Quality-of-life issues, especially fertility preservation, are just as important as oncologic outcomes in young women with endometrial cancer. Based on the results of numerous studies, fertility-sparing progestin therapy can be safely performed in endometrioid adenocarcinoma confined to the endometrium and can also be reasonably recommended to selected women with more advanced disease and recurrent disease. However, careful follow-up is important because of the high rate of recurrence.
Keywords: Endometrial cancer, Fertility-sparing, Conservative, Progestin
Abstract
Endometrial cancer is the most common gynecologic cancer in developed countries. Approximately 3%–14% of endometrial cancers are diagnosed in young women under 40 who want to preserve their fertility. The incidence of endometrial cancer in this age group is increasing, for which fertility-sparing therapy is increasingly used because it is one of the most important quality of life issues in these women. Progestin therapy is the most common type of fertility-sparing therapy. In this review, the most up-to-date findings regarding fertility-sparing progestin therapy for young women with primary and recurrent endometrial cancer is addressed in terms of diagnosis, treatment, follow-up, and oncologic and reproductive outcomes. Fertility-sparing progestin therapy is highly effective in selected young women with primary and recurrent endometrial cancer. The selection of appropriate patients through comprehensive pretreatment evaluation is of paramount importance to achieve the best outcomes without compromising survival. Because of the high rate of recurrence after successful fertility-sparing therapy, close surveillance is mandatory, and prophylactic hysterectomy is the best option for patients who have completed family planning. Pregnancy outcomes are very promising with the aid of assisted reproductive technologies. Continuous daily oral medroxyprogesterone acetate and megestrol acetate are the preferred progestins for fertility-sparing therapy, but future studies should be performed to determine the optimal dose and treatment duration of these agents.
Implications for Practice:
In young women with endometrial cancer, the cure rate is very high. Therefore, the efficacy of treatment should not be limited to the oncologic outcomes. The quality-of-life issue is as important as oncologic outcomes in these patients. Fertility preservation is one of the most important quality-of-life issues. Based on the results of numerous studies, fertility-sparing progestin therapy can be safely performed in endometrioid adenocarcinoma confined to the endometrium. It also can be reasonably recommended to selected women with more advanced disease and recurrent disease. However, careful follow-up is important because of the high rate of recurrence.
Introduction
Endometrial cancer is the most common gynecologic malignancy in Western countries [1, 2]. In the U.S., 49,560 new endometrial cancer cases and 8,190 deaths from endometrial cancer are projected to occur in 2013 [2]. In Eastern countries, the incidence of endometrial cancer is rapidly increasing, and it will be the most common gynecologic malignancy in the near future [3–5]. Endometrial cancer is a disease of perimenopausal women. However, approximately 3%–14% of endometrial cancer cases are diagnosed in women equal to or under 40 years of age who want to preserve their fertility [6, 7]. Endometrial cancers diagnosed at this age group are increasing in frequency and are typically early-stage, well-differentiated, endometrioid type adenocarcinomas [8, 9]. Hence, the incidence of myometrial invasion or lymph node metastasis is very rare in these cases [10, 11]. Because the cure rate is very high for endometrial cancer diagnosed at this age group, quality of life is as important as survival outcomes in these patients, and the preservation of fertility is one of the most important quality of life issues. Fertility-sparing management using various agents has thus been increasingly adopted as an alternative treatment.
Current fertility-sparing treatment modalities mainly comprise hormonal therapies involving progestins [12–16], progestin-releasing intrauterine devices [17–21], natural progesterone [22], oral contraceptives [23], selective estrogen receptor modulators [24–26], gonadotropin-releasing hormone agonist [24, 27], and aromatase inhibitors [28]. Of these treatments, progestin therapy is the most commonly used, and its efficacy is well-known compared with other treatment modalities. We here review the most recent findings for fertility-sparing management with progestin in young women with early endometrial cancer who want to preserve their fertility.
Materials and Methods
We performed a Medline search of articles published in English between January 1969 and September 2013 with the key words: “endometrial cancer,” “fertility-sparing,” “fertility preservation,” “conservative management,” and “progestin.” We identified further articles from the bibliographies of these publications including case reports, case series, original articles, review articles, and meta-analyses. The most up-to-date findings regarding diagnosis, treatment, oncologic and reproductive outcomes, and follow-up after fertility-sparing progestin therapy in young women with primary or recurrent endometrial cancer were extracted from these reports.
Results
Progesterone is a steroid hormone that opposes estrogen-driven growth and carcinogenesis in the endometrium. Excessive estrogen stimulation, which is not opposed by progesterone, will cause the development of endometrial hyperplasia or cancer. The mechanism of the anticancer effect of progesterone has been studied in women who were treated with progestin, xenograft models, and various cell lines. Progestins are made of synthetic progesterone and have been used for hormone therapy in women with endometrial hyperplasia or cancer. Progestin was previously considered to exert anticancer effects through downregulation of estrogen receptors and activation of enzymes involved in estrogen metabolism [29]. Recently, it is considered that the anticancer effect of progestin is also exerted by involving cell cycle regulation by cyclin-dependent kinase, and antioncogene is an important factor for this process [29]. Progestin is known to enhance p27 expression, resulting in inhibition of cyclin E-Cdk2 function and suppression of the cell cycle [30].
Indications for Fertility-Sparing Progestin Therapy
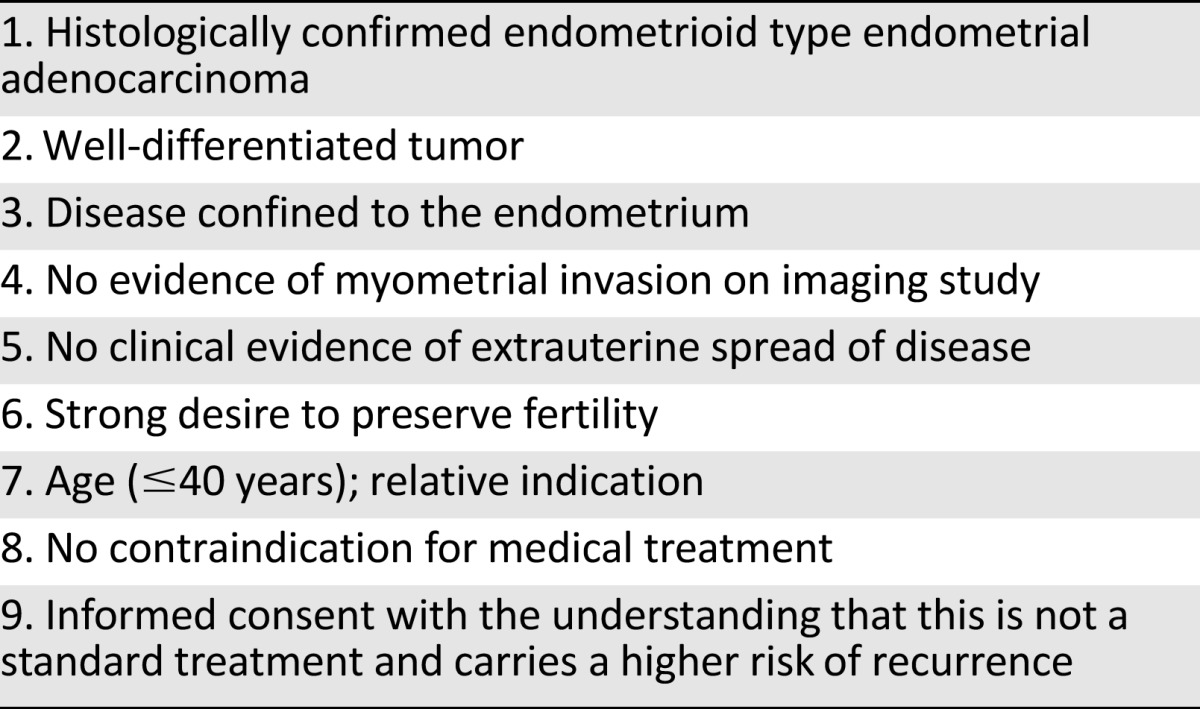
The selection of endometrial cancer patients for whom fertility-sparing progestin therapy is appropriate is of paramount importance to achieve the best outcomes. In almost all relevant studies on this issue, fertility-sparing progestin therapy has been recommended for patients with presumed early-stage, well-differentiated, endometrioid type endometrial adenocarcinoma with no evidence of myometrial invasion or extrauterine spread. According to the revised International Federation of Obstetrics and Gynecology staging system (2009), stage IA (confined to endometrium), grade 1 endometrioid adenocarcinoma cases are eligible for fertility-sparing progestin therapy.
It is important that well-differentiated tumors are verified and documented by an experienced gynecologic pathologist. Well-differentiated tumors have a very low risk of myometrial invasion and extrauterine spread including lymph node, ovarian, or peritoneal metastasis [10, 31]. In addition, well-differentiated tumor cells are more likely to express progesterone receptors and therefore respond to progestin therapy [32]. The absence of myometrial invasion is also an important clinical aspect of endometrioid adenocarcinoma and implies a very low risk of extrauterine spread [10, 11]. If these two criteria are met, the risk of extrauterine disease is likely to be extremely rare, a positive response to progestin therapy is expected, and fertility-sparing progestin therapy can be safely recommended [10, 11].
Well-differentiated tumor cells are more likely to express progesterone receptors and therefore respond to progestin therapy. The absence of myometrial invasion is also an important clinical aspect of endometrioid adenocarcinoma and implies a very low risk of extrauterine spread.
Table 1 lists the optimal indications for fertility-sparing progestin therapy. Navarria et al. [33] have reported the estimated number of patients who may need fertility-sparing progestin therapy in a population-based population as a rate of 0.3 in 100,000 women for these criteria. However, because the incidence of young women with endometrial caner is increasing, as is the number of women who want to delay having children until their late 30s, the future need for fertility-sparing progestin therapy will necessarily increase [34].
Table 1.
Optimal indications for fertility-sparing progestin therapy
Pretreatment Evaluation
To reduce the incidence of life-threatening sequelae, a comprehensive pretreatment evaluation to select appropriate patients for progestin therapy without compromising curability is vital. Careful history taking and physical examination should be carried out to obtain possible clues for extrauterine spread of the disease. To assess the risk of familial cancer, careful family history taking and an appropriate genetic work-up are required. Endometrial cancer diagnosed in young women harbors the additional risk of cancers associated with the Lynch/hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, as well as synchronous or metachronous ovarian cancers occurring outside the setting of Lynch/HNPCC syndrome. Because patients with endometrial cancer diagnosed prior to age 50 will have a risk greater than 5%–10% of having an inherited predisposition to a Lynch/HNPCC syndrome, genetic counseling may play an important role in the treatment of all young women with endometrial cancer considering conservative management [35]. If Lynch/HNPCC syndrome is diagnosed, this will not only have important implications for the patient herself, but it may lead to life-saving interventions for close family members as well. Appropriate laboratory testing should also be considered before commencing progestin therapy. The exact histologic diagnosis and accurate estimation of the disease extent is essential before deciding to administer fertility-sparing progestin therapy.
Histologic Diagnosis
Dilatation and curettage biopsy (DCBx), office endometrial biopsy, and hysteroscopic biopsy can be performed for the histologic diagnosis of endometrial cancer and grading of histologic differentiation of tumor. However, DCBx is the preferred method of histologic diagnosis before commencing fertility-sparing progestin therapy. Office endometrial biopsy is an accurate and convenient diagnostic method that can detect over 90% of endometrial cancers [36, 37]. However, its diagnostic accuracy is limited for small localized tumor [38]. It is very difficult to differentiate grade 1 adenocarcinoma from atypical complex hyperplasia [39] and to accurately determine the histologic grade of tumor using the small tissue samples that are obtained by office endometrial biopsy [40]. The histologic grade of tumor determined by office endometrial biopsy was upgraded after hysterectomy in 26% of cases, whereas the grade determined by DCBx was upgraded in 10% of cases after hysterectomy [40]. DCBx can also provide a therapeutic benefit by removing all, or at least most, of the cancer tissues in the endometrial cavity, whereas office endometrial biopsy serves a diagnostic role only. In a previous study of hysterectomy specimens, there was no residual tumor found in 11% of patients who underwent office endometrial biopsy and in 2% of patients who underwent DCBx [39]. There are two reported cases of successful fertility-sparing management via the therapeutic effects alone of DCBx [41, 42].
Hysteroscopic biopsy is also an accurate diagnostic method for endometrial adenocarcinoma. It provides direct assessment of the extent of the endometrial lesion and an accurate diagnosis when previous methods have been equivocal [43]. Some studies have suggested an increased risk of the peritoneal spread of endometrial cancer during hysteroscopy caused by the use of liquid distension medium [44], although others have not supported this [45, 46]. We thus contend that it would be better to use hysteroscopic evaluation as an adjunct to DCBx in cases with an equivocal diagnosis.
The pitfall in the histologic diagnosis of endometrial cancer and grading of the histologic differentiation of these tumors from biopsy material (regardless of the type of biopsies) without hysterectomy is that the diagnosis, histologic type, and grade can change after hysterectomy. There is also a significant inter- and intraobserver discrepancy of up to 40% in the differentiation between atypical endometrial hyperplasia and well-differentiated adenocarcinoma [47, 48] and in the determination of the histologic grade of tumor [49]. It is reported that the grade of tumor may change in 25% of cases and be upgraded in 10% of cases of endometrial cancer after hysterectomy [40, 50]. In addition, studies have shown that the endometrioid histology may change to other histologic types or other primary cancers after a hysterectomy [51–53].
Determination of Disease Extent
Before administering fertility-sparing progestin therapy, the extent of disease should be fully determined. The absence of cervical, myometrial, adenexal, lymph node, or peritoneal involvement and also the lack of any distant metastasis should be thoroughly verified. Clinical staging can only be done using imaging, although such clinical staging will be upstaged in up to 13%–22% of cases after surgical staging [54].
In the evaluation of myometrial invasion, contrast enhanced magnetic resonance imaging (MRI) is the preferred method as it has better accuracy compared with transvaginal ultrasonography, computed tomography (CT) and noncontrast enhanced MRI [54–59]. The accuracy of contrast enhanced MRI in the evaluation of cervical invasion, adnexal involvement, and lymph node metastasis has been reported also. The sensitivity and specificity of contrast-enhanced MRI is reported as 75%–80% and 94%–96%, respectively, in detecting myometrial invasion; 75%–80% and 94%–96%, respectively, in detecting cervical invasion; and 50% and 95%, respectively, in detecting lymph node metastasis [60]. This imaging modality can be reasonably used to assess disease extent in endometrial cancer patients. Recently, the utility of positron emission tomogram (PET) or PET-CT in the detection of lymph node metastases in early-stage endometrial cancer cases has been reported, with a sensitivity and specificity of 63% and 94.7%, respectively [61]. This level of accuracy is comparable to that of contrast enhanced MRI [62], but further evaluations are required to fully validate this.
The risk of adnexal, lymph node, and peritoneal metastasis in low risk endometrial cancer patients with well-differentiated tumors and no myometrial invasion detected by imaging technology is extremely low. Hence, laparoscopic evaluation of the adnexa, lymph node, and peritoneal cavity is not recommended unless imaging studies suggest a suspicious involvement of these regions in tumor spread.
Synchronous ovarian cancer is one of the concerns to consider before initiating fertility-sparing progestin therapy. Some investigators have suggested diagnostic laparoscopy because the incidence of synchronous ovarian cancer ranged between 11% and 29% in their patient series [6, 8, 17, 63–65]. However, most of these were small, single-institution studies. Other studies have suggested a much lower incidence range of 2.2%–6.9% [66–68], and a recent population-based study and additional large multicenter studies have reported an incidence range of approximately 3%–4.5% [69–72]. Hence, the incidence of synchronous ovarian cancer among candidates for fertility-sparing progestin therapy appears to be relatively low, and a diagnostic laparoscopy would not be required unless there was some clinical evidence of an ovarian tumor.
Preferred Progestins
Oral medroxyprogesterone acetate (MPA) and megestrol acetate (MA) are the most commonly used progestins for fertility-sparing therapy, with approximately 80% of the treated patients receiving continuous daily oral doses of these agents [73–75]. The potency of these two drugs in terms of an endometrial response has been reported to be similar [76, 77]. However, there has been no specific study comparing the efficacy of these two oral agents in fertility-sparing therapy. Although Park et al. [14] suggested that the complete response rate was similar between MPA and MA and that the recurrence rate was lower for MPA-treated cases in their subgroup analysis, further evaluation is required.
Recently, a progestin-containing intrauterine device has been used as a sole agent or in conjunction with oral progestin for fertility-sparing therapy [78]. This device can deliver a higher dose of progestin to the endometrium than orally administered progestin [79] and can avoid systemic complications associated with high doses of oral progestin including thromboembolism, weight gain, mood and libido changes, headaches, breast tenderness, sleep disorders, and leg cramps [50, 51, 80].
Optimal Dose and Duration of Progestin Therapy
The optimal dose of oral MPA and MA for fertility-sparing therapy is not currently well defined. In previous studies, the progestin doses varied from 60 to 1,800 mg/day for MPA and 10 to 400 mg/day for MA [73–75]. The most frequently used dose ranges of MPA and MA were 200-800 and 40-400 mg/day, respectively, with most patients receiving doses of ≥400 and <200 mg/day, respectively [73–75]. A high daily dose of oral progestin is typically used in clinical practice, but it is not clear whether low- or high-dose progestin is more effective. In a previous Gynecologic Oncology Group randomized trial of advanced and recurrent endometrial cancer, the response rate and progression-free survival outcome following MPA therapy was higher in low-dose group (200 mg/day) than in high-dose group (1,000 mg/day) [81]. However, this comparison has never been investigated for fertility-sparing therapy in a randomized trial. In a study by Park et al. [14], subgroup analysis of the response rate and recurrence rate in endometrial cancer patients did not differ between low-dose (MPA or MA, <250 mg/day) and high-dose (MPA or MA, >250 mg/day) fertility-sparing treatment groups. Further evaluations are warranted to elucidate the optimal dose of progestin for fertility-sparing therapy.
The median treatment duration to a complete response has differed between studies of progestin-treated endometrial cancer patients. Ramirez et al. [73] report that the median time interval to a complete response in this context is 12 weeks (range, 4–60 weeks). Hence, a treatment period of at least 3 months is required to determine treatment failure. If the patient shows disease progression at this time point, definitive surgical management is warranted. However, if the patient has persistent disease without progression at this time point, further treatment with progestin can be performed as some instances of a complete response after 9–12 months of treatment have been reported [14, 23, 51]. Therefore, although it is not clear when progestin treatment failure should be determined in patients with persistent disease without progression, this therapy can be extended to 9–12 months. In this regard, changes to the progestin dose and type can be considered when the response is incomplete at the first evaluation of treatment respone. However, the efficacy of this strategy remains to be fully evaluated.
The total progestin treatment duration varies between 3 and 36 months in previous studies [74, 75]. Chiva et al. [82] have reviewed this issue and reported a median of approximately 6 months. It is not yet clear when progestin therapy should be discontinued in patients who achieve a complete response because this ranges from immediately to several months in different cases. However, the benefit of additional progestin therapy for several months after a complete response was not clear in a previous study [14].
Monitoring of Progestin Therapy
Because the impact of progestins on endometrial cancer cells becomes apparent as early as 10 weeks after the start of treatment [83], and an initial exposure period of at least 12 weeks should be allowed before the response is evaluated [84], a reasonable time point for the first pathologic response evaluation is 3 months after the start of treatment. Subsequently, pathologic responses should be evaluated every 3 months during the progestin treatment course until a complete response is achieved. The treatment response should be assessed by histologic evaluation of the endometrium. As the initial diagnosis, DCBx is the preferred method to evaluate the response to progestin therapy. DCBx was also found to be more accurate than office endometrial biopsy in the evaluation of treatment response for progestin-containing intrauterine devices [85]. Frequent use of a hysteroscopic biopsy of the endometrium may adversely impact on future pregnancy outcomes because of the destruction of the basal layer of the endometrium [86], the subsequent replacement of the endometrial lining with fibrosis [87], and potential thermal injury to the myometrium [88].
Surveillance After Progestin Therapy
Surveillance after successful progestin therapy should include periodic interviews to explore any symptoms, physical examinations, and transvaginal ultrasonography at 3-month intervals. However, periodic pathologic evaluations of the endometrium, using office endometrial biopsy, DCBx, or hysteroscopy, need not be recommenced in patients who do not have symptoms or signs of recurrence. Frequent pathologic evaluation of the endometrium may not be effective and may adversely affect pregnancy outcomes by causing intrauterine adhesion or destruction of the basal layer of the endometrium [86]. Hence, endometrial pathologic evaluation is recommended only for patients with symptoms or signs suggesting recurrence.
If the patients wish to conceive after achieving a complete response to progestin, pregnancy trials can be attempted immediately. However, if the patients want to delay pregnancy, maintenance therapy using low-dose cyclic progestin, oral medications, or a progestin-containing intrauterine device can be recommended. Because young women with endometrial cancer often have an excessive unopposed estrogen milieu, elimination of this condition using maintenance therapy would be helpful in preventing either recurrence or de novo endometrial cancer [8, 9]. Park et al. [14] have reported in this regard that maintenance therapy is associated with decreased recurrence.
A prophylactic hysterectomy should be recommended after the completion of family planning because of a high reported rate of disease recurrence [73–75]. The safety of alternative strategies such as the delay of hysterectomy until recurrence has not yet been evaluated.
Oncologic Outcomes After Progestin Therapy
The complete response rate to fertility-sparing therapy is reported to range between 25% and 89% in previous case reports and case series [74, 75]. However, recent review articles and previous studies report a mean complete response rate ranging from 66.7% to 79.7% [73–75, 78, 82, 89–97]. The most recent meta-analysis, which included 408 patients from 32 studies published between 1983 and 2011, reported a pooled complete response rate of 76.2% (95% confidence interval, 68%–85.3%) [98]. However, various types of fertility-sparing therapy were included in these review articles, and meta-analysis and the indication for fertility-sparing therapy were not limited to stage IA (confined to endometrium), grade 1 endometrioid endometrial cancer. Recently, Park et al. [14] reported the largest series of fertility-sparing therapy cases including only oral progestin therapy and using strict inclusion criteria as shown in Table 1. The complete response rate to progestin therapy was 77.7% in that study [14]. Hence, fertility-sparing progestin therapy is highly effective in stage I (confined to endometrium), grade 1 endometrioid endometrial adenocarcinoma.
Montz et al. [80] have reported for the first time the use of a progestin-containing intrauterine device for stage IA (without myometrial invasion), grade 1 endometrial cancer. However, this was for inoperable cases caused by medical morbidity and not for fertility-sparing therapy [80]. According to a recent review, 17 of 37 patients with stage IA (without myometrial invasion), grade 1 endometrial cancer achieved a complete response with a progestin-containing intrauterine device with a pooled complete response rate of 46% (95% confidence interval, 29%–63%) [78], which is a somewhat disappointing outcome. Hence, some investigators have reported the outcomes of a combined use of progestin-containing intrauterine device with oral progestin [18] or gonadotropin-releasing hormone agonist [17] for fertility-sparing therapy to achieve better outcomes than either agent alone. Kim et al. [18] conducted a prospective observational study of 16 patients with stage IA (confined to endometrium), grade 1 endometrial cancer who were treated with progestin-containing intrauterine device with oral progestin. The results of that study were promising because 14 patients (87%) achieved complete response, and only 2 patients (14.3%) had recurrent disease [18]. Further evaluations are warranted in this regard.
Although the initial response rate of endometrial cancer patients to progestins is very promising, a significant proportion of these cases subsequently show recurrence. According to the findings reported in previous review articles, the endometrial cancer recurrence rate after successful fertility-sparing therapy ranges between 19.2% and 33.8% [73–75, 78, 82, 89–97]. The most contemporary meta-analysis of these patients reported a pooled recurrence rate of 40.6% (95% confidence interval, 33.1%–49.8%) [98]. In the study of Park et al. [14], the recurrence rate after successful progestin therapy was 30.4%, so that the durable complete response rate to progestin therapy was 54.5%. High recurrence rates after fertility-sparing therapy reflect the fact that the goal of this treatment approach is to delay any definitive surgical management to allow child bearing and not to cure the disease. Close surveillance is therefore mandatory after achieving a complete response to progestin treatment.
The safety of fertility-sparing therapy is supported by the findings that subsequent disease progression is extremely rare even in patients who did not respond and that almost all recurrences are well-differentiated tumors confined to the endometrium and are thus still curable with definitive surgical management. In the literature, only 10 patients with stage II or higher disease after fertility-sparing therapy have been reported [98], 4 of whom died of disease [99–102]. However, it is not clear whether these cases had true early endometrial cancer at their initial diagnosis and whether fertility-sparing therapy compromised their survival. Park et al. [14] report that of 148 patients in their series with stage IA (confined to endometrium), grade 1, endometrioid endometrial adenocarcinoma who commenced fertility-sparing progestin therapy, no cases of disease progression over stage IA, grade 1 disease emerged during or after progestin therapy. All cases of treatment failure and recurrent disease in that cohort were successfully salvaged by definitive surgical management or progestin retreatment [14].
Progestin Therapy for More Advanced Disease
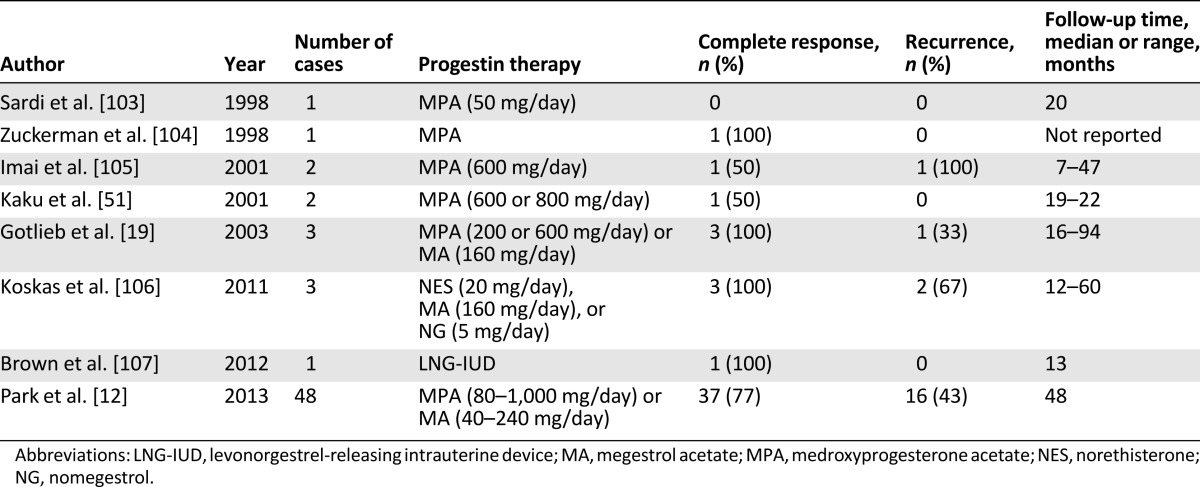
As indicated in Table 1, the optimal indication for fertility-sparing progestin therapy is stage IA, grade 1 endometrial cancer without myometrial invasion. Sometimes, however, patients with superficial myometrial invasion and/or grade 2–3 disease may want to preserve their fertility. Only a few studies have reported the outcomes of fertility-sparing treatment in patients with stage IA, grade 2–3 disease without myometrial invasion as a part of their wider analyses (Table 2) [19, 51, 103–107]. Recently, Park et al. [12] reported the oncologic and reproductive outcomes in a cohort of endometrial cancer patients with superficial myometrial invasion and/or grade 2–3 disease. The complete response rates to progestin therapy in that study were 76.5%, 73.9%, and 87.5%, respectively, for patients with stage IA (without myometrial invasion), grade 2–3 disease; patients with stage IA (with superficial myometrial invasion), grade 1 disease; and patients with stage IA (with superficial myometrial invasion), grade 2–3 disease [12]. The recurrence rates after progestin therapy in that study were 23.1%, 47.1%, and 71.4%, respectively, with no evidence of disease progression after fertility-sparing progestin therapy [12]. Fertility-sparing progestin therapy is therefore a viable treatment option in patients with stage IA (without myometrial invasion), grade 2–3 disease and in patients with stage IA (with superficial myometrial invasion), grade 1 disease. However, further evaluations are still required before recommending fertility-sparing progestin therapy to endometrial cancer patients with more advanced disease in routine practice.
Table 2.
Published studies showing the efficacy of progestin therapy in endometrial cancer with myometrial invasion and/or grade 2–3 differentiation
Progestin Therapy for Recurrent Disease
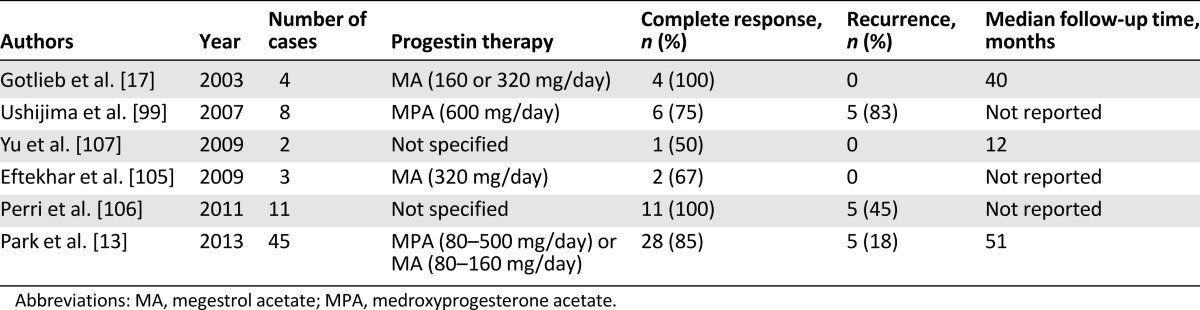
Most endometrial cancer patients who have recurrent disease undergo definitive surgical management including hysterectomy. If these patients have not had a successful pregnancy at the time of recurrence, they may still want to preserve their fertility. Because most recurrent disease in endometrial cancer cases involves well-differentiated tumors confined to the endometrium, a second round of fertility-sparing progestin therapy can be considered. However, the treatment outcomes are not well known in such cases, and few studies have addressed this as a part of their wider analyses (Table 3) [19, 102, 108–110]. The complete response rate to progestin retreatment is reported to range from 52% to 100% for recurrent disease. Recently, Park et al. [13] reported their findings for the largest endometrial cancer series yet analyzed regarding this subject. Of 33 patients with recurrent endometrial cancer in that study who received a second round of fertility-sparing progestin therapy, a complete response rate of 89% and rerecurrence rate of 42% were recorded, with no disease progression [13]. These outcomes were similar to those of the primary fertility-sparing progestin therapy. Again, progestin retreatment in patients with recurrent disease can therefore be considered a safe and effective intervention for patients who still want to preserve their fertility.
Table 3.
Published studies showing the efficacy of progestin therapy for recurrent endometrial cancer
Pregnancy Outcomes After Progestin Therapy
The pregnancy outcomes after fertility-sparing progestin therapy are not well known because most previous studies were case reports or involved small case series and mostly focused on the oncologic safety of the treatment rather than pregnancy outcomes. In a previous meta-analysis that included 325 women from 26 studies, 75 women achieved at least 1 live birth, with a pooled live birth rate of 28% (95% confidence interval, 21.6%–36.3%) [98]. However, the live birth rate would be higher than this if only women who tried to conceive after successful fertility-sparing therapy were considered. Park et al. [111] reported the largest series to be evaluated in terms of pregnancy outcome after progestin therapy in women with stage IA (confined to endometrium), grade 1 endometrioid endometrial adenocarcinoma. In that study, of the 144 patients who achieved complete remission, 70 patients attempted to conceive, 51 patients achieved at least 1 pregnancy, and 46 patients gave birth to a healthy child [111]. The pregnancy rate was therefore 73%, and live birth rate was 66% when considering only women who tried to conceive [111]. In that study, the spontaneous abortion rate was slightly higher than and the ectopic pregnancy and preterm delivery rates were similar to those of general population [111].
Because anovulatory disorders including polycystic ovary syndrome are a frequent predisposing factor for endometrial adenocarcinoma in young women, the incidence of subfertility or infertility is higher in these women than in the general population [8, 9, 111]. Therefore, assisted reproductive technologies are often required in these cases to achieve pregnancy [98, 111]. The pregnancy rate and live birth rate were found to be significantly higher in women who received assisted reproductive technology than in women who attempted natural pregnancy [98, 111]. The use of fertility drugs during assisted reproductive technologies increases estrogen production [112]. However, it is controversial as to whether this would increase the risk of recurrence after fertility-sparing progestin therapy for early endometrial cancer [113–116]. Park et al. [111] assessed the association between the use of fertility drugs and an increased risk of recurrence after successful progestin therapy but did not find any such association. Instead, these authors found that patients who achieved at least one pregnancy had a lower risk of disease recurrence regardless of the use of fertility drugs [111]. Fertility drugs can therefore be used safely after successful fertility-sparing progestin therapy, and a history of subfertility or infertility should not be a contraindication for such therapy.
Because anovulatory disorders including polycystic ovary syndrome are a frequent predisposing factor for endometrial adenocarcinoma in young women, the incidence of subfertility or infertility is higher in these women than in the general population. Therefore, assisted reproductive technologies are often required in these cases to achieve pregnancy.
Conclusion
Fertility-sparing progestin therapy is highly effective in selected young women with primary and recurrent endometrial cancer. The selection of appropriate patients through comprehensive pretreatment evaluations is of paramount importance to achieve the best outcomes without compromising survival outcomes. Because of the high rate of recurrence after successful fertility-sparing management, close surveillance is mandatory, and prophylactic hysterectomy is the best option after a successful pregnancy. Pregnancy outcomes are very promising in these cases with the aid of assisted reproductive technologies. Continuous daily oral MPA are MA are the preferred progestins for fertility-sparing therapy. However, future studies should be performed to determine the optimal dose and treatment duration of these agents.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Jeong-Yeol Park, Joo-Hyun Nam
Provision of study material or patients: Jeong-Yeol Park, Joo-Hyun Nam
Collection and/or assembly of data: Jeong-Yeol Park, Joo-Hyun Nam
Data analysis and interpretation: Jeong-Yeol Park, Joo-Hyun Nam
Manuscript writing: Jeong-Yeol Park, Joo-Hyun Nam
Final approval of manuscript: Jeong-Yeol Park, Joo-Hyun Nam
Disclosures
The authors indicated no financial relationships.
References
- 1.Bray F, Loos AH, Oostindier M, et al. Geographic and temporal variations in cancer of the corpus uteri: Incidence and mortality in pre- and postmenopausal women in Europe. Int J Cancer. 2005;117:123–131. doi: 10.1002/ijc.21099. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: Incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY, Chen CA, Chen YL, et al. Nationwide surveillance in uterine cancer: Survival analysis and the importance of birth cohort: 30-year population-based registry in Taiwan. PLoS One. 2012;7:e51372. doi: 10.1371/journal.pone.0051372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol. 2009;20:67–71. doi: 10.3802/jgo.2009.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crissman JD, Azoury RS, Barnes AE, et al. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. 1981;57:699–704. [PubMed] [Google Scholar]
- 7.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–420. [PubMed] [Google Scholar]
- 8.Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105:575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 9.Duska LR, Garrett A, Rueda BR, et al. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83:388–393. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 10.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Boronow RC, Morrow CP, Creasman WT, et al. Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–832. [PubMed] [Google Scholar]
- 12.Park JY, Kim DY, Kim TJ, et al. Hormonal therapy for women with stage IA endometrial cancer of all grades. Obstet Gynecol. 2013;122:7–14. doi: 10.1097/AOG.0b013e3182964ce3. [DOI] [PubMed] [Google Scholar]
- 13.Park JY, Lee SH, Seong SJ, et al. Progestin re-treatment in patients with recurrent endometrial adenocarcinoma after successful fertility-sparing management using progestin. Gynecol Oncol. 2013;129:7–11. doi: 10.1016/j.ygyno.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Kim DY, Kim JH, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur J Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Shan W, Wang C, Zhang Z, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol. 2014;25:214–220. doi: 10.3802/jgo.2014.25.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MK, Seong SJ. Conservative treatment for atypical endometrial hyperplasia: What is the most effective therapeutic method? J Gynecol Oncol. 2014;25:164–165. doi: 10.3802/jgo.2014.25.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minig L, Franchi D, Boveri S, et al. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Seong SJ, Kim YS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013;209:358. doi: 10.1016/j.ajog.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Gotlieb WH, Beiner ME, Shalmon B, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102:718–725. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]
- 20.Bahamondes L, Ribeiro-Huguet P, de Andrade KC, et al. Levonorgestrel-releasing intrauterine system (Mirena) as a therapy for endometrial hyperplasia and carcinoma. Acta Obstet Gynecol Scand. 2003;82:580–582. [PubMed] [Google Scholar]
- 21.Abu Hashim H, Zayed A, Ghayaty E, et al. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: A randomized controlled trial. J Gynecol Oncol. 2013;24:128–134. doi: 10.3802/jgo.2013.24.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Signorelli M, Caspani G, Bonazzi C, et al. Fertility-sparing treatment in young women with endometrial cancer or atypical complex hyperplasia: A prospective single-institution experience of 21 cases. BJOG. 2009;116:114–118. doi: 10.1111/j.1471-0528.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 23.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–440. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang CB, Wang CJ, Huang HJ, et al. Fertility-preserving treatment in young patients with endometrial adenocarcinoma. Cancer. 2002;94:2192–2198. doi: 10.1002/cncr.10435. [DOI] [PubMed] [Google Scholar]
- 25.Lai CH, Hsueh S, Chao AS, et al. Successful pregnancy after tamoxifen and megestrol acetate therapy for endometrial carcinoma. Br J Obstet Gynaecol. 1994;101:547–549. doi: 10.1111/j.1471-0528.1994.tb13162.x. [DOI] [PubMed] [Google Scholar]
- 26.Kung FT, Chen WJ, Chou HH, et al. Conservative management of early endometrial adenocarcinoma with repeat curettage and hormone therapy under assistance of hysteroscopy and laparoscopy. Hum Reprod. 1997;12:1649–1653. doi: 10.1093/humrep/12.8.1649. [DOI] [PubMed] [Google Scholar]
- 27.Huang SY, Jung SM, Ng KK, et al. Ovarian metastasis in a nulliparous woman with endometrial adenocarcinoma failing conservative hormonal treatment. Gynecol Oncol. 2005;97:652–655. doi: 10.1016/j.ygyno.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Burnett AF, Bahador A, Amezcua C. Anastrozole, an aromatase inhibitor, and medroxyprogesterone acetate therapy in premenopausal obese women with endometrial cancer: A report of two cases successfully treated without hysterectomy. Gynecol Oncol. 2004;94:832–834. doi: 10.1016/j.ygyno.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Banno K, Kisu I, Yanokura M, et al. Progestin therapy for endometrial cancer: The potential of fourth-generation progestin. Int J Oncol. 2012;40:1755–1762. doi: 10.3892/ijo.2012.1384. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu Y, Takeuchi T, Mita S, et al. Dienogest, a synthetic progestin, inhibits the proliferation of immortalized human endometrial epithelial cells with suppression of cyclin D1 gene expression. Mol Hum Reprod. 2009;15:693–701. doi: 10.1093/molehr/gap042. [DOI] [PubMed] [Google Scholar]
- 31.Quick CM, May T, Horowitz NS, et al. Low-grade, low-stage endometrioid endometrial adenocarcinoma: A clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31:337–343. doi: 10.1097/PGP.0b013e31823ff422. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich CE, Young PC, Stehman FB, et al. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 33.Navarria I, Usel M, Rapiti E, et al. Young patients with endometrial cancer: How many could be eligible for fertility-sparing treatment? Gynecol Oncol. 2009;114:448–451. doi: 10.1016/j.ygyno.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 34.Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 35.Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107:159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Dijkhuizen FP, Mol BW, Brölmann HA, et al. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: A meta-analysis. Cancer. 2000;89:1765–1772. [PubMed] [Google Scholar]
- 37.Stovall TG, Photopulos GJ, Poston WM, et al. Pipelle endometrial sampling in patients with known endometrial carcinoma. Obstet Gynecol. 1991;77:954–956. [PubMed] [Google Scholar]
- 38.Guido RS, Kanbour-Shakir A, Rulin MC, et al. Pipelle endometrial sampling. Sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553–555. [PubMed] [Google Scholar]
- 39.Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: A Gynecologic Oncology Group study. Cancer. 2006;106:812–819. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 40.Larson DM, Johnson KK, Broste SK, et al. Comparison of D&C and office endometrial biopsy in predicting final histopathologic grade in endometrial cancer. Obstet Gynecol. 1995;86:38–42. doi: 10.1016/0029-7844(95)00105-Z. [DOI] [PubMed] [Google Scholar]
- 41.Kempson RL, Pokorny GE. Adenocarcinoma of the endometrium in women aged forty and younger. Cancer. 1968;21:650–662. doi: 10.1002/1097-0142(196804)21:4<650::aid-cncr2820210416>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Shibahara H, Shigeta M, Toji H, et al. Successful pregnancy in an infertile patient with conservatively treated endometrial adenocarcinoma after transfer of embryos obtained by intracytoplasmic sperm injection. Hum Reprod. 1999;14:1908–1911. doi: 10.1093/humrep/14.7.1908. [DOI] [PubMed] [Google Scholar]
- 43.Symonds I. Ultrasound, hysteroscopy and endometrial biopsy in the investigation of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:381–391. doi: 10.1053/beog.2000.0183. [DOI] [PubMed] [Google Scholar]
- 44.Obermair A, Geramou M, Gucer F, et al. Does hysteroscopy facilitate tumor cell dissemination?: Incidence of peritoneal cytology from patients with early stage endometrial carcinoma following dilatation and curettage (D&C) versus hysteroscopy and D&C. Cancer. 2000;88:139–143. [PubMed] [Google Scholar]
- 45.Selvaggi L, Cormio G, Ceci O, et al. Hysteroscopy does not increase the risk of microscopic extrauterine spread in endometrial carcinoma. Int J Gynecol Cancer. 2003;13:223–227. doi: 10.1046/j.1525-1438.2003.13044.x. [DOI] [PubMed] [Google Scholar]
- 46.Biewenga P, de Blok S, Birnie E. Does diagnostic hysteroscopy in patients with stage I endometrial carcinoma cause positive peritoneal washings? Gynecol Oncol. 2004;93:194–198. doi: 10.1016/j.ygyno.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Bergeron C, Nogales FF, Masseroli M, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23:1102–1108. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 49.Nofech-Mozes S, Ismiil N, Dube V, et al. Interobserver agreement for endometrial cancer characteristics evaluated on biopsy material. Obstet Gynecol Int. 2012;2012:414086. doi: 10.1155/2012/414086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jadoul P, Donnez J. Conservative treatment may be beneficial for young women with atypical endometrial hyperplasia or endometrial adenocarcinoma. Fertil Steril. 2003;80:1315–1324. doi: 10.1016/s0015-0282(03)01183-x. [DOI] [PubMed] [Google Scholar]
- 51.Kaku T, Yoshikawa H, Tsuda H, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: Central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 52.Jacques SM, Qureshi F, Munkarah A, et al. Interinstitutional surgical pathology review in gynecologic oncology: II. Endometrial cancer in hysterectomy specimens. Int J Gynecol Pathol. 1998;17:42–45. doi: 10.1097/00004347-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Fujiwara H, Shibahara H, Usui R, et al. Unsuspected uterine carcinosarcoma (heterologous) diagnosed following conservative therapies with medroxyprogesterone acetate for presumed early-stage endometrial carcinoma. Am J Reprod Immunol. 2002;47:129–131. doi: 10.1034/j.1600-0897.2002.1c068.x. [DOI] [PubMed] [Google Scholar]
- 54.Kinkel K, Kaji Y, Yu KK, et al. Radiologic staging in patients with endometrial cancer: A meta-analysis. Radiology. 1999;212:711–718. doi: 10.1148/radiology.212.3.r99au29711. [DOI] [PubMed] [Google Scholar]
- 55.Varpula MJ, Klemi PJ. Staging of uterine endometrial carcinoma with ultra-low field (0.02 T) MRI: A comparative study with CT. J Comput Assist Tomogr. 1993;17:641–647. doi: 10.1097/00004728-199307000-00023. [DOI] [PubMed] [Google Scholar]
- 56.Hardesty LA, Sumkin JH, Hakim C, et al. The ability of helical CT to preoperatively stage endometrial carcinoma. AJR Am J Roentgenol. 2001;176:603–606. doi: 10.2214/ajr.176.3.1760603. [DOI] [PubMed] [Google Scholar]
- 57.DelMaschio A, Vanzulli A, Sironi S, et al. Estimating the depth of myometrial involvement by endometrial carcinoma: Efficacy of transvaginal sonography vs MR imaging. AJR Am J Roentgenol. 1993;160:533–538. doi: 10.2214/ajr.160.3.8430547. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita Y, Mizutani H, Torashima M, et al. Assessment of myometrial invasion by endometrial carcinoma: Transvaginal sonography vs contrast-enhanced MR imaging. AJR Am J Roentgenol. 1993;161:595–599. doi: 10.2214/ajr.161.3.8352114. [DOI] [PubMed] [Google Scholar]
- 59.Zarbo G, Caruso G, Caruso S, et al. Endometrial cancer: Preoperative evaluation of myometrial infiltration magnetic resonance imaging versus transvaginal ultrasonography. Eur J Gynaecol Oncol. 2000;21:95–97. [PubMed] [Google Scholar]
- 60.Barwick TD, Rockall AG, Barton DP, et al. Imaging of endometrial adenocarcinoma. Clin Radiol. 2006;61:545–555. doi: 10.1016/j.crad.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Chang MC, Chen JH, Liang JA, et al. 18F-FDG PET or PET/CT for detection of metastatic lymph nodes in patients with endometrial cancer: A systematic review and meta-analysis. Eur J Radiol. 2012;81:3511–3517. doi: 10.1016/j.ejrad.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 62.Park JY, Kim EN, Kim DY, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008;108:486–492. doi: 10.1016/j.ygyno.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 63.Evans-Metcalf ER, Brooks SE, Reale FR, et al. Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol. 1998;91:349–354. doi: 10.1016/s0029-7844(97)00668-6. [DOI] [PubMed] [Google Scholar]
- 64.Gitsch G, Hanzal E, Jensen D, et al. Endometrial cancer in premenopausal women 45 years and younger. Obstet Gynecol. 1995;85:504–508. doi: 10.1016/0029-7844(95)00001-8. [DOI] [PubMed] [Google Scholar]
- 65.Walsh C, Holschneider C, Hoang Y, et al. Coexisting ovarian malignancy in young women with endometrial cancer. Obstet Gynecol. 2005;106:693–699. doi: 10.1097/01.AOG.0000172423.64995.6f. [DOI] [PubMed] [Google Scholar]
- 66.Yamanoi K, Mandai M, Suzuki A, et al. Synchronous primary corpus and ovarian cancer: High incidence of endometriosis and thrombosis. Oncol Lett. 2012;4:375–380. doi: 10.3892/ol.2012.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Zhao Q, Lv X. Characteristics and prognosis of coexisting adnexa malignancy with endometrial cancer: A single institution review of 51 cases. Arch Gynecol Obstet. 2011;283:1133–1137. doi: 10.1007/s00404-010-1574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee TS, Jung JY, Kim JW, et al. Feasibility of ovarian preservation in patients with early stage endometrial carcinoma. Gynecol Oncol. 2007;104:52–57. doi: 10.1016/j.ygyno.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Williams MG, Bandera EV, Demissie K, et al. Synchronous primary ovarian and endometrial cancers: A population-based assessment of survival. Obstet Gynecol. 2009;113:783–789. doi: 10.1097/AOG.0b013e31819c7bdf. [DOI] [PubMed] [Google Scholar]
- 70.van Niekerk CC, Vooijs GP, Bulten J, et al. Increased risk of concurrent primary malignancies in patients diagnosed with a primary malignant epithelial ovarian tumor. Mod Pathol. 2007;20:384–388. doi: 10.1038/modpathol.3800752. [DOI] [PubMed] [Google Scholar]
- 71.Beard CM, Hartmann LC, Keeney GL, et al. Endometrial cancer in Olmsted County, MN: Trends in incidence, risk factors and survival. Ann Epidemiol. 2000;10:97–105. doi: 10.1016/s1047-2797(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 72.Song T, Seong SJ, Bae DS, et al. Synchronous primary cancers of the endometrium and ovary in young women: A Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013;131:624–628. doi: 10.1016/j.ygyno.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez PT, Frumovitz M, Bodurka DC, et al. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: A literature review. Gynecol Oncol. 2004;95:133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 74.Tangjitgamol S, Manusirivithaya S, Hanprasertpong J. Fertility-sparing in endometrial cancer. Gynecol Obstet Invest. 2009;67:250–268. doi: 10.1159/000209324. [DOI] [PubMed] [Google Scholar]
- 75.Erkanli S, Ayhan A. Fertility-sparing therapy in young women with endometrial cancer: 2010 update. Int J Gynecol Cancer. 2010;20:1170–1187. doi: 10.1111/igc.0b013e3181e94f5a. [DOI] [PubMed] [Google Scholar]
- 76.Kumar N, Koide SS, Tsong Y, et al. Nestorone: A progestin with a unique pharmacological profile. Steroids. 2000;65:629–636. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 77.Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2003;46(suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Baker J, Obermair A, Gebski V, et al. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: A meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. doi: 10.1016/j.ygyno.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 79.Nilsson CG, Haukkamaa M, Vierola H, et al. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf) 1982;17:529–536. doi: 10.1111/j.1365-2265.1982.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 80.Montz FJ, Bristow RE, Bovicelli A, et al. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–657. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 81.Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 82.Chiva L, Lapuente F, González-Cortijo L, et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111(suppl):S101–S104. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 83.Saegusa M, Okayasu I. Progesterone therapy for endometrial carcinoma reduces cell proliferation but does not alter apoptosis. Cancer. 1998;83:111–121. doi: 10.1002/(sici)1097-0142(19980701)83:1<111::aid-cncr15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 84.Reifenstein EC., Jr The treatment of advanced endometrial cancer with hydroxyprogesterone caproate. Gynecol Oncol. 1974;2:377–414. doi: 10.1016/0090-8258(74)90029-8. [DOI] [PubMed] [Google Scholar]
- 85.Kim MK, Seong SJ, Song T, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecol Oncol. 2013;130:470–473. doi: 10.1016/j.ygyno.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 86.Yu D, Wong YM, Cheong Y, et al. Asherman syndrome: One century later. Fertil Steril. 2008;89:759–779. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 87.Taskin O, Onoglu A, Inal M, et al. Long-term histopathologic and morphologic changes after thermal endometrial ablation. J Am Assoc Gynecol Laparosc. 2002;9:186–190. doi: 10.1016/s1074-3804(05)60130-2. [DOI] [PubMed] [Google Scholar]
- 88.Sentilhes L, Sergent F, Roman H, et al. Late complications of operative hysteroscopy: Predicting patients at risk of uterine rupture during subsequent pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;120:134–138. doi: 10.1016/j.ejogrb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Kesterson JP, Fanning J. Fertility-sparing treatment of endometrial cancer: Options, outcomes and pitfalls. J Gynecol Oncol. 2012;23:120–124. doi: 10.3802/jgo.2012.23.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiva de Agustín L, Lapuente Sastre F, Corraliza Galán V, et al. Conservative management of patients with early endometrial carcinoma: A systematic review. Clin Transl Oncol. 2008;10:155–162. doi: 10.1007/s12094-008-0173-1. [DOI] [PubMed] [Google Scholar]
- 91.Gadducci A, Spirito N, Baroni E, et al. The fertility-sparing treatment in patients with endometrial atypical hyperplasia and early endometrial cancer: A debated therapeutic option. Gynecol Endocrinol. 2009;25:683–691. doi: 10.1080/09513590902733733. [DOI] [PubMed] [Google Scholar]
- 92.Gunderson CC, Fader AN, Carson KA, et al. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 93.Dorais J, Dodson M, Calvert J, et al. Fertility-sparing management of endometrial adenocarcinoma. Obstet Gynecol Surv. 2011;66:443–451. doi: 10.1097/OGX.0b013e31822f8f66. [DOI] [PubMed] [Google Scholar]
- 94.Bovicelli A, D’Andrilli G, Giordano A, et al. Conservative treatment of early endometrial cancer. J Cell Physiol. 2013;228:1154–1158. doi: 10.1002/jcp.24292. [DOI] [PubMed] [Google Scholar]
- 95.Rackow BW, Arici A. Endometrial cancer and fertility. Curr Opin Obstet Gynecol. 2006;18:245–252. doi: 10.1097/01.gco.0000193012.11523.c5. [DOI] [PubMed] [Google Scholar]
- 96.Benshushan A. Endometrial adenocarcinoma in young patients: Evaluation and fertility-preserving treatment. Eur J Obstet Gynecol Reprod Biol. 2004;117:132–137. doi: 10.1016/j.ejogrb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Kalogiannidis I, Agorastos T. Conservative management of young patients with endometrial highly-differentiated adenocarcinoma. J Obstet Gynaecol. 2011;31:13–17. doi: 10.3109/01443615.2010.532249. [DOI] [PubMed] [Google Scholar]
- 98.Gallos ID, Yap J, Rajkhowa M, et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Cormio G, Martino R, Loizzi V, et al. A rare case of choroidal metastasis presented after conservative management of endometrial cancer. Int J Gynecol Cancer. 2006;16:2044–2048. doi: 10.1111/j.1525-1438.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 100.Ferrandina G, Zannoni GF, Gallotta V, et al. Progression of conservatively treated endometrial carcinoma after full term pregnancy: A case report. Gynecol Oncol. 2005;99:215–217. doi: 10.1016/j.ygyno.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 101.Ota T, Yoshida M, Kimura M, et al. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int J Gynecol Cancer. 2005;15:657–662. doi: 10.1111/j.1525-1438.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 102.Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 103.Sardi J, Anchezar Henry JP, Paniceres G, et al. Primary hormonal treatment for early endometrial carcinoma. Eur J Gynaecol Oncol. 1998;19:565–568. [PubMed] [Google Scholar]
- 104.Zuckerman B, Lavie O, Neuman M, et al. Endoemtrial cancer stage I-grade II: Conservative treatment followed by a healthy twin pregnancy. Int J Gynecol Cancer. 1998;8:172–174. [Google Scholar]
- 105.Imai M, Jobo T, Sato R, et al. Medroxyprogesterone acetate therapy for patients with adenocarcinoma of the endometrium who wish to preserve the uterus-usefulness and limitations. Eur J Gynaecol Oncol. 2001;22:217–220. [PubMed] [Google Scholar]
- 106.Koskas M, Yazbeck C, Walker F, et al. Fertility-sparing management of grade 2 and 3 endometrial adenocarcinomas. Anticancer Res. 2011;31:3047–3049. [PubMed] [Google Scholar]
- 107.Brown AJ, Westin SN, Broaddus RR, et al. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet Gynecol. 2012;119:423–426. doi: 10.1097/AOG.0b013e318234d97c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eftekhar Z, Izadi-Mood N, Yarandi F, et al. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: Our experiences with 21 patients. Int J Gynecol Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 109.Perri T, Korach J, Gotlieb WH, et al. Prolonged conservative treatment of endometrial cancer patients: More than 1 pregnancy can be achieved. Int J Gynecol Cancer. 2011;21:72–78. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- 110.Yu M, Yang JX, Wu M, et al. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil Steril. 2009;92:2122–2124. doi: 10.1016/j.fertnstert.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 111.Park JY, Seong SJ, Kim TJ, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121:136–142. doi: 10.1097/aog.0b013e31827a0643. [DOI] [PubMed] [Google Scholar]
- 112.Sovino H, Sir-Petermann T, Devoto L. Clomiphene citrate and ovulation induction. Reprod Biomed Online. 2002;4:303–310. doi: 10.1016/s1472-6483(10)61821-4. [DOI] [PubMed] [Google Scholar]
- 113.Althuis MD, Moghissi KS, Westhoff CL, et al. Uterine cancer after use of clomiphene citrate to induce ovulation. Am J Epidemiol. 2005;161:607–615. doi: 10.1093/aje/kwi084. [DOI] [PubMed] [Google Scholar]
- 114.Dor J, Lerner-Geva L, Rabinovici J, et al. Cancer incidence in a cohort of infertile women who underwent in vitro fertilization. Fertil Steril. 2002;77:324–327. doi: 10.1016/s0015-0282(01)02986-7. [DOI] [PubMed] [Google Scholar]
- 115.Jensen A, Sharif H, Kjaer SK. Use of fertility drugs and risk of uterine cancer: Results from a large Danish population-based cohort study. Am J Epidemiol. 2009;170:1408–1414. doi: 10.1093/aje/kwp290. [DOI] [PubMed] [Google Scholar]
- 116.Silva IS, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: A long-term follow-up of a British cohort. Br J Cancer. 2009;100:1824–1831. doi: 10.1038/sj.bjc.6605086. [DOI] [PMC free article] [PubMed] [Google Scholar]


