Abstract
Metastatic cases of breast cancer pose the primary challenge in clinical management of this disease, demanding the identification of effective therapeutic strategies which remain wanting. In this study, we report that elevated levels of α-tubulin acetylation are a sufficient cause of metastatic potential in breast cancer. In suspended cell culture conditions, metastatic breast cancer cells exhibited high α-tubulin acetylation levels that extended along microtentacle protrusions. Mutation of the acetylation site on α-tubulin and enzymatic modulation of this post-translational modification exerted a significant impact on microtentacle frequency and the re-attachment of suspended tumor cells. Reducing α-tubulin acetylation significantly inhibited migration but did not affect proliferation. In an analysis of over 140 matched primary and metastatic tumors from patients, we found that acetylation was maintained and in many cases increased in lymph node metastases compared to primary tumors. Proteomic analysis of an independent cohort of over 390 patient specimens further documented the relationship between increased α-tubulin acetylation and the aggressive behaviors of basal-like breast cancers, with a trend toward increased risk of disease progression and death in patients with high intensity α-tubulin acetylation in primary tumors. Taken together, our results identify a tight correlation between acetylated α-tubulin levels and aggressive metastatic behavior in breast cancer, with potential implications for the definition of a simple prognostic biomarker in breast cancer patients.
Keywords: breast cancer, metastasis, microtentacles, acetylated tubulin, circulating tumor cells
Introduction
Advances in detection and treatment have greatly increased survival rates of patients diagnosed with localized or regional breast cancer. However, for patients diagnosed with metastatic breast cancer, survival rates drop dramatically (1). Current treatments are largely successful at prolonging patient survival, but they are not sufficient to prevent metastasis (2). Because most breast cancer deaths are due to secondary disease, finding targets to treat or prevent metastasis is an important therapeutic priority.
In order for a primary tumor to metastasize, cancer cells must leave the breast and enter the blood or lymphatic system. Once detached, these circulating tumor cells (CTCs) attach and/or arrest at secondary sites before extravasation and metastatic outgrowth. The cytoskeleton, composed of actin microfilaments, microtubules, and intermediate filaments, plays a vital role in metastatic dissemination (3). Tumor cell reattachment has been shown to be dependent on stable microtubules in vivo (4–8), while the stability of the microtubule network has also been implicated in the control of migration (9, 10), highlighting a potential therapeutic target for both attached and suspended disseminated cells.
Breast cancer cells produce long and dynamic microtubule-based membrane protrusions, termed microtentacles (McTNs), upon detachment (8, 11–13). Importantly, invasive breast tumor cells produce significantly higher frequencies of McTNs compared to non-invasive cell lines (14). These protrusions encircle adjacent cells to promote cell-cell aggregation and facilitate reattachment of tumor cells to an extracellular matrix, endothelial monolayer, and retention in the lungs of mice (7, 13, 14). McTNs can be enhanced by actin depolymerization but are dependent upon microtubule stability (12, 13). Data support a model in which McTNs are generated when the physical force generated by outwardly expanding microtubules overcomes the contractile force of the actin cortex underlying the plasma membrane (8). Inhibition of McTNs by microtubule-destabilizing drugs significantly reduces cell-cell and cell-substrate reattachment efficiency of breast tumor cells (11). Conversely, increased microtubule stability enhances reattachment for in vitro and in vivo metastasis models (7, 15).
Post-translational modifications (PTMs) of α-tubulin can control diverse microtubule functions like signaling, trafficking, and cellular tensegrity (16, 17), but we are only beginning to uncover the many functions that could impact cancer progression and metastasis. Acetylation of α-tubulin, a well-known marker of stabilized microtubules, occurs on lysine 40 (K40) by the α-tubulin acetyltransferase 1 (αTAT1) (16, 18, 19) and can be reversed by histone deacetylase 6 (HDAC6) and sirtuin 2 (SIRT2) (20). Studies suggest high HDAC6 levels and low acetylated α-tubulin are associated with good prognosis and increased disease-free survival of breast cancer patients (21, 22), but the mechanisms behind this correlation and the role of this PTM in metastatic breast cancer are not clear.
Detyrosination is the only α-tubulin PTM associated with microtubule stability that has been found to play a significant role in McTN formation and reattachment of suspended breast tumor cells (11). However, previous studies could not establish a correlative trend between cancer invasiveness and detyrosination of α-tubulin (14). Because CTC reattachment is dependent upon stable microtubules in vivo (5), an alternative α-tubulin PTM associated with microtubule stability was investigated. In this study, we present a novel role for α-tubulin acetylation in breast cancer. We find a significant association between metastatic breast cancer cell lines and high acetylation of α-tubulin that extends along the length of McTN protrusions. Mutation of the specific lysine 40 acetylation site on α-tubulin as well as enzymatic modulation of this PTM has a significant impact on McTN frequency and cancer cell reattachment. Investigation into chemotaxis of attached breast tumor cells finds acetylated α-tubulin is also necessary for migration. Furthermore, matched primary and metastatic tumor arrays containing tissue from over 140 patients show acetylation is maintained and increased in many nodal metastases while large-scale proteomic studies of over 390 patients link this modification to the aggressive basal-like subtype. There is also a trend of increased risk of disease progression and death when α-tubulin acetylation is high in a patient’s primary tumor. Acetylation of α-tubulin may promote a more metastatic phenotype through its effects on reattachment and migration while serving as a marker for basal-like breast cancer and a potential prognostic indicator.
Materials and Methods
Cell culture
MCF-7, BT-20, BT-549, and Hs578T cells were obtained from American Type Culture Collection. MDA-MB-231 cells were kindly provided by Dr. X. Zhan (University of Maryland, Baltimore). MDA-MB-453 and MDA-MB-231 cells were authenticated by Bio-Synthesis Inc. on 23 May 2013 (Lewisville, TX). Cells were maintained at 37°C, 5% CO2 in Dulbecco’s Modified Eagles Medium (DMEM) (CellGro), except BT-20 maintained in Eagle’s Minimum Essential Medium (EMEM) (CellGro), supplemented with 10% FBS and 1% penicillin-streptomycin. Stable cell lines were maintained in 1% Geneticin.
Plasmids and transfections
EGFP-Tubulin.K40R (plasmid 30488, Tso-Pang Yao), EGFP-Tubulin wt (plasmid 30487, Tso-Pang Yao), and pEF5B-FRT-GFP-αTAT1 (plasmid 27099, Maxence Nachury) were obtained from Addgene. AcGFP1-C1 was obtained from Clontech. Transient transfections utilized Fermentas ExGen 500 in vivo transfection reagent (Thermo Scientific) according to manufacturer’s protocol. Stable cell lines were selected 3 days post-transfection. Stable pooled clones were verified after antibiotic selection by GFP expression and immunoblot.
Immunoblot
Cells were harvested as previously described (14). 20 μg protein was separated by SDS-PAGE on 4–12% NuPage MES Bis-Tris gels (Life Technologies). Membranes were blocked with 5% milk/TBST for 1h at room temperature before overnight incubation at 4°C with primary antibody: Acetyl-α-tubulin (Lys40) (D20G3) XP Rabbit mAb (1:1000 Cell Signaling), Anti-detyrosinated alpha tubulin (1:1000 Abcam), Monoclonal Anti-α-tubulin Clone DM1A (1:5000 Sigma Aldrich), GFP (1:5000 Santa Cruz) in 5% milk/TBST. Densitometry was calculated using 3 independent immunoblots with ImageJ (NIH).
Attached and suspended immunofluorescence
Cells were suspended for 30min in ultra low-attach plates (Corning) then spun down onto poly-L-lysine coated coverslips. Cells were fixed with 3.7% formaldehyde/PBS, washed, permeabilized in 0.25% Triton-X 100/PBS, blocked in 5% BSA/NP40/PBS, and incubated overnight at 4°C in 2.5%BSA/NP40/PBS with primary antibody. Secondary antibody was added 1:500 in PBS with Hoechst. Images were acquired using an Olympus FV1000 confocal microscope (Olympus) and analyzed with ImageJ.
Live cell imaging and McTN scoring
Live cell imaging and McTN scoring was performed as previously described (7). Briefly, cells were stained with CellMask Orange plasma membrane stain (Life Technologies) and suspended for 30min in ultra low-attach plates (Corning). Cells were blindly scored for McTNs using an Olympus CKX41 inverted fluorescent microscope. Images were taken using MicroSuite Five software (Olympus). Transiently transfected cells were confirmed to be GFP-positive before counting.
Cell proliferation assay
The number of viable cells in proliferation was determined by the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega) according to manufacturer’s protocol. Briefly, 10,000 cells/well were plated in triplicate/time point in a 96-well plate in full-serum media. Cell Titer was added at t=0 and every 24h thereafter for 120h; absorbance was read at 490nm. Viability in serum-free conditions was monitored over the same time, except t=0 was marked by the washout of full-serum media and the addition of serum-free media. Average absorbance at each time point was divided by the average absorbance reading at Day 0 to account for differences in cell number at plating.
Cell re-attachment and migration assays
Real-time cell reattachment and chemotaxis was measured using the xCELLigence RTCA DP device (Acea Biosciences, Inc.) according to manufacturer’s protocol. Briefly, 20,000 cells/well were added to electrode-containing microtiter plates (E-plate 16) for re-attachment. 40,000 cells/well of MDA-MB-231/MCF-7 and 20,000 cells/well of BT-549 were added to each CIM-plate 16 for migration. CIM-plates are similar to a Boyden chamber, containing an upper and a lower chamber separated by a polyethylene terephthalate membrane with 8 μm pore size. The membrane has gold electrodes on the bottom of the upper chamber to detect cells moving into the lower chamber. The chemoattractant used was 5% FBS. Reattachment and migration rates were quantitatively recorded as Cell Index (a change in electrical impedance of the current flowing through the electrodes). Impedance was recorded every 1–5min for 2h for reattachment and every 15min for 24h for chemotaxis. Raw data was exported to Microsoft Excel. Graphs shown are representative of a single experimental run +/− standard deviation of 3 wells. The underside of the CIM-plate upper chamber was stained with Cell Stain (Millipore) to visualize migrated cells at 24h with a Nikon SMZ1500 stereomicroscope attached to a Nikon digital camera DXM1200 using ACT-1 Software Version 2.62 (Nikon).
Patient samples
Breast cancer and matched metastatic carcinoma of lymph node tissue arrays BR1005a, BR10010a, BR1001 were obtained from US BioMax, Inc (Rockville, MD). Samples from 144 patients were stained by the University of Maryland Greenebaum Cancer Center (UMGCC) Pathology Biorepository and Research Core using the anti-acetylated tubulin antibody. Blind scoring of samples was carried out by Dr. Olga Ioffe, head clinical pathologist for breast and gynecologic cancers. Images were scanned using the Aperio System and captured using ImageScope Viewer (www.aperio.com). Supplementary Figure S4 contains patient ages and histologies.
Reverse phase protein array (RPPA)
412 breast cancer patient primary tumors from The Cancer Genome Atlas (TCGA) Breast Invasive Carcinoma RPPA Set 041011-0035 were assayed for acetylated α-tubulin intensity as previously described (23). Briefly, proteins extracted from patient tumors were printed on slides, probed with anti-acetylated tubulin, and a signal was obtained and visualized by colorimetric reaction. Scanned slides were analyzed using Microvigene software (VigeneTech Inc.). Dilution curves were fitted with a logistic model developed by MD Anderson Cancer Center and concentrations were normalized to correct for loading. Read-out of loading-corrected intensity was calculated as “Normalized Linear Value”. Data from 10 patients was excluded because molecular subtype (classified via PAM50 (24)) was not determined. Data from 5 male patients and 5 patients subtyped as “normal” were also excluded. 392 patients were matched with clinical information (current as of July 2014) presented by the TCGA Research Network (TCGA Data Portal: https://tcga-data.nci.nih.gov/tcga/). Acetylation intensity was classified as high vs. low if it was above or below the median acetylation intensity, respectively, for n=392 patients. Supplementary Figure S5 contains clinical information.
Survival analysis
The study population for survival analysis included 277 patients analyzed through RPPA. Exclusion criteria included prior treatment or neoadjuvant chemotherapy, unknown nodal status, and follow-up time of less than 1yr (<365 days). Patients diagnosed prior to 1998 were also excluded from survival analysis due to FDA approval of trastuzumab and letrozole that year (25, 26). Overall survival (OS) was time from date of diagnosis to date of death from any cause, censored at date of last contact. Progression-free survival (PFS) was time from date of diagnosis to the earlier of disease recurrence or death from any cause. Patients alive without recurrence were censored at date of last contact. Supplementary Table S1 contains clinical information.
Statistics
The McNemar’s test was used to test the equality of binary acetylation rates from two populations (primary tumor and metastases) with the data that are paired and dependent, since tumor and metastases rates are obtained from the same patients. Welch two-sample t-test was applied to determine significance of basal-like acetylation intensity vs. non-basal subtypes. Significance testing of densitometry and McTN scoring was assessed using t-test.
Survival analysis was conducted by Dr. Olga Goloubeva, faculty member of the Biostatistics and Bioinformatics Shared Service of UMGCC. The Kaplan-Meier approach was used to estimate overall survival (OS) and progression-free survival (PFS) functions. Hazard ratios with the corresponding 95% confidence intervals were estimated using the Cox regression model to assess plausible association between tubulin acetylation and patients’ OS as well as PFS. The proportionality assumption for hazards was tested using martingale and Schoenfeld residuals. Acetylation intensity was log-transformed to smooth the distribution and decrease variability. Patients were grouped according to acetylation intensity using the 25th, 50th, and 75th quartiles of the marker’s distribution. OS and PFS were estimated for the predefined patient groups and compared by the log-rank test. Event times were truncated at 8.5yr. Statistical analysis was conducted in R (3.0.3, x64) and SAS (v.9.3, SAS Institute Inc., Cary, NC).
Results
Acetylation of α-tubulin is significantly increased in metastatic breast cancer cells, correlates with increased McTN frequency, and is enriched in McTNs
Given previous data showing that stable microtubules are associated with increased CTC reattachment in vivo (5), we sought to determine if α-tubulin PTMs associated with microtubule stability are differentially represented in breast cancer cells. Acetylation and detyrosination of α-tubulin were investigated in non-metastatic (MCF-7, MDA-MB-453, BT-20) and metastatic (MDA-MB-231, BT-549, Hs578T) cell lines (27). Only acetylation of α-tubulin was significantly associated with the metastatic cell lines, whereas detyrosination was not (Fig. 1A and B, Supplementary Fig. S1A). There is over a three-fold difference between the average acetylation in non-metastatic cell lines compared to metastatic cell lines (Fig. 1B).
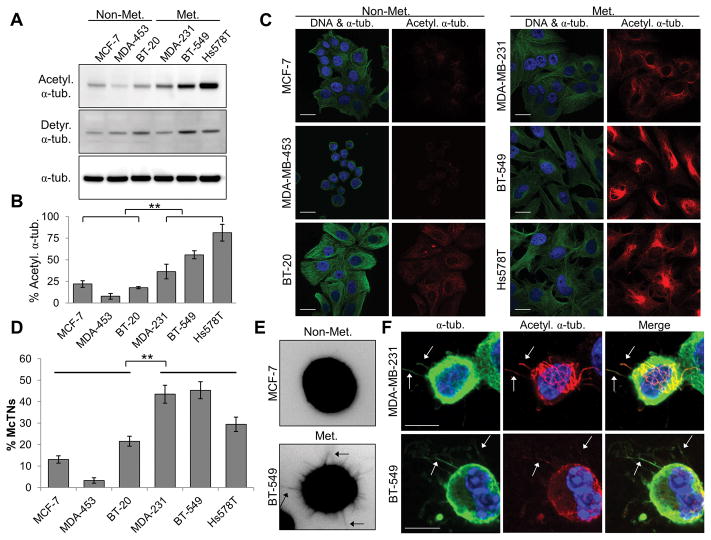
Figure 1. Metastatic breast tumor cell lines have high acetylation of α-tubulin that is enriched in more numerous McTNs.
(A) Lysates from non-metastatic (Non-Met.) and metastatic (Met.) breast cancer cell lines were subjected to immunoblot analysis for α-tubulin post-translational modifications. (B) Densitometric analysis of acetylated α-tubulin, as compared to total α-tubulin. Error bars indicate +/− standard deviation, n=3. Non-Met. vs. Met. breast tumor cells: **p<0.01 (C) Immunofluorescence for DNA (blue), α-tubulin (green), and acetylated α-tubulin (red). Scale bar=20 μm. (D) McTN counts, with data represented as the mean +/− standard deviation of n=3 experiments with at least 100 cells scored blindly in each independent experiment. **p<0.01 between Non-Met. and Met. McTN counts. (E) Representative suspended-cell images highlighting McTN protrusions (arrows). (F) Suspended cell immunofluorescence indicates acetylated α-tubulin (red) localizes along α-tubulin (green)-based protrusions in metastatic cells. DNA is stained in blue. Arrows indicate McTNs. Scale bar=10 μm.
Immunofluorescence was performed to visualize differences in localization and structure of acetylated α-tubulin, as compared to total α-tubulin in the cell panel. The non-metastatic cell lines MCF-7 and MDA-MB-453 exhibit low basal α-tubulin acetylation, with only BT-20 showing minimal acetylation (Fig. 1C). In contrast, metastatic MDA-MB-231, BT-549, and Hs578T cell lines display robust acetylation of α-tubulin with bundling or increased density of acetylated microtubules radiating from the perinuclear region.
Upon suspension, breast tumor cells produce McTNs, membrane-based protrusions dependent upon microtubule stability that can aid in suspended cell reattachment (7, 11–14). Given the increased tubulin acetylation detected in more metastatic cell lines that remains elevated under suspended conditions (Supplementary Fig. S1B), blinded quantitation of McTN frequencies was carried out. The metastatic cell lines with higher acetylation of α-tubulin have significantly more McTNs than the non-metastatic lines (Fig. 1D). Figure 1E shows representative images of non-metastatic and metastatic suspended cells where the metastatic cells produce increased McTNs. The remainder of the suspended cell panel is shown in Supplementary Figure S1C.
Detyrosination is the only α-tubulin PTM to date that has been reported to be enriched in McTNs; however, detyrosination does not directly correlate with increased McTN occurrence or invasiveness (11). Given that increased acetylation correlated with higher McTN frequency in metastatic lines, the role of α-tubulin acetylation in McTN formation was examined. Immunofluorescence of suspended metastatic cell lines shows acetylated α-tubulin extends along the lengths of McTNs (Fig. 1F, arrows), highlighting that this PTM is a constituent of McTNs.
K40R α-tubulin mutation decreases endogenous acetylation and McTN frequency
The increase in acetylation observed in metastatic cell lines and enrichment of this PTM in McTNs propelled further investigation into the mechanistic role of α-tubulin acetylation in McTN formation. We utilized a point mutation on α-tubulin to determine if decreasing α-tubulin acetylation could affect McTNs. Acetylation occurs on lysine 40 of α-tubulin (K40), a highly conserved site that affects microtubule stability (28, 29). Previous research has shown the lysine 40 to arginine α-tubulin point mutation (K40R) is acetylation-resistant but can still incorporate into the microtubule polymer (30).
MDA-MB-231 and BT-549 cells were selected to investigate the effects of the K40R mutation given their high endogenous acetylation and McTN frequency. Immunofluorescence was carried out on transient transfections with either K40R α-tubulin-GFP or a control α-tubulin-GFP to compare the effects of this mutant on acetylation of the α-tubulin network. MDA-MB-231 and BT-549 cells expressing the K40R α-tubulin-GFP showed major disruption of acetylated α-tubulin filaments after 24h (Fig. 2A, top row, arrows), compared to adjacent untransfected cells (Fig. 2A, top row, arrowheads) or transfection of the α-tubulin-GFP control (Fig. 2A, bottom row). Under attached and suspended conditions, MDA-MB-231 and BT-549 cells stably expressing the K40R α-tubulin-GFP mutant show decreased endogenous acetylation of α-tubulin, as compared to α-tubulin-GFP control cells (Fig. 2B). We found no significant difference in proliferation between cells stably expressing K40R α-tubulin-GFP and the α-tubulin-GFP control (Supplementary Fig. S2A and B).
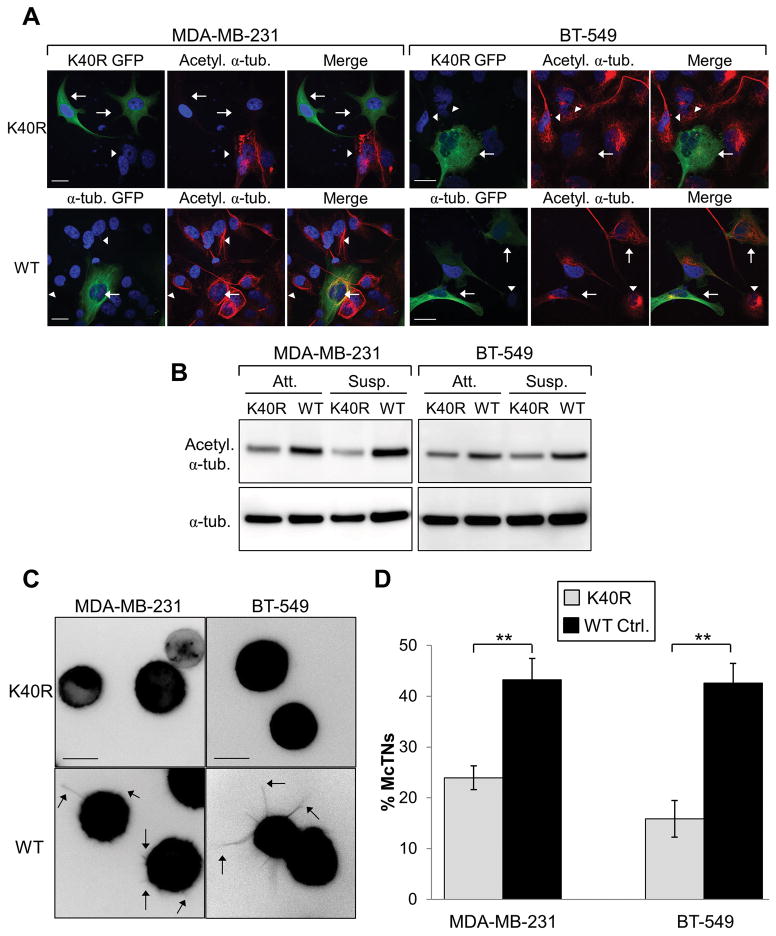
Figure 2. K40R α-tubulin mutant decreases acetylation and McTN frequency.
(A) MDA-MB-231 and BT-549 cells were transiently transfected with GFP-labeled K40R α-tubulin (arrows, top row) or GFP α-tubulin control (arrows, bottom row) and subjected to immunofluorescence for acetylated α-tubulin (red). DNA is stained in blue. Arrowheads indicate non-transfected cells. Scale bar=20 μm. (B) Stable cell lysates were taken of both attached (Att.) and suspended (Susp.) cells expressing the K40R α-tubulin mutant or wild-type (WT) α-tubulin control and subjected to immunoblot. (C) Representative McTN images of each stable cell line suspended for 30min under low-attach conditions. Arrows indicate McTNs. Scale bar=10 μm. (D) McTN counts were carried out on suspended stable cell lines. Error bars indicate +/− standard deviation of n=3 in triplicate. **p<0.01.
Since K40R expression decreased endogenous acetylation and significantly reduced acetylated microtubules, the impact of reducing α-tubulin acetylation on McTN formation in cell lines with high McTN frequency was investigated. MDA-MB-231 and BT-549 control cells exhibit numerous long McTNs when suspended (Fig. 2C, arrows). However, when these metastatic cells stably express the non-acetylatable K40R α-tubulin mutant, the McTN protrusions are significantly reduced (Fig. 2C, top panels, and D). McTN frequency was reduced by more than 45% in MDA-MB-231 and 62% in BT-549 K40R expressing cells, as compared to controls (Fig. 2D). Additional McTN counts were carried out on transiently transfected MDA-MB-231 and BT-549 cells to ensure the process of creating the stable cell lines did not adversely affect McTNs. Similar to what was seen in the stable lines, both metastatic cell lines significantly decreased McTN frequency when transiently expressing the K40R mutant (Supplementary Fig. S2C).
Overexpression of αTAT1 increases tubulin acetylation and enhances McTN frequency
Since reducing acetylation of α-tubulin decreased McTN formation and frequency in highly acetylated metastatic breast tumor cells, we reversed this molecular mechanism to determine if increasing acetylation in a non-metastatic cell line would promote McTNs. The α-tubulin acetyltransferase 1 (αTAT1) was recently demonstrated to specifically acetylate α-tubulin on lysine 40 (18, 19). MCF-7 cells were selected to overexpress αTAT1 because they have low endogenous acetylation and McTN frequency.
Overexpression of αTAT1-GFP (19) in MCF-7 cells caused robust acetylation of α-tubulin throughout the cytoplasm of transfected cells (Fig. 3A, top row, arrows) while the GFP control (Fig. 3A, bottom row, arrows) and non-transfected cells (arrowheads) were unaffected. Overexpression of αTAT1 or the GFP control did not affect the overall α-tubulin network (Supplementary Fig. S3A). Concurrently, immunoblot shows that αTAT1 overexpression greatly increased endogenous acetylation of α-tubulin in both attached and suspended MCF-7 cells, as compared to the GFP-control (Fig. 3B). Transient overexpression of αTAT1-GFP or the GFP control did not significantly affect proliferation (Supplementary Fig. S3B).
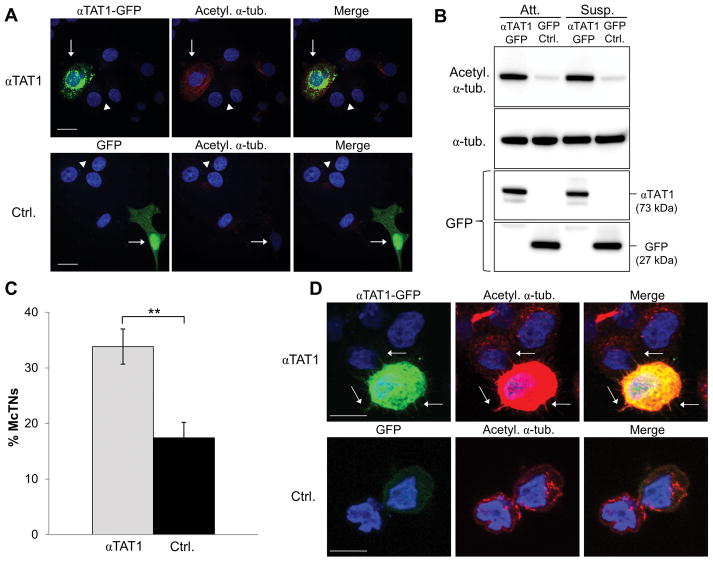
Figure 3. αTAT1 significantly increases α-tubulin acetylation and McTNs.
(A) MCF-7 cells were transiently transfected with αTAT1-GFP or GFP control and subjected to immunofluorescence for acetylated α-tubulin (red). Transfected cells are indicated by arrows; non-transfected cells are indicated by arrowheads. DNA is stained in blue. Scale bar=20 μm. (B) Immunoblot of cells lysed after 24h transfection under attached or 30min suspended conditions. (C) McTN counts of MCF-7 cells transiently transfected with αTAT1-GFP or GFP control. Data represents n=3 in triplicate +/− standard deviation. **p<0.01. (D) Cells were subjected to suspended cell immunofluorescence for acetylated α-tubulin (red). Arrows indicate McTNs. Scale bar=10 μm.
αTAT1-GFP or the GFP control MCF-7 cells were then suspended to determine the effects of increased acetylation on McTN formation and function. We found that overexpression of αTAT1 significantly increased McTN frequency by approximately two-fold over control (Fig. 3C). Because of the significant difference in McTN frequency, we then examined if αTAT1-induced acetylation of α-tubulin localized along the lengths of McTNs. Suspended cell immunofluorescence revealed that acetylated α-tubulin extended within McTNs in the αTAT1 overexpressing cells (Fig. 3D, top row, arrows) but not in the GFP-transfected controls (Fig. 3D, bottom row).
Altering acetylation of α-tubulin influences the reattachment of suspended breast tumor cells
McTN function is evaluated by the ability of detached cells to reattach in order to model one of the early steps for CTC retention in distant tissues (7). To assess how the reduction in acetylation affects reattachment of metastatic cell lines, MDA-MB-231 and BT-549 cells expressing the K40R stable mutation were analyzed. The K40R mutant α-tubulin expressing cells reattached at a significantly decreased rate over 2h, as compared to controls (Fig. 4A and B). It is interesting to note that BT-549 cells had a greater difference in McTN frequency between those stably expressing the K40R mutant and the α-tubulin control (Fig. 2D). This could explain the larger difference in reattachment rates, as compared to the significant but less drastic reduction in reattachment in the MDA-MB-231 cells.
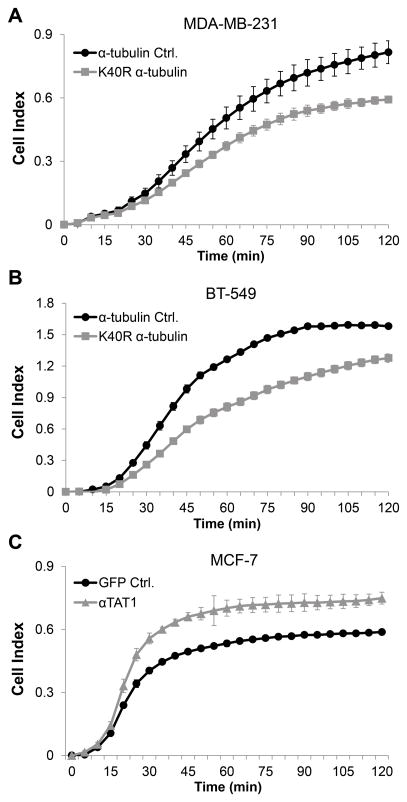
Figure 4. α-tubulin acetylation significantly affects reattachment rates of suspended breast tumor cells.
(A–C) Real-time cell reattachment was analyzed using the xCELLigence system. Each cell line was plated in triplicate and error bars indicate +/− standard deviation of those 3 wells. The graphs shown are representative of n=3 independent runs/cell line. Cell Index represents the change in electrical impedance over time. (A and B) Representative graphs of suspended cell reattachment for stable pooled clones expressing the mutant K40R α-tubulin or the α-tubulin wild-type control. (C) Representative graph of MCF-7 cells transiently transfected with αTAT1-GFP or GFP control 24h prior to analysis.
In complementary experiments, increasing acetylation by overexpressing αTAT1 in low acetylated and non-metastatic MCF-7 cells revealed that increased acetylation significantly increases reattachment, compared to the GFP control (Fig. 4C). This elevation in attachment efficiency parallels the increased McTN counts in Figure 3C, not only confirming McTN function but demonstrating for the first time the importance of acetylation in cell reattachment.
Decreasing α-tubulin acetylation with the K40R mutant inhibits migration
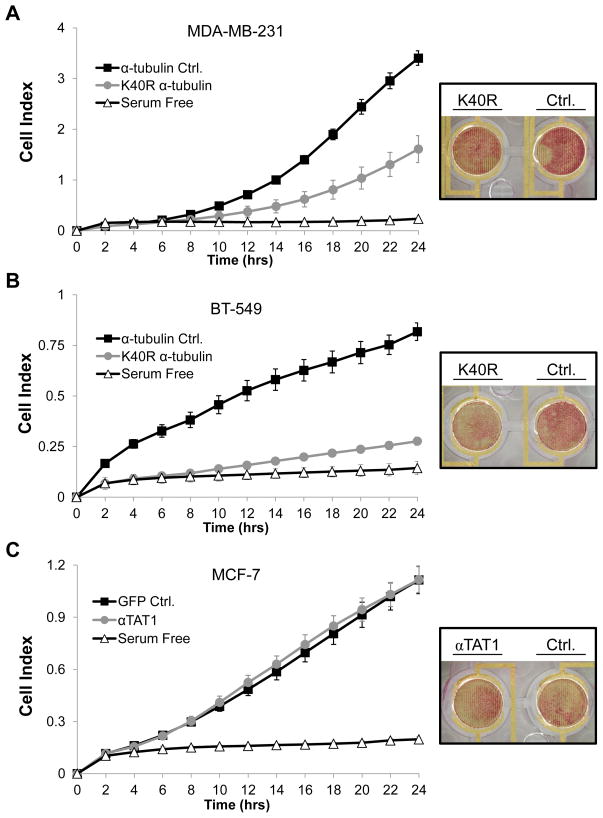
Reattachment is only one step in the metastatic cascade that could promote disseminated disease progression. Next, we investigated the role of acetylated α-tubulin in another metastatic process dependent upon cytoskeletal coordination, migration. MDA-MB-231 and BT-549 cell lines stably expressing the K40R α-tubulin mutant or the α-tubulin control were subjected to a real-time migration assay. This assay utilized plates similar to a Boyden chamber, where chemotaxis from the upper (serum-free) to the lower chamber (with serum) was monitored continuously. Over a period of 24h, both stable cell lines expressing the K40R α-tubulin mutant migrated significantly less, as compared to cells expressing the α-tubulin control (Fig. 5A and B). The underside of the upper chamber was stained at 24h to visualize cells that migrated toward the serum and representative images are shown.
Figure 5. Chemotactic movement of breast tumor cells is affected by reducing acetylated α-tubulin.
(A–C) Real-time migration was analyzed with the xCELLigence system. Modified electronic chamber plates (CIM-plates) were utilized. Each cell line was plated in triplicate into serum-free media (upper chamber) and allowed to migrate towards the lower chamber (containing 5% FBS) for 24h. The serum-free control represents control cells plated in duplicate into serum-free media (upper chamber) where the lower chamber also contained serum-free media. Error bars indicate +/− standard deviation of the 3 experimental or 2 control wells/run. Graphs shown are representative of n=3 independent runs/cell line. Cell Index represents the change in electrical impedance representing movement from the upper to lower chamber. (A and B) Representative chemotaxis graphs of MDA-MB-231 or BT-549 stable pooled clones expressing the K40R α-tubulin mutant or the α-tubulin control and (C) MCF-7 cells transiently transfected with αTAT1-GFP or GFP control. The underside of the upper chamber was stained at 24h and representative images are shown to the right of each graph.
MCF-7 cells transiently overexpressing αTAT1-GFP or the GFP control showed no significant difference in chemotaxis over 24h (Fig. 5C). These data support a model that transient increases in acetylated tubulin are not sufficient to promote migration in a non-metastatic cell line, but acetylation of α-tubulin may be necessary for chemotaxis of more motile and metastatic breast tumor cells.
Acetylation of α-tubulin increases from primary tumor to metastasis in breast cancer patients
To extend the in vitro findings that tubulin acetylation promotes McTN generation, tumor cell reattachment, and affects migration, tubulin acetylation was examined in patient tumor samples. Tumor microarrays of primary lesions and matched lymphatic metastases of 144 breast cancer patients were examined to determine if changes in tubulin acetylation status from primary to metastasis could reflect a selective advantage for primary tumor cells during the metastatic process. Patient age and pathologic diagnosis can be viewed in Supplementary Figure S4.
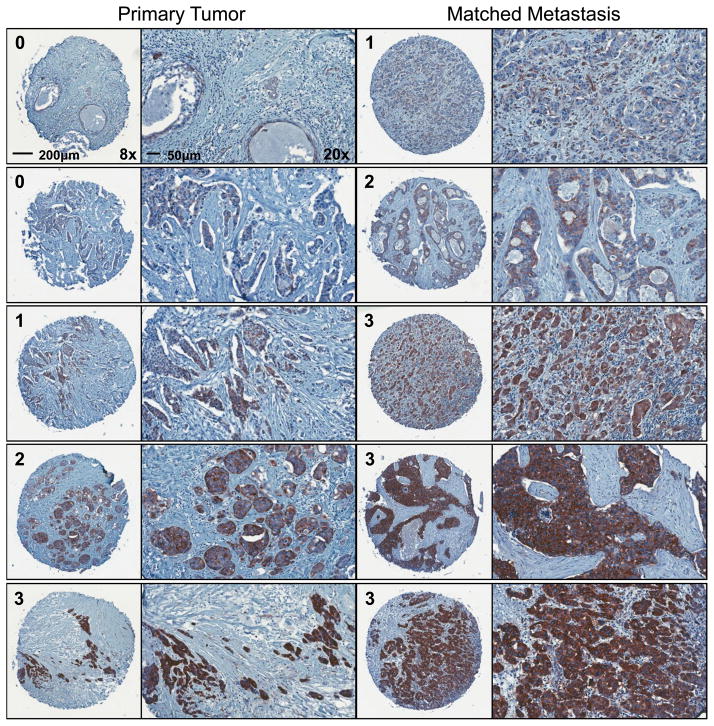
Immunohistochemistry was carried out for acetylated α-tubulin and each primary and metastatic tumor sample was blindly scored by a board-certified pathologist. The two-sided exact McNemar’s test for paired data revealed that based on the 144 samples, there is a statistically significant increase (p=0.03) in acetylated α-tubulin score from the primary to matched metastasis in almost 30% of patients (42 total) (Table 1). 17 patients also maintained high acetylation in their metastases, as compared to their primary tumor. Only 17% of patients (24/144) decreased in acetylation intensity, as compared to the 41% of patients (59/144) that increased or remained strongly positive from primary tumor to matched lymph node metastasis. Figure 6 shows representative images of matched tumor core samples probed for acetylated α-tubulin at 8x and 20x magnification. Each horizontal set of tumors represents matched primary and metastatic samples from a single patient.
Table 1. Acetylated α-tubulin is increased from patient primary to matched metastatic tumors.
Tumor scores for primary and matched lymph node metastases were compared for 144 patients. Tumor score of 0–1 is “Low”; 2–3 is “High” α-tubulin acetylation.
| Metastasis Score | ||||
|---|---|---|---|---|
| Low | High | Total | ||
| Primary Score | Low | 61 | 42* | 103 |
| High | 24 | 17 | 41 | |
McNemar’s test was carried out and the two-sided exact test revealed that these rates were statistically different: *p=0.03.
Figure 6. Acetylated α-tubulin is detected in patient primary and matched metastatic tumors.
Representative images of matched patient primary invasive ductal carcinomas and lymph node metastases stained for acetylated α-tubulin. Horizontal pairings represent the primary tumor and the matched lymphatic metastasis from the same patient. 8x magnification image contains the tumor score (scale bar=200 μm) and is compared to 20x of the same sample (scale bar=50 μm).
High α-tubulin acetylation is associated with the basal-like breast cancer subtype and patient survival
The tissue microarray results demonstrate that acetylation of α-tubulin is a clinically relevant modification that increases in breast cancer metastases. Therefore, we investigated if this modification could be linked to tumor characteristics in a larger patient cohort. Breast cancers can be molecularly classified by one of four subtypes: luminal A (LumA), luminal B (LumB), human epithelial growth factor receptor-2 positive (HER2), and basal-like (31, 32). These subtypes are associated with significantly different patient outcomes and can influence therapeutic strategies (33, 34). Basal-like breast cancers have poor patient prognosis with high metastasis rates, while the luminal A subtype is associated with a better response to therapy and higher survival rates (35, 36). Given our previous findings that there is a strong correlation between high acetylation of α-tubulin and metastatic breast cancer cell lines as well as an increase in acetylation in patient metastases, we investigated the possibility that acetylation may be associated with more aggressive breast cancer subtypes. A primary tumor tissue sample set from The Cancer Genome Atlas (TCGA) was probed for acetylated α-tubulin using the Reverse Phase Protein Array (RPPA) (37).
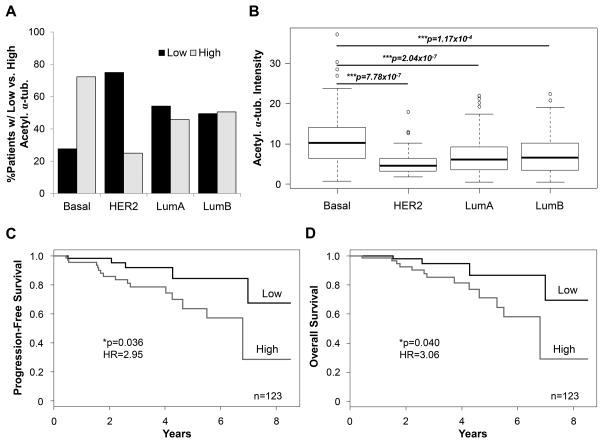
A large patient cohort (n=392) representing all subtypes as well as a variety of ages and histologies was investigated (Supplementary Fig. S5A). When patients were separated by subtype, over 72% (60/83) of patients diagnosed with basal-like breast cancer had high acetylated α-tubulin in their primary tumor (Fig. 7A). Only 25% of HER2 patients had high acetylation. The luminal subtypes showed a relatively even distribution between high and low acetylation intensity in the patients’ primary tumors (Fig. 7A). Basal-like tumors had an average acetylated α-tubulin intensity around 1.5x higher than the HER2 and luminal subtype tumors that was statistically significant (Fig. 7B).
Figure 7. High acetylation of α-tubulin in patient primary tumors is linked to the basal-like subtype and an increased risk of disease progression and death.
(A) Percentage of patients in each subtype with low or high acetylated α-tubulin intensity in the patient primary tumor. Acetylation was considered “Low” if it was below and “High” if it was above the median acetylation of n=392 patients. Basal (n=83), HER2 (n=48), luminal A (LumA) (n=168), and luminal B (LumB) (n=93). (B) Raw intensity values for acetylated α-tubulin in all patient primary tumors are compared by subtype. ***p<0.001. (C) Kaplan-Meier progression-free survival (PFS) and (D) overall survival (OS) for n=123 non-HER2 patients in the high and low acetylation categories. *p<0.05, HR= hazard ratio.
It has been reported that 14 of the 392 patients in this sample set formed metastases after initial diagnosis, so we investigated these patients’ primary tumor acetylation intensity. HER2 patients that formed metastases had low acetylation in the primary tumor and the luminal subtypes were split around the median. All basal-like breast cancer patients that formed metastases had high α-tubulin acetylation intensity in their primary tumor (Supplementary Fig. S5B).
To investigate whether acetylated α-tubulin is associated with patient prognosis, the Kaplan-Meier analysis was used to estimate overall survival (OS) and progression-free survival (PFS) functions. Patients in this cohort (n=277, Supplementary Table S1) were grouped using quartiles and separated into low, low-medium, medium-high, and high α-tubulin acetylation categories. The Cox regression model was utilized to assess the strength of association between OS (PFS) and acetylated tubulin intensity. The regression model indicated a trend toward increased risk of disease progression and death in patients with high α-tubulin acetylation, but acetylation intensity was not a statistically significant predictor of survival (Supplementary Fig. S5C and D). However, we previously found HER2 patients had the lowest average acetylation intensity (Fig. 7B), suggesting there may be an inverse association between acetylated α-tubulin and HER2 overexpression. In non-HER2 patients, high acetylation intensity was significantly associated with worse PFS (p=0.055) and OS (p=0.03), when compared to patients with lower acetylation level (Supplementary Fig. S5E and F). Among non-HER2 patients, those in the highest acetylation category are approximately 3 times more likely to be at risk of disease progression and death when compared to subjects with the lowest level of the marker. The estimated hazard ratios (HR) for PFS and OS are HR=2.95 with the 95% confidence interval (CI): 1.07–8.12 (p=0.036, Fig. 7C), and HR=3.06 with the 95% CI: 0.98–9.52 (p=0.04, Fig. 7D), respectively.
Discussion
This report investigates the specific role of α-tubulin acetylation at lysine 40 in the formation of suspended cell protrusions and tumor cell reattachment. Metastatic breast tumor cell lines have high acetylation of α-tubulin that is enriched in McTN protrusions upon suspension. Specifically reducing acetylation of α-tubulin with the K40R mutant significantly decreases McTN frequency in metastatic cell lines, while elevating acetylation via αTAT1 overexpression increases McTNs in a less aggressive cell line. Manipulation of acetylated α-tubulin through mutation and enzymatic regulation also demonstrates that suspended cell reattachment is dependent upon this modification. Stable McTNs have been associated with enhanced reattachment to endothelial monolayers (15) and CTC lung trapping in a murine metastasis model (7). Here we find the specific PTM of α-tubulin acetylation enhances McTN formation to promote suspended cell reattachment, one key step in the metastatic cascade.
Migration is another necessary step in cancer dissemination. We find that chemotaxis is significantly reduced with overexpression of the K40R α-tubulin mutant in metastatic breast tumor cell lines. This suggests acetylation may be necessary for proper chemotaxis in more invasive breast cancer cells. However, the role of stabilized microtubules in migration is greatly understudied, as compared to the more established role of actin. It has been shown that a reduction in acetylated α-tubulin impairs migration in neuronal cell lines (38, 39), but little is known about how it affects cancer cell motility. Conflicting reports have suggested the cytoskeletal alterations caused by HDAC inhibitors, specifically targeting HDAC6’s tubulin deacetylase activity, reduces cancer migration by increasing α-tubulin acetylation (22). However, effects of α-tubulin acetylation have been largely determined by non-specific chemical inhibition or knockdown of deacetylases that also affect actin polymerization and severing (40, 41). Others have reported acetylated microtubules orient towards the leading edge of migrating MDA-MB-231 breast cancer cells to direct cell motility (42). Our results showing that α-tubulin acetylation enhances migration, McTN formation, and reattachment indicate α-tubulin acetylation may actually promote a metastatic phenotype.
Our results indicate αTAT1 overexpression increases McTNs and reattachment in a non-metastatic breast cancer cell line while others have shown αTAT1 enhances extracellular matrix invasion of the highly metastatic MDA-MB-231 cell line (42, 43). These studies suggest inhibition of αTAT1 could potentially impede reattachment and migration in tumors with high α-tubulin acetylation. However, there are no known inhibitors of αTAT1 for current investigative use. Until inhibitors are developed, insight can be gained from current tools that could affect α-tubulin acetylation. αTAT1 is thought to be the major tubulin acetyltransferase in vivo, but histone acetyltransferases (HATs) ELP3 (39) and Gcn5 (44) have also been shown to acetylate α-tubulin. Interestingly, overexpression of these enzymes are linked to progression of acute lymphoblastic leukemia (ALL) (45), enhanced lung cancer growth (46), and increased motility in melanoma cells (47). HAT inhibitors have also been shown to have potent anti-tumor activity against triple-negative breast cancer xenografts in vivo (48). These targeted therapies are mainly aimed at inhibiting histone acetyltransferase activity but should be further investigated to determine if HAT inhibition could also reduce tubulin acetylation at clinically tolerable levels. This dual mechanism of action may prove to be more efficacious in certain cancers, specifically those with high acetylation of α-tubulin.
Our current results indicate α-tubulin acetylation is increased from the primary tumor to the matched lymph node metastasis in 30% of patients (42/144) while high acetylation was detected in 41% of screened breast cancer metastases (59/144). The dependence upon endogenous acetylation for reattachment and chemotaxis of metastatic breast tumor cell lines and the significant increase of α-tubulin acetylation in metastatic tumors support a model in which tubulin acetylation confers a selective advantage for metastatic potential. This model is further supported by very recent evidence that high α-tubulin acetylation is associated with a more invasive phenotype in human pancreatic cancer cell lines (49) as well as lymph node metastasis and poor prognosis in patients with head and neck squamous cell carcinoma (50).
We report for the first time that α-tubulin acetylation is also associated with the basal-like breast cancer subtype in patients. Basal-like breast cancers are negative for estrogen receptor (ER) or progesterone receptor (PR)-responsive genes and genes associated with HER2 amplification (31). This aggressive subtype is correlated with increased risk of metastatic spread and poor patient prognosis (33, 36). Although there is consensus for what characteristics basal-like tumors lack (ER/PR/HER2 amplification), there are very few positive markers that can define the basal-like subtype (36). We found that over 72% of basal-like tumors had high α-tubulin acetylation. The average acetylated tubulin intensity for basal-like tumors was approximately 1.5x higher than the HER2 and luminal subtype tumors, and all basal-like primary tumors that metastasized had high α-tubulin acetylation. Our results also indicate high α-tubulin acetylation in the primary tumor is significantly associated with an increased risk of disease progression and death in non-HER2 patients. We believe this could be due to an inverse relationship between α-tubulin acetylation and HER2, since previous studies have shown that HER2 overexpression reduces α-tubulin acetylation (51, 52). Additional studies utilizing a larger patient cohort and further investigation into the inverse relationship within the HER2 subtype are necessary to determine the clinical significance of α-tubulin acetylation as a patient prognostic factor.
Despite the need for treatments to prevent metastatic dissemination, there are a number of challenges in developing such therapies. One major challenge in targeting metastasis is that tumor cells can leave the primary site and begin metastatic progression before cancer is clinically detected (53). Although CTCs can enter the bloodstream before diagnosis, early steps of the metastatic cascade are still reasonable targets for therapeutic intervention. Tumor cells have been shown in animal models to reenter the circulation and seed other metastatic sites as well as self-seed the primary tumor throughout cancer progression (54, 55). Surgery to remove a primary tumor seeds millions of cells into the patient’s bloodstream (56) and CTCs can be detected in a patient’s blood for years after primary tumor resection (57). Anti-metastatic therapies may be most effective prior to surgery on the primary tumor or in patients at high risk of disseminated disease (55). A key advantage of targeting disseminated and circulating cells is that they are more accessible for chemotherapeutics in the bloodstream (55). Ideally, targeted therapies against dissemination, reattachment, or invasion could be successfully combined with existing cancer treatments to control or prevent metastatic disease (10, 55).
The current results define a novel mechanism where acetylation of lysine 40 of α-tubulin promotes microtentacle generation, tumor cell reattachment, and chemotaxis that are selective advantages for metastatic potential and particularly enriched in basal-like breast cancers. The resulting opportunity for α-tubulin acetylation to serve as both a diagnostic and therapeutic target for metastatic breast cancer will be an important avenue of ongoing investigation.
Supplementary Material
Acknowledgments
Financial Support: This research is supported by R01-CA154624 and R01-124704 from the National Cancer Institute, KG100240 from the Susan G. Komen Foundation and an Era of Hope Scholar Award from the Department of Defense (BC100675). MIV is supported by NIH grant K01-CA166576.
We would like to thank Drs. Tso-Pang Yao & Maxence Nachury for plasmids deposited at Addgene, Carol Robles for assistance with tumor microarray staining and scanning, and Dr. Steven Munger and Dr. Stephan Vigues for use of and assistance with the stereomicroscope. We would also like to thank Dr. Katherine Hoadley (University of North Carolina, Chapel Hill), Dr. Han Liang (MD Anderson) and Dr. Mark Jensen (National Cancer Institute) for feedback on TCGA clinical data.
Footnotes
Conflicts of Interest: There are no conflicts of interest to disclose
References
- 1.ACS. American Cancer Society Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–97. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 3.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 4.Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest. 2008;118:3170–80. doi: 10.1172/JCI34279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korb T, Schluter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, et al. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–47. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Thamilselvan V, Basson MD. The role of the cytoskeleton in differentially regulating pressure-mediated effects on malignant colonocyte focal adhesion signaling and cell adhesion. Carcinogenesis. 2005;26:1687–97. doi: 10.1093/carcin/bgi135. [DOI] [PubMed] [Google Scholar]
- 7.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29:3217–27. doi: 10.1038/onc.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matrone MA, Whipple RA, Balzer EM, Martin SS. Microtentacles tip the balance of cytoskeletal forces in circulating tumor cells. Cancer Res. 2010;70:7737–41. doi: 10.1158/0008-5472.CAN-10-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden A, Rida PC, Aneja R. Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration. Cancer Metastasis Rev. 2013;32:269–87. doi: 10.1007/s10555-012-9413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313:1326–36. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2010;121:65–78. doi: 10.1007/s10549-009-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitolo MI, Boggs AE, Whipple RA, Yoon JR, Thompson K, Matrone MA, et al. Loss of PTEN induces microtentacles through PI3K-independent activation of cofilin. Oncogene. 2013;32:2200–10. doi: 10.1038/onc.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–88. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whipple RA, Matrone MA, Cho EH, Balzer EM, Vitolo MI, Yoon JR, et al. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010;70:8127–37. doi: 10.1158/0008-5472.CAN-09-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 17.Perdiz D, Mackeh R, Pous C, Baillet A. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal. 2011;23:763–71. doi: 10.1016/j.cellsig.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–22. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–22. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004;10:6962–8. doi: 10.1158/1078-0432.CCR-04-0455. [DOI] [PubMed] [Google Scholar]
- 22.Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531–9. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 23.Iadevaia S, Lu Y, Morales FC, Mills GB, Ram PT. Identification of optimal drug combinations targeting cellular networks: integrating phospho-proteomics and computational network analysis. Cancer Res. 2010;70:6704–14. doi: 10.1158/0008-5472.CAN-10-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkelstein JB. FDA panel recommends two new cancer drugs for approval. J Natl Cancer Inst. 2001;93:175–6. doi: 10.1093/jnci/93.3.175. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–18. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 27.Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2:706–21. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22:1066–74. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topalidou I, Keller C, Kalebic N, Nguyen KC, Somhegyi H, Politi KA, et al. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol. 2012;22:1057–65. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J Biol Chem. 2010;285:11219–26. doi: 10.1074/jbc.M109.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 34.Sasa M, Bando Y, Takahashi M, Hirose T, Nagao T. Screening for basal marker expression is necessary for decision of therapeutic strategy for triple-negative breast cancer. J Surg Oncol. 2008;97:30–4. doi: 10.1002/jso.20906. [DOI] [PubMed] [Google Scholar]
- 35.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentin MD, da Silva SD, Privat M, Alaoui-Jamali M, Bignon YJ. Molecular insights on basal-like breast cancer. Breast Cancer Res Treat. 2012;134:21–30. doi: 10.1007/s10549-011-1934-z. [DOI] [PubMed] [Google Scholar]
- 37.Tabchy A, Hennessy BT, Gonzalez-Angulo AM, Bernstam FM, Lu Y, Mills GB. Quantitative proteomic analysis in breast cancer. Drugs Today (Barc) 2011;47:169–82. doi: 10.1358/dot.2011.47.2.1576695. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Wei D, Wang Q, Pan J, Liu R, Zhang X, et al. MEC-17 deficiency leads to reduced alpha-tubulin acetylation and impaired migration of cortical neurons. J Neurosci. 2012;32:12673–83. doi: 10.1523/JNEUROSCI.0016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–64. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 40.Garnham CP, Roll-Mecak A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton (Hoboken) 2012;69:442–63. doi: 10.1002/cm.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–76. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 42.Montagnac G, Meas-Yedid V, Irondelle M, Castro-Castro A, Franco M, Shida T, et al. alphaTAT1 catalyses microtubule acetylation at clathrin-coated pits. Nature. 2013;502:567–70. doi: 10.1038/nature12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91:950–60. doi: 10.1016/j.ejcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Conacci-Sorrell M, Ngouenet C, Eisenman RN. Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation. Cell. 2010;142:480–93. doi: 10.1016/j.cell.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmlund T, Lindberg MJ, Grander D, Wallberg AE. GCN5 acetylates and regulates the stability of the oncoprotein E2A-PBX1 in acute lymphoblastic leukemia. Leukemia. 2013;27:578–85. doi: 10.1038/leu.2012.265. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Wei T, Si X, Wang Q, Li Y, Leng Y, et al. Lysine acetyltransferase GCN5 potentiates the growth of non-small cell lung cancer via promotion of E2F1, cyclin D1, and cyclin E1 expression. J Biol Chem. 2013;288:14510–21. doi: 10.1074/jbc.M113.458737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Close P, Gillard M, Ladang A, Jiang Z, Papuga J, Hawkes N, et al. DERP6 (ELP5) and C3ORF75 (ELP6) regulate tumorigenicity and migration of melanoma cells as subunits of Elongator. J Biol Chem. 2012;287:32535–45. doi: 10.1074/jbc.M112.402727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, Pinello CE, Luo J, Li D, Wang Y, Zhao LY, et al. Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents. Mol Cancer Ther. 2013;12:610–20. doi: 10.1158/1535-7163.MCT-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–56. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saba NF, Magliocca KR, Kim S, Muller S, Chen Z, Owonikoko TK, et al. Acetylated Tubulin (AT) as a Prognostic Marker in Squamous Cell Carcinoma of the Head and Neck. Head Neck Pathol. 2013 doi: 10.1007/s12105-013-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitamura T, Connolly K, Ruffino L, Ajiki T, Lueckgen A, DiGiovanni J, et al. The therapeutic effect of histone deacetylase inhibitor PCI-24781 on gallbladder carcinoma in BK5.erbB2 mice. J Hepatol. 2012;57:84–91. doi: 10.1016/j.jhep.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki J, Chen YY, Scott GK, Devries S, Chin K, Benz CC, et al. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res. 2009;15:3163–71. doi: 10.1158/1078-0432.CCR-08-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–9. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown DC, Purushotham AD, Birnie GD, George WD. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery. 1995;117:95–101. doi: 10.1016/s0039-6060(05)80235-1. [DOI] [PubMed] [Google Scholar]
- 57.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.