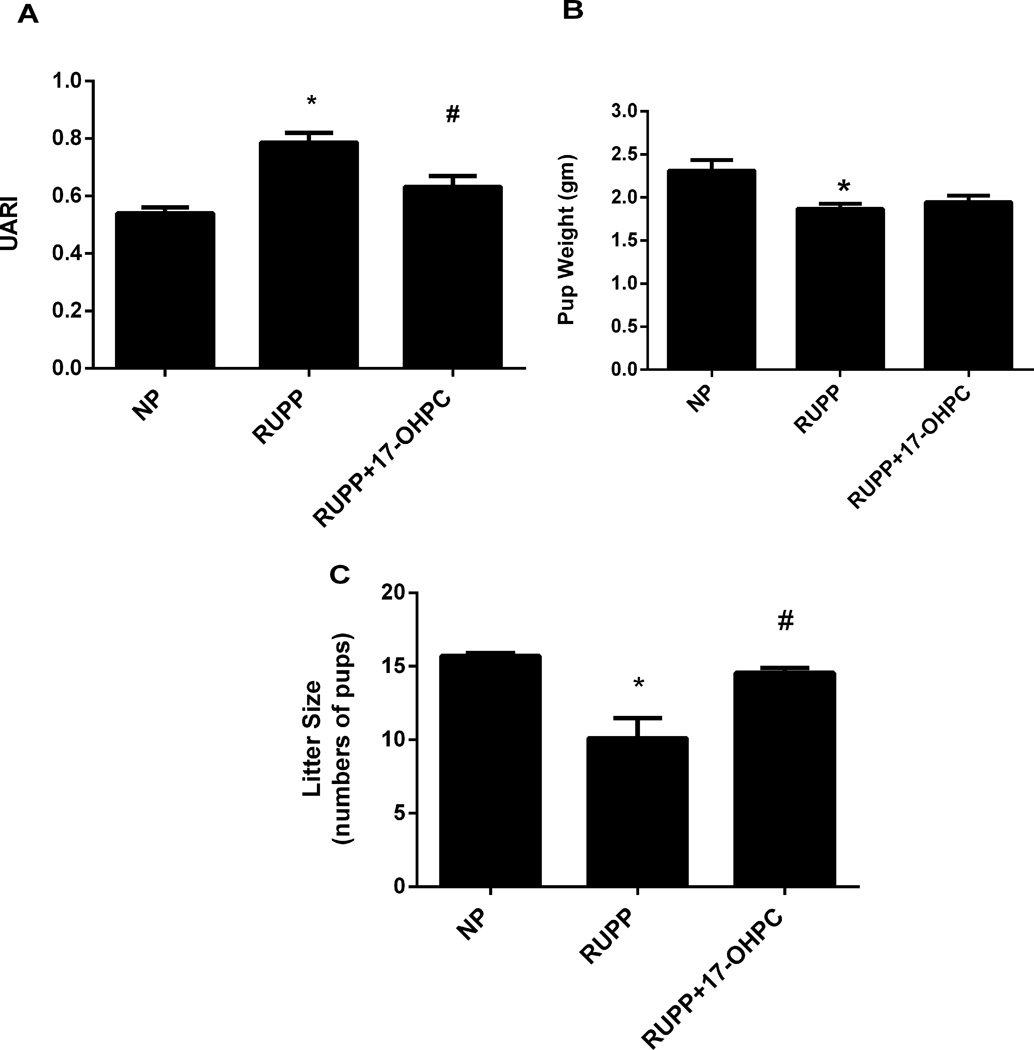
Administration of 17-OHPC improved Uterine Artery Resistance and Litter Size in RUPP rats
Consistent with PE, we have shown the rise in UARI in response to induction of chronic placental ischemia to be associated with the RUPP rat. UARI was 0.54±0.02 in NP (n=3) which increased to 0.79±0.03 (n=8, P<0.05) in RUPP rats. Interestingly, the UARI was improved to 0.63±0.04 in RUPP rats treated with 17-OHPC (n=8) compared to untreated RUPP rats (P<0.05, Figure 4A). Pup weight from RUPP rats (1.87 ± 0.06 g) was significantly lower than that of NP rats (2.31 ± 0.12 g, P < 0.05, Figure 4B). Importantly, 17-OHPC supplementation of RUPP rats improved pup weight, however, this did not reach significance (1.95 ± 0.07g). However, litter size from RUPP rats (10.10 ± 1.40), which is significantly lower than that of NP rats (15.71 ± 0.18, P < 0.05, Figure 4C) was improved to 14.56 ±0.34, P < 0.05 in RUPPS+17-OHPC.
Figure 4.
17-OHPC supplementation improves Uterine Arterial Resistance (UARI) and Litter Size in RUPP rats (A, C). Pup weights were decreased in RUPP rats; however 17-OHPC did not improve weights of offspring in RUPP rats (B). Data are shown as means ± S.E.M. (n=6–8/group).
*P <0.05 versus NP group
# P<0.05 versus RUPP group
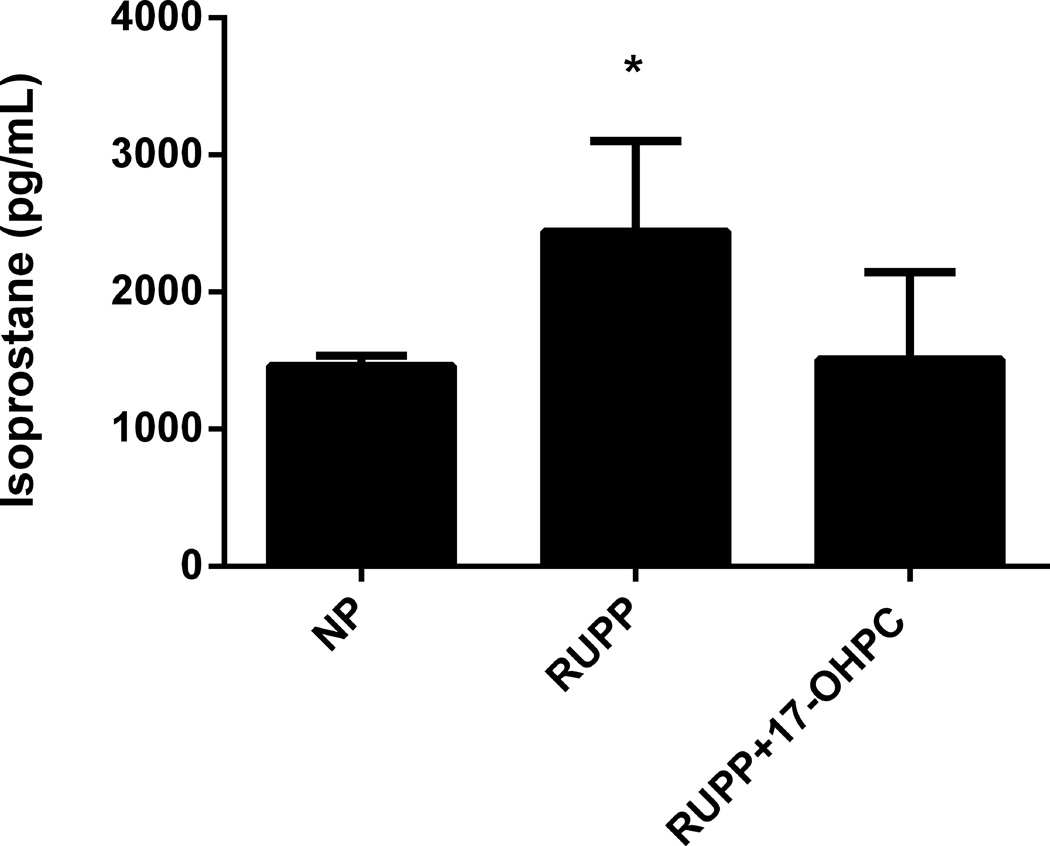
Administration of 17-OHPC improved plasma 8-isoprostane concentrations
To determine whether 17-OHPC improved oxidative stress, we measured 8-isoprostane (8-isoPGF2α) concentrations in plasma collect at day 19 of gestation. 8-isoprostane levels were increased in RUPP rats (n=6) compared with NP (n=3, P<0.05, Figure 5) but were reduced in RUPP+17-OHPC, however, this was not significantly different between the RUPP and RUPP+17 OHPC.
Figure 5.
17-OHPC improved plasma 8-isoprostane concentrations in RUPP rats. Data are shown as means ± S.E.M. (n=3–6/group).
*P <0.05 versus NP group
# P<0.05 versus RUPP group
DISCUSSION
Some clinical characteristics of preeclampsia (PE) are new-onset hypertension during pregnancy and increased uterine artery resistance (UARI) as measured by Doppler waveform sonography2, 6, 27. In addition, many preeclamptic women are in a state of chronic inflammation, characterized by moderate increases in inflammatory cytokines1, 3, 12, 28.
Our current findings support previous conclusions that the hypertension associated with reductions in uterine perfusion pressure (RUPP) results in an increase of UARI. Determining increased UARI is instrumental in diagnosing placental deficiencies in pregnant women and is often used to identify those who may develop PE. Importantly, in this study we demonstrated that administration of 17-OHPC improved UARI, hypertension, inflammation, and nitric oxide bioavailability in response to placental ischemia during pregnancy.
Growing evidence indicates that altered immune mechanisms play an important role in the pathophysiology of PE. Furthermore, PE is associated with increased CD4+ T cells, inflammatory mediators such as TNF-α, IL-6, and decreased regulatory mechanisms such as T regulatory cells and IL-1026, 29–32. We have recently shown that T cells from RUPP rat model of PE causes hypertension, inflammation, and increases in plasma AT1-AA and anti-angiogenic factor, sFlt-1 when transferred to normal pregnant rats33, 34. In addition, we have reported that pharmacological inhibition of inflammatory cytokines or lymphocytes (T and B cells) with Etanercept, Abetacept or Rituximab, reduced blood pressure and ET-1 expression and improved pup weight in RUPP rats35, 36. However, none of these agents would be considered obstetrically safe for use in preeclamptic women.
While 17-OHPC is obstetrically safe and used for the attenuation of preterm labor, and may have anti-inflammatory and vasodilatory actions, its utility in the treatment of PE is unclear. Interestingly, our unpublished data demonstrates from cultured placental explants in the presence of hypoxia and 1 uMole progesterone that IL-6, TNF alpha, IL-17 and sFlt-1 were all reduced when compared to hypoxic cultures alone, thereby indicating an important anti-inflammatory role progesterone could play at the level of the placenta. In addition, our previous in vitro studies demonstrate a profound effect of progesterone to inhibit TNF alpha induced ET-1 secretion and to inhibit ET-1 secretion from HUVECs stimulated with PE serum within a short 6 hour period of progesterone treatment21, 22. These study demonstrate an important effect progesterone on vascular cells. In the current study we demonstrated that CD4+ T cells were increased in the circulation of RUPP rats and 17-OHPC administration significantly reduced these pro-inflammatory cells. In addition, concurring with previous data, we demonstrate that 17 OHPC significantly reduced TNF alpha in response to placental ischemia. These data support a role for the anti-inflammatory properties of 17-OHPC to modulate decreases in maternal blood pressure. Although the reduction in blood pressure in RUPP rats treated with 17 OHPC may be considered modest, this decrease could improve maternal health and thereby increase time to delivery. This is important because furthering the pregnancy leads to overall preparedness of the baby for birth such as increased fetal weight and fetal lung maturation. Small improvements in maternal health and well being could lead to ever larger improvements in the health of babies born to PE moms.
Another effect of 17-OHPC could be vasodilatory by improving nitric oxide synthesis. We found increased vascular eNOS expression and nitrate/nitrite levels with 17-OHPC administration in response to placental ischemia. These data suggest that 17-OHPC may affect the vascular function which depends on the balanced production/bioavailability of nitric oxide (NO), which is maintained by the normal activity of endothelial nitric oxide synthase (eNOS)14. Consistent with this suggestion, it is well known that hormones may impact production of NO both by rapid effects on the activity of eNOS through phosphorylation of the enzyme and longer term modulation through changes in amount of eNOS protein24, 37.
Previous studies have demonstrated that increased nitric oxide synthase (eNOS) activity and expression have been shown to play an important role during normal pregnancy38. Levels of eNOS and NO are elevated in the uterine artery during pregnancy and higher circulating of hormone may be in part responsible for modulating these NO levels and uterine vasculature changes38, 39 In fact, in this study, the rise in UARI following the RUPP procedure may reflect a lack of autoregulation of uteroplacental blood flow. In addition to RUPP procedure, it is possible that release of vascular mediators, including nitric oxide, has been impaired. Although the mechanism of 17-OHPC is not completely understood, it is expected that 17-OHPC interacts with the progesterone receptors to increase nitric oxide production which could cause relaxation of the uterus and slow contractions during preterm labor. Indeed, we demonstrated that 17-OHPC administration improved UARI in response to placental ischemia during pregnancy. This could be a mechanism of improved litter size in this group. Although 17-OHPC did not improve individual pup weight, it normalized the number of pups in RUPP rats to that seen in NP rats. These data indicate improved intrauterine growth restriction in response to placental ischemia. It could be that pup weight would have increased if 17-OHPC had been given at an earlier time point in gestation to RUPP rats however, this has not yet been perform but will be the subject of future studies. In fact, future studies are under way to determine the effects of 17-OHPC administered on GD 15 in the RUPP rat model of PE in order to determine the beneficial effects of 17-OHPC on preterm PE. In addition future studies will examine how 17-OHPC interacts with progesterone receptors in the systemic or uterine vasculature to allow for vasodilation.
Interestingly, whether progesterone could reduce the incidence of preeclampsia and its complications still unclear. At present progesterone is not being used for this purpose in clinical practice since there are insufficient data to be able to say the benefits of progesterone for the mother and child. In fact, a previous review has shown that there is not good evidence showing that progesterone, both oral and vaginal, could help to reduce the incidence of preeclampsia40. Furthermore, there is little information about potential adverse outcomes. In fact, more studies will be necessary to obtain the better understanding about the effects of progesterone for prevention or management of this disease. In our present study we have studied the effects of progesterone on late gestation and further studies in patients are warranted to examine whether intervention such as progesterone supplementation to enhance current management for affected patients could be positive for mother and child.
In conclusion, our findings may have important clinical implications because they suggest that attenuation of CD4+ T cells and pro-inflammatory cytokines accompany an increase of NO bioavailability, and an improvement of UARI, litter size and IUGR and hypertension in response to placental ischemia with 17-OHPC administration, which could profoundly affect pregnancy outcomes during PE. Thus, we believe that 17-OHPC should be considered further for addition to the clinical management of PE
PERSPECTIVE
The discovery of mechanisms that are relevant to the pathophysiology of preeclampsia may offer new therapeutic targets. Our findings suggest that 17-OHPC administration into RUPP rats may regulate inflammation and nitric oxide bioavailability resulting in decreased blood pressure, inflammation and increased uterine artery resistance and IUGR in response to placental ischemia during pregnancy. Thus, an intervention such as 17-OHPC supplementation to enhance current management for affected patients could be positive for mother and child.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
17-OHPC administered on day 18 attenuated hypertension by suppressing pro-inflammatory cells while improving uterine artery resistance index (UARI) and nitric oxide (NO) bioavailability and litter size in response to placental ischemia during pregnancy.
What is relevant?
The suppressed CD4+ cells population and an up-regulation of eNOS with 17-OHPC supplementation may have a clinical role to improve factors implicated in mediating the pathophysiology associated with preeclampsia.
Summary
Progesterone supplementation in the form of 17-OHPC is currently used obstetrically to reduce the risk of recurrent preterm labor if the prior preterm birth was not undertaken because of PE. A role for 17-OHPC in the treatment of PE is debated. Our study illustrates that 17-OHPC could be a viable addition to the treatment of PE demonstrated by it effect to decrease blood pressure, inflammation and improve uterine artery resistance index and nitric oxide bioavailability in response to placental ischemia during pregnancy.
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Institutes of Health grants RO1HD067541 and T32HL105324 and the department of Pharmacology, University of Mississippi Medical Center.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. American journal of reproductive immunology. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. American journal of physiology. Heart and circulatory physiology. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 3.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010;62:105–120. [PMC free article] [PubMed] [Google Scholar]
- 4.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res. 2010;36:239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 5.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol. 2005;1:98–114. doi: 10.1038/ncpneph0035. quiz 120. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Sandrim VC, Montenegro MF, Palei AC, Metzger IF, Sertorio JT, Cavalli RC, Tanus-Santos JE. Increased circulating cell-free hemoglobin levels reduce nitric oxide bioavailability in preeclampsia. Free Radic Biol Med. 2010;49:493–500. doi: 10.1016/j.freeradbiomed.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JMPG, Cutler J, Lindheimer M. Summary of the nhlbi working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 10.Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: Current understanding of the molecular basis of vascular dysfunction. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- 11.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the at1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 12.Granger JP. Inflammatory cytokines, vascular function, and hypertension. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;286:R989–R990. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 13.Herse F, LaMarca B. Angiotensin ii type 1 receptor autoantibody (at1-aa)-mediated pregnancy hypertension. American journal of reproductive immunology. 2013;69:413–418. doi: 10.1111/aji.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 15.Sandrim VC, Palei AC, Luizon MR, Izidoro-Toledo TC, Cavalli RC, Tanus-Santos JE. Enos haplotypes affect the responsiveness to antihypertensive therapy in preeclampsia but not in gestational hypertension. Pharmacogenomics J. 2010;10:40–45. doi: 10.1038/tpj.2009.38. [DOI] [PubMed] [Google Scholar]
- 16.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN, Jr, Lamarca B. Endothelin-1, oxidative stress, and endogenous angiotensin ii: Mechanisms of angiotensin ii type i receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62:886–892. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meis PJ. The role of 17 alpha-hydroxyprogesterone caproate in the prevention of preterm birth. Womens Health (Lond Engl) 2006;2:819–824. doi: 10.2217/17455057.2.6.819. [DOI] [PubMed] [Google Scholar]
- 19.Merlob P, Stahl B, Klinger G. 17alpha hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. Reprod Toxicol. 2012;33:15–19. doi: 10.1016/j.reprotox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19:763–772. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 21.Kiprono LV, Wallace K, Moseley J, Martin J, Jr, Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am J Obstet Gynecol. 2013;209:44.e1–44.e6. doi: 10.1016/j.ajog.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN, Jr, Lamarca B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. American journal of hypertension. 2009;22:1120–1125. doi: 10.1038/ajh.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veillon EW, Jr, Keiser SD, Parrish MR, Bennett W, Cockrell K, Ray LF, Granger JP, Martin JN, Jr, LaMarca B. 17-hydroxyprogesterone blunts the hypertensive response associated with reductions in uterine perfusion pressure in pregnant rats. Am J Obstet Gynecol. 2009;201:324 e321–324 e326. doi: 10.1016/j.ajog.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selles J, Polini N, Alvarez C, Massheimer V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life Sci. 2001;69:815–827. doi: 10.1016/s0024-3205(01)01174-2. [DOI] [PubMed] [Google Scholar]
- 25.Simoncini T, Fu XD, Caruso A, Garibaldi S, Baldacci C, Giretti MS, Mannella P, Flamini MI, Sanchez AM, Genazzani AR. Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors. Hum Reprod. 2007;22:2325–2334. doi: 10.1093/humrep/dem109. [DOI] [PubMed] [Google Scholar]
- 26.Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J, Wallukat G, Martin JN, Jr, Dechend R, Lamarca B. Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin ii type i receptor in response to elevated interleukin-6 during pregnancy. Am J Obstet Gynecol. 2014;211:158.e1–158.e6. doi: 10.1016/j.ajog.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TamTam KB1GE, Cockrell K, Arany M, Speed J, Martin JN, Jr, Lamarca B, Granger JP. Endothelin type a receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol. 2011 Apr;204(4):330.e331–330.e334. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to il-6 during pregnancy: Role of at1-receptor activation. Int J Infereron Cytokine Mediator Res. 2011;2011:65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. Preeclampsia is associated with lower percentages of regulatory t cells in maternal blood. Hypertension in pregnancy: official journal of the International Society for the Study of Hypertension in Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 30.Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, Treszl A. Decreased number of foxp3+ regulatory t cells in preeclampsia. Acta obstetricia et gynecologica Scandinavica. 2008;87:1229–1233. doi: 10.1080/00016340802389470. [DOI] [PubMed] [Google Scholar]
- 31.Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B, Rigo J, Jr, Molvarec A. The frequency of peripheral blood cd4+ cd25high foxp3+ and cd4+ cd25− foxp3+ regulatory t cells in normal pregnancy and pre-eclampsia. American journal of reproductive immunology. 2012;68:175–180. doi: 10.1111/j.1600-0897.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 32.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Current hypertension reports. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 33.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. Cd4+ t-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin ii type i receptor play an important role in mediating hypertension in response to adoptive transfer of cd4+ t lymphocytes from placental ischemic rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R1197–R1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novotny SWK, Herse F, Moseley J, Darby M, Heath J, Gill J, Wallukat G, Martin JN, Dechend R, Lamarca B . Cd4+ t cells play a critical role in mediating hypertension in response to placental ischemia. Journal of Hypertension. 2013;2:116. doi: 10.4172/2167-1095.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: Effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas P, Pang Y. Protective actions of progesterone in the cardiovascular system: Potential role of membrane progesterone receptors (mprs) in mediating rapid effects. Steroids. 2013;78:583–588. doi: 10.1016/j.steroids.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87:406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- 39.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. Vii. Estrogen and progesterone effects on enos. American journal of physiology. Heart and circulatory physiology. 2001;280:H1699–H1705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- 40.Meher S, Duley L. Progesterone for preventing pre-eclampsia and its complications. The Cochrane database of systematic reviews. 2006 doi: 10.1002/14651858.CD006175. CD006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.