This study aimed to subdivide the M1 stage of patients with nasopharyngeal carcinoma (NPC) and bone-only metastases and to identify the patients who may benefit from combined chemoradiotherapy (CRT). Metastasis to the spine and having more than three bone metastatic sites are independent unfavorable predictors for overall survival in patients with NPC and bone-only metastasis. Combined CRT should be considered for patients with single bone metastasis.
Keywords: Nasopharyngeal carcinoma, Metastasis, TNM staging, Prognosis, Chemoradiotherapy
Abstract
Background.
The current M1 stage in nasopharyngeal carcinoma (NPC) does not differentiate patients based on metastatic site and number of metastases. This study aims to subdivide the M1 stage of NPC patients with bone-only metastases and to identify the patients who may benefit from combined chemoradiotherapy (CRT).
Methods.
Between 1998 and 2007, 312 patients diagnosed with bone-only metastasis at Sun Yat-sen University Cancer Center were enrolled. Various possible subdivisions of M1 stage were considered, including by the time order of metastasis (synchronous vs. metachronous), involvement of specific bone metastatic site, the number of metastatic sites, and the number of metastases. The correlation of the subdivisions of M1 stage with overall survival (OS) was determined by Cox regression.
Results.
The median OS was 23.4 months. Patients with more than three metastatic sites had significantly poorer OS than patients with three or fewer metastatic sites (16.2 vs. 32.4 months; p < .001). Metastasis to the spine was significantly associated with unfavorable OS (20.4 vs. 37.9 months; p < .001). Multivariate analysis showed that number of metastatic sites (more than three vs. three or fewer), spine involvement (present vs. absent), and treatment modality (CRT vs. chemotherapy or radiotherapy only) were independent prognostic factors for OS. In stratified analysis, compared with chemotherapy or radiotherapy alone, combined chemoradiotherapy could significantly benefit the patients with single bone metastasis (hazard ratio: 0.21; 95% confidence interval: 0.09–0.50).
Conclusion.
Metastasis to the spine and having more than three bone metastatic sites are independent unfavorable predictors for OS in NPC patients with bone-only metastasis. Combined chemoradiotherapy should be considered for patients with single bone metastasis.
Abstract
摘要
背景。鼻咽癌 (NPC) 的现行 M1 期不基于转移部位和转移灶数目区分患者。这项研究旨在细分仅具有骨转移的 NPC 患者的 M1 期并鉴定可能从联合化放疗 (CRT) 中获益的患者。
方法。1998 年至 2007 年在中山大学肿瘤防治中心招募了 312 位确诊仅具有骨转移的患者。考虑了 M1 期的多种可能细分,包括按照转移(同步与异时)的时间顺序、涉及的特定骨转移部位、转移部位数目和转移灶数目细分。通过 Cox 回归确定 M1 期细分与总生存期 (OS) 的相关性。
结果。中位数 OS 是 23.4 个月。存在超过三个转移部位的患者比存在三个或更少转移部位的患者显著具有更差的 OS(16.2 个月对比 32.4 月;p < 0.001)。脊柱转移与不利 OS 显著相关(20.4 个月对比 37.9 月;p < 0.001)。多变量分析显示转移部位数目(超过三个对比三个或更少)、脊柱累及(存在对比不存在)和治疗模式(CRT 对比仅化疗或放疗)是 OS 的独立预后因素。在分层分析中,与单一化疗或放疗相比,联合化放疗可能显著有益于具有单一骨转移的患者(风险比:0.21;95% 置信区间:0.09–0.50)。
结论。脊柱转移和具有超过三个骨转移部位是仅具有骨转移的 NPC 患者 OS 的独立不利预测因素。联合化放疗应当考虑用于具有单一骨转移的患者。The Oncologist 2015; 20:291–298
Implication for Practice:
Establishing rigorous subdivision of the M1 stage of bone-only metastatic nasopharyngeal carcinoma (NPC) patients will facilitate disease assessment and treatment planning for clinicians. In our study, we found that the number of metastatic sites and spine involvement were independent prognostic factors for overall survival of NPC patients with bone-only metastasis; combined chemoradiotherapy (CRT) could potentially benefit the patients with single bone metastasis. By subdividing the M1 stage of the patients according to the two prognostic factors, we can better predict the prognosis of patients and facilitate treatment planning. Moreover, combined CRT is recommended for NPC patients with single bone metastasis.
Introduction
The tumor-node-metastasis (TNM) classification, which describes the anatomic extent of cancer, is widely used to aid clinicians and investigators in planning treatment, assessing prognosis, and facilitating communication [1, 2]. T refers to the extent of the primary tumor, N refers to the lymphatic involvement, and M describes distant metastasis. A series of modifications of the primary tumor (T) and local node (N) elements were introduced in the TNM staging system of nasopharyngeal carcinoma (NPC) in recent years [3–6], primarily because of the progress in diagnostic imaging, radiation techniques, and chemotherapy regimens. The metastatic (M1) stage is still a “catch-all” classification, with patients who differ in terms of the specific organs involved and the number and location of lesions in each organ. Subdividing the M1 stage for patients with metastatic NPC may help clinicians stratify patients according to prognosis and make therapeutic decisions [7, 8].
Bone is the most frequent involved organ by metastasis among patients with NPC, with an estimated incidence rate of 54%–80% in that group [9–12]. The frequently involved metastatic sites included spine, pelvis, and ribs, and the prognosis of patients with NPC and bone metastasis varied, with occasional long-term survivors [13–15]. Past research in identifying prognostic factors among different types of cancer, such as breast cancer [16], hepatocellular carcinoma [17], and non-small cell lung cancer [18], suggested that bone metastasis patients with different metastatic sites and numbers of lesions may differ greatly in terms of survival. In NPC, growing evidence shows that long-term survival and complete response can be obtained among a small proportion of patients with bone metastasis, especially for those with only a solitary lesion and received aggressive treatment [19]. To date, no study specifically evaluating the associated prognostic factors from M1 stage among patients with bone-metastatic NPC has been reported. In addition, there is no standard treatment for NPC patients who developed bone metastasis because of the limited number of studies with small numbers of cases due to their rarity.
In this study, we aimed to subdivide the M1 stage of NPC patients with bone-only metastasis at diagnosis and to evaluate the treatment effect of combined chemoradiotherapy (CRT) among different M1 patient groups.
Patients and Methods
Patients
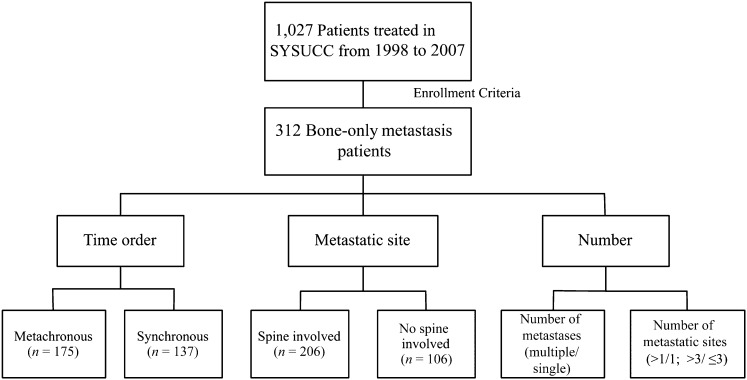
The medical records of 1,027 patients with NPC who were diagnosed with distant metastasis at Sun Yat-sen University Cancer Center (SYSUCC) from January 1998 to December 2007 were reviewed. This study was approved by the SYSUCC hospital ethics committee, which waived the need for written informed consent because of the retrospective nature of the study. The inclusion criteria included bone-only metastasis at the time of initial staging or developed bone metastasis as the first recurrence site during follow-up and receipt of systemic chemotherapy and/or local radiotherapy after metastasis. The exclusion criteria included treatment at another hospital after diagnosis of metastasis before admission to our institution and development of other distant metastasis within 2 months of diagnosis of bone-only metastasis. A total of 312 patients were enrolled (Fig. 1). The histology in 92.9% of the patients was nonkeratinizing or undifferentiated carcinoma (World Health Organization type 2 or 3 histology) [20]. The median duration of follow-up for the population was 16 months (range: 1–120 months).
Figure 1.
Flowchart of study design. A total of 312 patients met the enrollment criteria. The population was divided into subgroups according to the metastatic time order, site, and number of metastases to evaluate the factors associated with M staging.
Abbreviation: SYSUCC, Sun Yat-sen University Cancer Center.
Definitions and Grouping
Bone metastasis was diagnosed based on patient history, physical examination, and imaging studies using a bone scan and/or positron emission tomography/computed tomography and/or magnetic resonance imaging (MRI). Bone-only metastasis was defined as patients with bone metastasis and without nonskeletal distant metastases at the time of initial diagnosis of metastatic NPC. Metastasis onset was categorized as synchronous or metachronous metastasis to define whether patients presented with distant metastasis at first diagnosis of NPC. The number of metastases refers to the number of bones involved by metastases based on imaging reports from a bone scan and/or positron emission tomography/computed tomography imaging at the initial diagnosis of bone metastasis and further confirmed on MRI or computed tomography scan. The bone metastatic sites were generally divided into following categories: skull, cervical vertebrae, upper limb, thoracic vertebrae, rib, sternum, lumbar vertebrae, sacrum, pelvic girdle, and lower limb. The number of metastatic sites was defined as the total number of sites described above that were involved by metastases. The primary diseases of patients with NPC were staged according to the seventh edition of the TNM staging system [21].
Treatment
Treatment modalities for bone metastasis were defined as any kind of treatment performed from the time of diagnosis of bone metastasis to the time of development of other systemic metastasis or mortality, with the aim of eradicating or inhibiting tumor cells. Patients with previous chemotherapy and radiotherapy before the diagnosis of bone-only metastasis and radiotherapy for primary disease of synchronous metastatic NPC were excluded. The primary chemotherapy (CT) for patients with bone-only metastatic NPC was nearly exclusively plastin based, with cisplatin in combination with one or two of the following drugs for four to six cycles: fluorouracil, cyclophosphamide, vincristine, paclitaxel, and bleomycin. A change of chemotherapy regimen would be conducted if the metastatic disease progressed under primary treatment. Treatment discontinuation occurred because of patient requests or unacceptable drug toxicities.
Radiotherapy (RT) with a dosage ranging from 30 to 54 Gy was an option for patients with localized bone metastatic lesion, pain, high risk for pathological fracture, or neurological complications arising from spinal cord compression or nerve root pain. RT was performed with a single dorsal posterior-anterior photon field in the 6-MV energy range. The planning target volume covered the specific bone affected and bones immediately above and below it. The median individual dose in all patients was 2 Gy; the median total dose was 40 Gy. The respective fraction and total doses were planned separately for each individual patient, depending on the patient’s general state of health and respective prognosis.
Bisphosphonate treatment, defined as intravenous administration of zoledronic acid or pamidronate every 4 weeks for >2 months after diagnosis of bone metastasis [22], was offered as an adjuvant therapy and evaluated as a separate variable.
Follow-Up and Endpoint
Patients were evaluated for response every two cycles during systemic chemotherapy and then every 3 months until death, based on computed tomography or isotopic bone scan. The primary outcome was overall survival (OS), which was defined as the time from diagnosis of distant metastasis to death by any cause.
Statistical Analysis
Pearson chi-square test was used to compare categorical variables between groups. Rates of OS were estimated by the Kaplan-Meier method and were compared between different subgroups with the use of the log-rank test. To determine the independent prognostic factors for survival, a multivariate Cox regression model with the backward conditioning method was used, with the covariates including age, gender, N stage, Karnofsky performance status (KPS), spine involvement, number of metastases (multiple vs. single), number of metastatic sites (more than one vs. one), number of metastatic sites (more than three vs. three for fewer), metastatic onset, and treatment modality. Further subgroup analysis using an adjusted Cox model was conducted to test the impact of combined therapy among patients with different anatomic distributions of bone metastases and included the covariates of age, gender, N stage, spine involvement, and number of metastatic sites (more than three vs. three or fewer), except the stratification factor. A p value <.05 was considered significant. Statistical analysis was performed using SPSS 20.0 software (IBM Corp, Armonk, NY, http://www.ibm.com).
Results
Characteristics of the Population
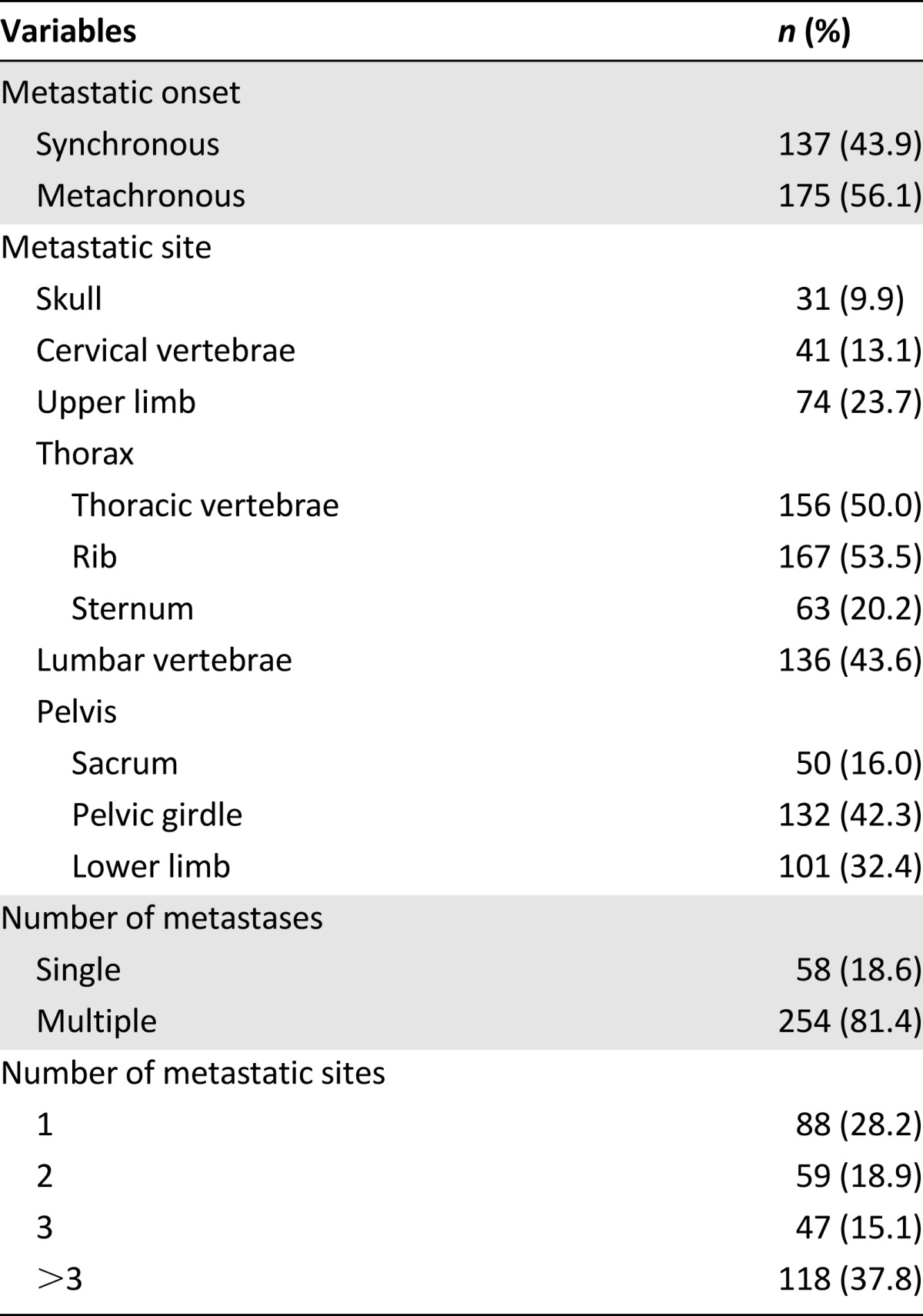
A total of 312 patients who were diagnosed with bone-only metastasis at the time of initial staging or who had developed bone metastasis as the first metastatic site were included in this study (Table 1). The median age was 46 years (range: 11–78 years). Overall, 269 patients (86.2%) were male and 43 (13.8%) were female; 137 (43.9%) had synchronous metastasis, and 175 (56.1%) had metachronous metastasis; 254 (81.4%) had multiple metastatic lesions, and 118 (37.8) had more than three bone metastatic sites. The most frequently involved bone metastatic sites were rib, thoracic vertebrae, lumbar vertebrae, pelvic girdle, and lower limb (Table 2).
Table 1.
Characteristics of study population
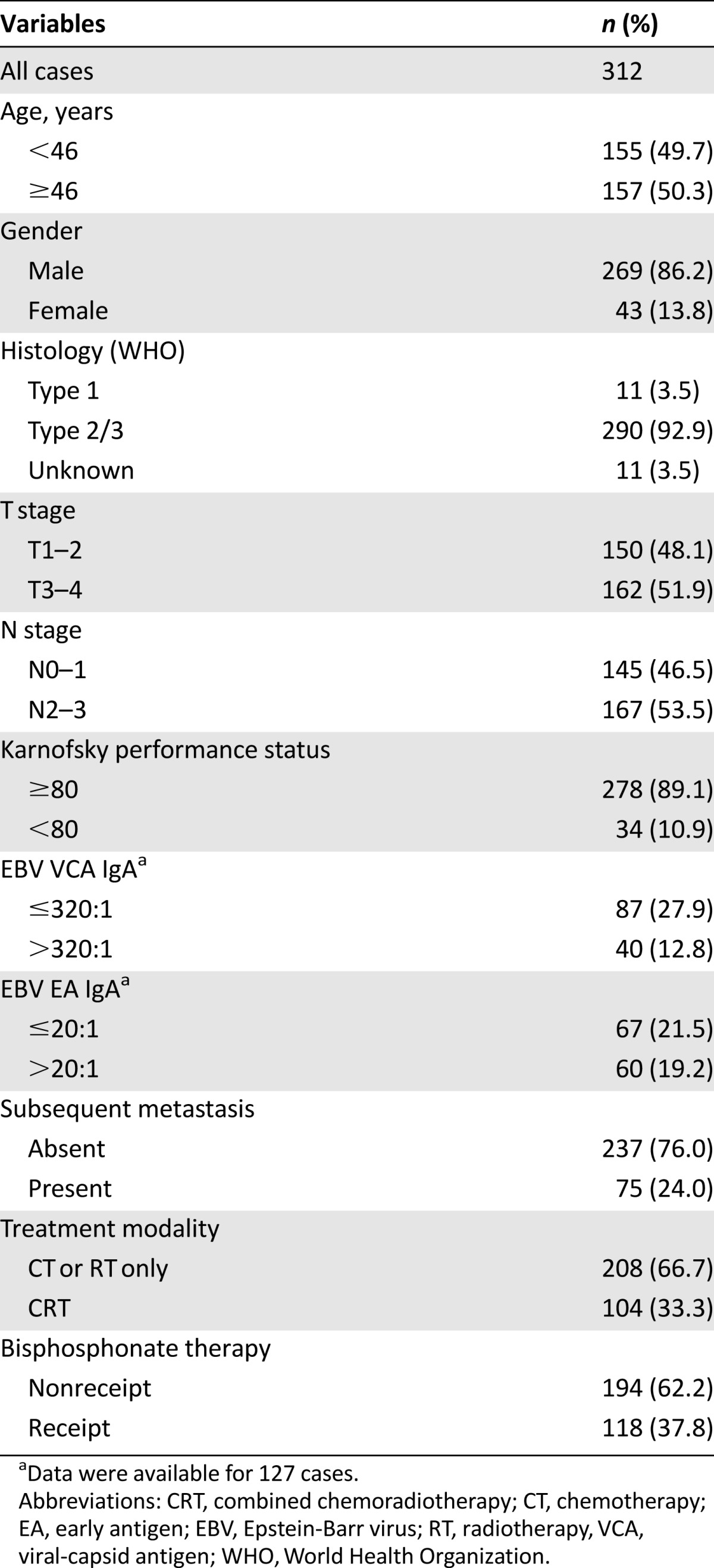
Table 2.
Onset, site, and number of metastases of the bone-only metastatic nasopharyngeal carcinoma patients
After diagnosis of metastasis, 188 patients (60.3%) received systemic chemotherapy only, 20 patients (6.4%) received local radiotherapy only, and 104 (33.3%) received combined CRT. A significantly higher proportion of patients with metachronous disease, lower age (<46 years), and single metastasis were found among those who received combined CRT compared with patients received CT or RT only (supplemental online Table 1). In terms of bisphosphonate treatment, 118 patients (37.8%) patients received treatment and 194 (62.2%) did not.
M1 Stage Subdividing and Survival
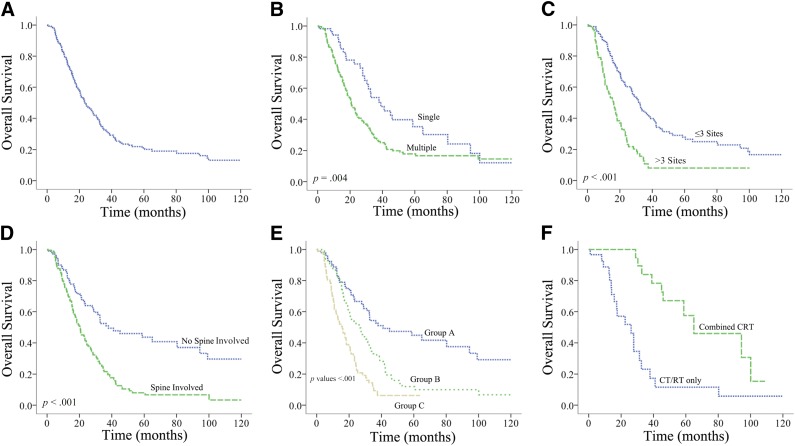
Overall, 184 deaths (59.0%) were recorded among the 312 patients included in this analysis. Median OS was 23.4 months (range: 1–120 months), and the 1-, 3-, and 5-year OS rates were 79.1%, 34.0%, and 22.1%, respectively, for the whole population (Fig. 2A).
Figure 2.
Kaplan-Meier plot showing overall survival for the entire cohort (A) and divided by number of metastases (multiple/single) (B), by number of metastatic sites (>3/≤3 sites) (C), by spine involvement (D), into three subsets according to independent prognostic factors (E), and by treatment modality in patients with single metastases (F).
Abbreviations: CRT, chemoradiotherapy; CT, chemotherapy; RT, radiotherapy.
In terms of metastatic site, univariate analysis showed that involvement of skull, upper limb, thoracic vertebrae, rib, lumbar vertebrae, sacrum, pelvic girdle, and lower limb were significantly associated with unfavorable OS among patients with bone metastasis (Table 3). Spine involvement, which refers to the involvement of any cervical vertebrae, thoracic vertebrae, lumbar vertebrae, or sacrum by metastases, was found in 206 patients (66.0%); those with metastases involving the spine had strikingly poorer OS (hazard ratio [HR]: 2.41; 95% confidence interval [CI]: 1.72–3.38) than with those without (Fig. 2D).
Table 3.
Univariate analysis of overall survival by clinical factors
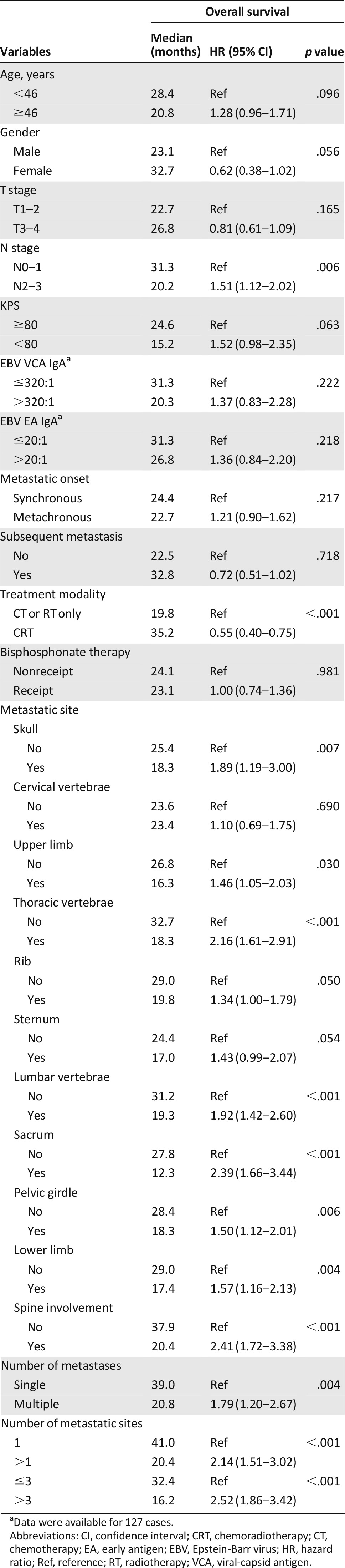
The numbers of metastases and metastatic sites were analyzed separately to identify the optimal grouping strategy. Univariate analysis showed that number of metastases (multiple vs. single), number of metastatic sites (more than one vs. one), and number of metastatic sites (more than three vs. three for fewer) were all significant in predicting OS. More than three or three or fewer metastatic sites achieved the highest hazard ratio in subdividing the patients by the number of metastases or metastatic sites (HR: 2.52; 95% CI: 1.86–3.42) (Fig. 2B, 2C). The other significant predictors for OS included N stage and treatment modality, whereas metastasis onset, subsequent metastasis, age, gender, T stage, KPS, Epstein-Barr virus (EBV) viral-capsid antigen-IgA and early antigen-IgA, and bisphosphonates treatment were not significant (Table 3).
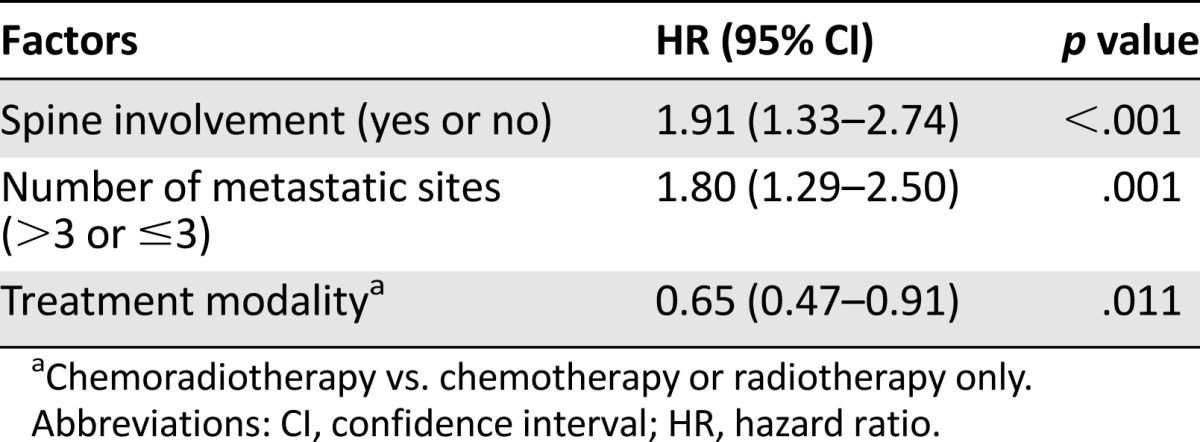
Multivariate analysis with the backward conditioning method was conducted using age, gender, N stage, KPS, spine involvement, skull involvement, number of metastases (multiple vs. single), number of metastatic sites (more than one vs. one), number of metastatic sites (more than three vs. three for fewer), metastatic onset, and treatment modality. The results showed that spine involvement, number of metastatic sites (more than three vs. three or fewer), and treatment modality were independent prognostic factors for patients with NPC and bone-only metastasis (Table 4). Based on the independent factors, we were able to subdivide the M1 stage of the 312 patients into three subdivisions: group A, three or fewer metastatic sites and no spine involvement; group B, three or fewer metastatic sites with spine involvement or more than three metastatic sites without spine involvement; group C, more than three metastatic sites with spine involvement. Overall, 98 patients (31.4%) were in group A, 104 patients (33.3%) were in group B, and 110 patients (35.3%) were in group C. The median OS was 41.0, 26.8, and 15.8 months, respectively; the 3-year OS rates were 54.9%, 34.7%, and 10.6%, respectively, and the 5-year OS rates were 45.3%, 12.7%, and 7.9%, respectively (Fig. 2E).
Table 4.
Independent prognostic factors from multivariate analysis for overall survival
M1 Stage Subdividing and Treatment Outcome
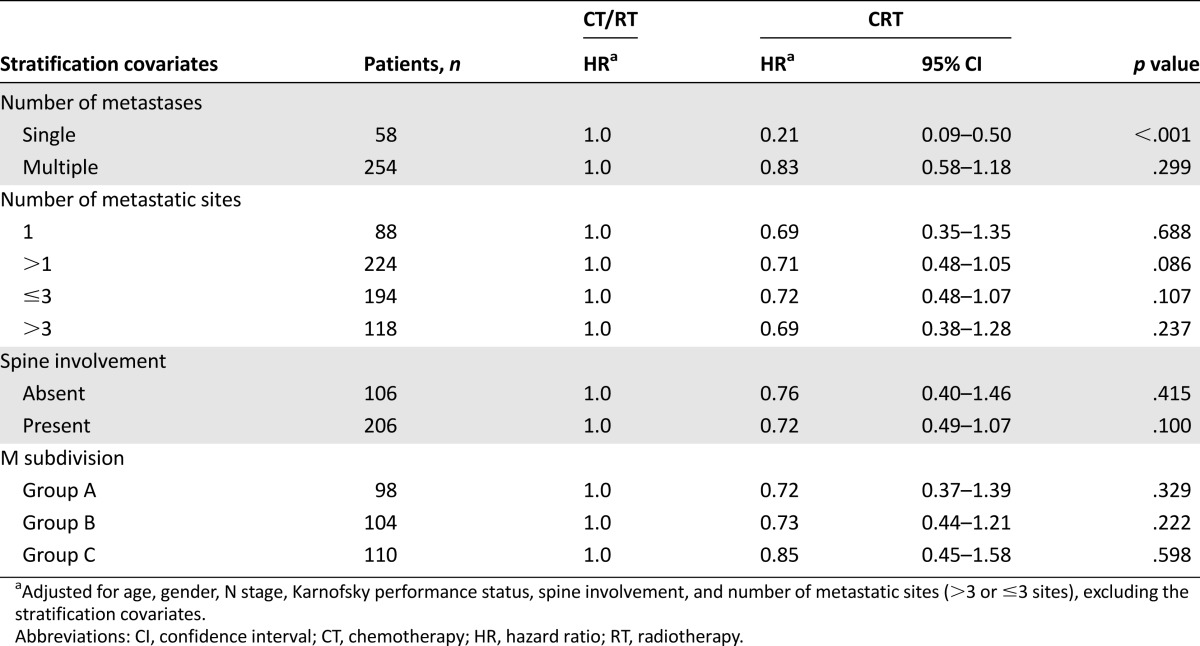
To investigate the impact of combined CRT among patients with bone-only metastatic NPC, further stratified analysis was conducted (Table 5). Of the 58 patients with solitary metastases, 30 (51.7%) received CT or RT only, and 28 (48.3%) received combined CRT. The 5-year OS rates for patients receiving CR or RT only and CRT were 11.2% and 57.3%, respectively (Fig. 2F). Multiple-adjusted analysis showed that combined CRT was associated with significantly favorable OS among patients with single bone metastasis (HR: 0.21; 95% CI: 0.09–0.50). For patients with multiple bone metastatic lesions, this impact was not significant (HR: 0.83; 95% CI: 0.58–1.18). No significance was reached for the impact of CRT among patients with different M1 subdivisions (Table 5).
Table 5.
Adjusted hazard ratios for overall survival by treatment modality stratified by covariates
Discussion
To our knowledge, this study is the first to document the subdivision of M1 stage and patient outcome in NPC with bone-only metastasis. Our study provided several notable findings. First, among 312 NPC with bone-only metastasis, the most frequently involved bone metastatic sites were rib (53.5%), thoracic vertebrae (50.0%), lumbar vertebrae (43.6%), pelvic girdle (42.3%), and lower limb (32.4%). Second, spine involvement, more than three or three or fewer metastatic sites, and treatment modality were independent prognostic factors for OS of NPC patient with bone-only metastasis. Third, combined CRT may benefit the patients with single bone metastasis.
The current study reflects the clinical course of bone-only metastasis in a large cohort of patients with NPC treated in the contemporary era. Overall survival after metastasis ranged from 1 to 120 months, indicating that long-term survival is possible for some bone metastatic patients. The median OS after metastasis was 23.4 months, which is slightly longer than the estimated 12 months reported by Hui et al. [23] and 17 months reported by Gao et al. [24]. These differences may be explained by the inclusion criteria of bone-only metastasis patients and the better performance status of the patients in our study.
Spine involvement has been reported to be associated with unfavorable prognosis in several solid tumors [25, 26]. Patients with spine metastasis were at risk for pathological fracture of spine and spinal cord compromise, which can lead to irreversible paralysis and poor prognosis [25, 27]. A recently published systematic review including 19 spine metastatic patients with squamous cell carcinoma as primary head and neck cancer showed that the mortality rate was 89.5% after follow-up of 13 months, and the mean time from treatment of spinal metastasis to mortality was only 3.4 months. In our study, multivariate analysis also showed that spine involvement was a strong predictor for unfavorable OS (HR: 1.91; 95% CI: 1.33–2.74) among patients with NPC and bone-only metastasis. These results suggested that it may be necessary for NPC patients with known vertebral metastases to receive frequent clinical evaluation to detect the risk for spinal cord compromise early and prevent oncological emergencies.
Patients with multiple bone metastases were associated with poor prognosis in various kinds of malignancies [13, 26, 28–30]; however, no previous study had specifically analyzed the prognostic value of the number of metastatic sites among patients with bone-only metastasis. It is intriguing that in our study, univariate analysis showed that both the number of metastases (multiple vs. single) and the number of metastatic sites (more than three vs. three or fewer) were associated with OS of patients with bone-only metastatic NPC; multivariate analysis including all significant covariates showed that the number of metastatic sites (more than three vs. three or fewer) but not the number of metastases (multiple vs. single) was an independent predictor for OS. These findings indicated that more than three or three or fewer metastatic sites was a better prognostic factor in evaluating the prognosis of patients with bone-only metastatic NPC.
The natural history and management of metastatic NPC has long been an area of controversy. Distant metastasis in patients with NPC has been conventionally regarded as incurable, and the aim of treatment has largely been palliative [27]. In contrast, the experience from our center and from the investigators in the French series [19] suggested that a small proportion of patients with metastatic NPC who were treated with aggressive therapy achieved long-term disease-free survival. Consequently, how therapies can be combined to achieve additive or synergistic effects and who will benefit from the combined treatment are the issues that need to be addressed. In stratified analysis, adjusted for age, gender, N stage, KPS, spine involvement, and more than three or three or fewer metastatic sites, we found that combined CRT may greatly improve OS among patients with NPC and single bone metastasis compared with CT or RT only (HR: 0.21; 95% CI: 0.09–0.50; 5-year OS: 57.3% vs. 11.2%). Because most patients with NPC are sensitive to radiotherapy [31–33], this preliminary finding suggested that a considerable proportion of patients with single bone metastasis receiving combined CRT could be cured. Future match-paired or even prospective studies are warranted to validate this finding.
This study showed no significant impact of bisphosphonate treatment on the survival of patients with bone-only metastatic NPC. Our results conflict with a recently published study by Jin et al. [34], who retrospectively analyzed the data of 307 patients with bone metastases at primary diagnosis or with recurrent bone disease attended in Sun Yat-sen University Cancer Center from 1999 to 2009 and concluded that bisphosphonates treatment combined with chemotherapy could improve OS for patients with NPC and bone metastases compared with patients receiving chemotherapy alone (HR: 0.548; 95% CI: 0.418–0.719). However, the population included in that study were not patients with bone-only metastatic NPC, and the confounding effects of the metastatic involvement of the other organs and radiotherapy for metastatic lesions were not fully considered. A multicenter randomized controlled trial evaluating the efficacy of bisphosphonate treatment among patients with NPC and bone-only metastasis at initial diagnosis may be needed in the future.
By subdividing the M1 stage of patients with bone-only metastatic NPC using independent factors, we were able to evenly divide the whole population into three subsets: group A, three or fewer metastatic sites and no spine involvement; group B, three or fewer metastatic sites and spine involvement or more than three metastatic sites and no spine involvement; and group C, more than three metastatic sites and spine involvement. The median OS was 41.0, 26.8, and 15.8 months in groups A, B, and C, respectively. Although patients in group A had M1 disease, our results showed that they were expected to live significantly longer than patients in groups B and C; therefore, more aggressive treatment (e.g., combined systemic agents and local therapies) was recommended. For patients in group C, as suggested by the American College of Radiology therapeutic guidelines for bone metastasis, management should aim to improve end-stage quality of life and maintain neurological function [35]. Ineffective, futile therapies that incur morbidity and cost and that provide little to no palliative benefit should not be administered [27]. The actual plan in dealing with every patient still needs multidisciplinary consideration.
Our study has several limitations. First, it is retrospective. Second, the cohort was obtained from a specific regional population and may be not representative of the general population of patients with bone-only metastatic NPC. Third, the modes of chemotherapy and radiotherapy applied varied, which might have had a confounding effect. Finally, only a small proportion of the patients (61 patients, 16.9%) had data of plasma EBV at the DNA level at diagnosis of metastasis; therefore, they were not included in our analysis. For these reasons, we need to validate our findings in a multi-institutional prospective study in the future.
Conclusion
Subdividing the M1 stage of NPC patients with bone-only metastasis into three subsets according to the number of bone metastatic sites and whether spine is involved may better predict prognosis of patients and facilitate treatment planning. Combined CRT should be considered for patients with single bone metastasis.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgment
This work was supported by grants from the National High Technology Research and Development Program of China (863Program), China (No. 2012AA022701).
Author Contributions
Conception/Design: Lujun Shen, Peihong Wu
Provision of study material or patients: Yunfei Xia, Peihong Wu
Collection and/or assembly of data: Lujun Shen, Jun Dong, Sheng Li, Yue Wang, Changchuan Pan
Data analysis and interpretation: Lujun Shen, Jun Dong, Wanhong Shu, Ming Wu
Manuscript writing: Lujun Shen, Jun Dong, Sheng Li, Yue Wang, Annan Dong, Wanhong Shu, Ming Wu, Changchuan Pan, Yunfei Xia, Peihong Wu
Final approval of manuscript: Lujun Shen, Jun Dong, Sheng Li, Yue Wang, Annan Dong, Wanhong Shu, Ming Wu, Changchuan Pan, Yunfei Xia, Peihong Wu
Disclosures
The authors indicated no financial relationships.
References
- 1.Greene FL, Sobin LH. The staging of cancer: A retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180–190. doi: 10.3322/CA.2008.0001. [DOI] [PubMed] [Google Scholar]
- 2.Gospodarowicz MK, Miller D, Groome PA, et al. The process for continuous improvement of the TNM classification. Cancer. 2004;100:1–5. doi: 10.1002/cncr.11898. [DOI] [PubMed] [Google Scholar]
- 3.Ng WT, Yuen KT, Au KH, et al. Staging of nasopharyngeal carcinoma - the past, the present and the future. Oral Oncol. 2014;50:549–554. doi: 10.1016/j.oraloncology.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.OuYang PY, Su Z, Ma XH, et al. Comparison of TNM staging systems for nasopharyngeal carcinoma, and proposal of a new staging system. Br J Cancer. 2013;109:2987–2997. doi: 10.1038/bjc.2013.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Q, Shen C, Kong L, et al. Involvement of both cervical lymph nodes and retropharyngeal lymph nodes has prognostic value for N1 patients with nasopharyngeal carcinoma. Radiat Oncol. 2014;9:7. doi: 10.1186/1748-717X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, Xu JM, Gong JS, et al. Relationship between dynamic contrast-enhanced and perfusion magnetic resonance imaging and T-staging of nasopharyngeal carcinoma. Zhonghua Yi Xue Za Zhi. 2013;93:1153–1155. [in Chinese] [PubMed] [Google Scholar]
- 7.Pan CC, Lu J, Yu JR, et al. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: A study of 1027 cases and review of the literature. Exp Ther Med. 2012;4:334–338. doi: 10.3892/etm.2012.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan C, He N, Zhao M, et al. Subdividing the M1 stage of liver metastasis for nasopharyngeal carcinoma to better predict metastatic survival. Med Oncol. 2011;28:1349–1355. doi: 10.1007/s12032-010-9643-8. [DOI] [PubMed] [Google Scholar]
- 9.Ong YK, Heng DM, Chung B, et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–1541. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 10.Bensouda Y, Kaikani W, Ahbeddou N, et al. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:79–85. doi: 10.1016/j.anorl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Gacani W, Bal IS, Babu MA, et al. Distant metastases from nasopharyngeal carcinoma at Kenyatta National Hospital, Nairobi. East Afr Med J. 2001;78:678–681. doi: 10.4314/eamj.v78i12.8940. [DOI] [PubMed] [Google Scholar]
- 12.Leung SF, Teo PM, Shiu WW, et al. Clinical features and management of distant metastases of nasopharyngeal carcinoma. J Otolaryngol. 1991;20:27–29. [PubMed] [Google Scholar]
- 13.Bhandari V, Jain RK. A retrospective study of incidence of bone metastasis in head and neck cancer. J Cancer Res Ther. 2013;9:90–93. doi: 10.4103/0973-1482.110385. [DOI] [PubMed] [Google Scholar]
- 14.Lim A, Corry J, Lau E, et al. Prolonged remission in a patient with nasopharyngeal carcinoma with a solitary bone metastasis. J Clin Oncol. 2011;29:e135–e137. doi: 10.1200/JCO.2010.31.9053. [DOI] [PubMed] [Google Scholar]
- 15.Farnia B, Louis CU, Teh BS, et al. Stereotactic body radiation therapy (SBRT) for an isolated bone metastasis in an adolescent male with nasopharyngeal carcinoma. Pediatr Blood Cancer. 2014;61:1520. doi: 10.1002/pbc.24963. [DOI] [PubMed] [Google Scholar]
- 16.Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: A single-institution retrospective analysis. The Oncologist. 2011;16:155–164. doi: 10.1634/theoncologist.2010-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Zeng ZC, Fan J, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma after liver transplantation. BMC Cancer. 2011;11:492. doi: 10.1186/1471-2407-11-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rief H, Muley T, Bruckner T, et al. Survival and prognostic factors in non-small cell lung cancer patients with spinal bone metastases: A retrospective analysis of 303 patients. Strahlenther Onkol. 2014;190:59–63. doi: 10.1007/s00066-013-0431-1. [DOI] [PubMed] [Google Scholar]
- 19.Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18:1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 20. Sobin LH, ed. World Health Organization. International Histological Classification of Tumours. 2nd ed. Berlin, Germany: Springer-Verlag, 2002. [Google Scholar]
- 21. Sobin L, Gospodarowicz M, Wittekind C, eds. TNM Classification of Malignant Tumors. 7th ed. Hoboken, NJ: John Wiley & Sons, 2009. [Google Scholar]
- 22.Niikura N, Liu J, Hayashi N, et al. Retrospective analysis of antitumor effects of zoledronic acid in breast cancer patients with bone-only metastases. Cancer. 2012;118:2039–2047. doi: 10.1002/cncr.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: A relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 24.Cao X, Han Y, He L, et al. Risk subset of the survival for nasopharyngeal carcinoma patients with bone metastases: Who will benefit from combined treatment? Oral Oncol. 2011;47:747–752. doi: 10.1016/j.oraloncology.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Katsenos S, Nikolopoulou M. Intramedullary thoracic spinal metastasis from small-cell lung cancer. Monaldi Arch Chest Dis. 2013;79:140–142. doi: 10.4081/monaldi.2013.5214. [DOI] [PubMed] [Google Scholar]
- 26.Knudson G, Grinis G, Lopez-Majano V, et al. Bone scan as a stratification variable in advanced prostate cancer. Cancer. 1991;68:316–320. doi: 10.1002/1097-0142(19910715)68:2<316::aid-cncr2820680218>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Janjan N, Lutz ST, Bedwinek JM, et al. Therapeutic guidelines for the treatment of bone metastasis: A report from the American College of Radiology appropriateness criteria expert panel on radiation oncology. J Palliat Med. 2009;12:417–426. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 28.Uccella S, Morris JM, Bakkum-Gamez JN, et al. Bone metastases in endometrial cancer: Report on 19 patients and review of the medical literature. Gynecol Oncol. 2013;130:474–482. doi: 10.1016/j.ygyno.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon A, Choi CH, Kim TH, et al. Bone metastasis in primary endometrial carcinoma: Features, outcomes, and predictors. Int J Gynecol Cancer. 2014;24:107–112. doi: 10.1097/IGC.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita K, Denno K, Ueda T, et al. Prognostic significance of bone metastases in patients with metastatic prostate cancer. Cancer. 1993;71:1297–1302. doi: 10.1002/1097-0142(19930215)71:4<1297::aid-cncr2820710421>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4:182–198. [PubMed] [Google Scholar]
- 32.Feng XP, Yi H, Li MY, et al. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res. 2010;70:3450–3462. doi: 10.1158/0008-5472.CAN-09-4099. [DOI] [PubMed] [Google Scholar]
- 33.Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: Review of current treatment options. CA Cancer J Clin. 2008;58:245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, An X, Cai YC, et al. Zoledronic acid combined with chemotherapy bring survival benefits to patients with bone metastases from nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2011;137:1545–1551. doi: 10.1007/s00432-011-1027-8. [DOI] [PubMed] [Google Scholar]
- 35.Trilling GM, Cho H, Ugas MA, et al. Spinal metastasis in head and neck cancer. Head Neck Oncol. 2012;4:36. doi: 10.1186/1758-3284-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.