This meta-analysis was carried out to ascertain the role of smoking status in influencing progression-free survival (PFS) outcomes in patients with the two common activating epidermal growth factor receptor mutations (EGFRm) who received first-line EGFR tyrosine kinase inhibitors (TKIs) in comparison with platinum-doublet chemotherapy. EGFRm non-small-cell lung cancer patients derived significant PFS benefit from TKI over platinum-doublet chemotherapy regardless of smoking status; however, PFS benefit is significantly better in never smokers by metaregression analysis.
Keywords: Meta-analysis, EGFR TKIs, Smoking status, EGFR mutant non-small cell lung cancer, Gefitinib, Erlotinib, Afatinib
Abstract
Background.
Univariate analyses from several randomized phase III trials seemed to suggest ever-smokers with advanced mutated epidermal growth factor receptor (EGFRm) non-small cell lung cancer (NSCLC) did not seem to benefit from EGFR tyrosine kinase inhibitors (TKIs) as first-line treatment when compared with platinum-doublet chemotherapy as measured by progression-free survival (PFS).
Methods.
A literature-based meta-analysis of PFS outcomes as measured by log-transformed pooled hazard ratio (HR) was performed using a random-effect model. Pooled HRs for smoking status, age, gender, ethnicity, type of EGFR mutation, and EGFR TKI were obtained. Comparison of the pooled HR was performed by metaregression analysis.
Results.
Among the 1,649 EGFRm NSCLC patients analyzed from 7 prospective randomized trials (WJTOG3405, NEJ002, EURTAC, OPTIMAL, LUX Lung-3, LUX Lung-6, and ENSURE), 83.7% were Asians, and 30.0% were ever-smokers. An equal percentage of ever-smokers received doublet chemotherapy (30.2%) or EGFR TKI (30.0%). The pooled HR for PFS was 0.29 (95% confidence interval [CI]: 0.21–0.39) for never-smokers and 0.54 (95% CI: 0.38–0.76) for ever-smokers (p < .007 by metaregression). The pooled PFS HR for exon 19 deletion was 0.25 (95% CI: 0.19–0.31) and 0.44 for exon 21 substitution (95% CI: 0.34–0.57) (p < .001 by metaregression analysis). The pooled PFS HR was 0.33 (95% CI: 0.24–0.46) for Asians and 0.48 for non-Asians (95% CI: 0.28–0.84) (p = .261 by metaregression analysis).
Conclusion.
EGFRm NSCLC patients derived significant PFS benefit from TKI over platinum-doublet chemotherapy as first-line treatment regardless of smoking status; however, PFS benefit is significantly better in never-smokers by metaregression analysis.
Abstract
摘要
背景。来自几项 III 期随机试验的单变量分析似乎提示:通过无进展生存期 (PFS) 进行测量,与铂类双药化疗相比时,患有表皮生长因子受体突变 (EGFRm) 晚期非小细胞肺癌 (NSCLC) 的过去吸烟者似乎未从一线EGFR 酪氨酸激酶抑制剂 (TKI) 治疗中获益。
方法。使用随机效应模型,基于文献对通过对数转换汇总风险比 (HR) 所测量的 PFS 转归进行meta分析。获得了吸烟状态、年龄、性别、种族、EGFR 突变类型和 EGFR TKI 的汇总 HR。通过meta回归分析比较汇总的 HR。
结果。从 7 项前瞻性随机试验(WJTOG3405、NEJ002、EURTAC、OPTIMAL、LUX Lung-3、LUX Lung-6 和 ENSURE)分析的 1 649 名 EGFRm NSCLC 患者当中,83.7% 患者是亚裔,并且 30.0% 患者是过去吸烟者。相等百分数的过去吸烟者接受双药化疗 (30.2%) 或 EGFR TKI (30.0%)。对于从不吸烟者,PFS 的汇总 HR 是 0.29 [95% 置信区间( CI):0.21–0.39],而对于过去吸烟者,是 0.54(95% CI:0.38-0.76)(通过meta回归分析,p < 0.007)。对于外显子 19 缺失,PFS 的汇总 HR 是 0.25(95% CI:0.19-0.31),而对于外显子 21 置换,是 0.44(95% CI:0.34-0.57)(通过meta回归分析,p < 0.001)。对于亚裔,PFS 的汇总 HR 是 0.33(95% CI:0.24-0.46),而对于非亚裔是 0.48 (95% CI:0.28-0.84)(通过meta回归分析,p = 0.261)。
结论。EGFRm NSCLC 患者无论吸烟状态如何,从一线 TKI 治疗的 PFS 获益优于铂类双药化疗;然而,meta 回归分析显示,PFS 获益在从不吸烟者中明显更好。The Oncologist 2015; 20:307–315
Implications for Practice:
The purpose of this meta-analysis was to ascertain the role of smoking status in influencing progression-free survival (PFS) outcomes in patients harboring the two common activating epidermal growth factor receptor mutations (EGFRm) who received first-line EGFR tyrosine kinase inhibitors (TKIs) in comparison with platinum-doublet chemotherapy. We found that EGFRm patients benefited from first-line EGFR TKIs regardless of smoking status, but EGFRm patients who were never-smokers benefited significantly more from EGFR TKIs than EGFRm patients with a history of smoking. Thus oncologists should be cognizant that the duration of PFS benefit from EGFR TKIs is likely to be shorter among ever-smokers with EGFRm, and close surveillance and alternative therapy should be formulated sooner.
Introduction
Based on results from seven prospective phase III randomized trials comparing first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) to platinum-doublet chemotherapy as first-line treatment of non-small cell lung cancer (NSCLC) patients harboring activating EGFR mutations (EGFRm), it is now well-established that EGFR TKI offers superior improvement in progression-free survival (PFS) [1–7]. Exploratory univariate analyses of three of the seven clinical trials (WJTOG3405, EURTAC, and LUX-Lung-3 [LL3]) suggested that EGFRm NSCLC patients who had a previous smoking history (former or current smoker) did not seem to derive a statistical PFS improvement when EGFR TKI was compared with platinum-doublet chemotherapy. In WJTOG3405, the hazard ratio (HR) for PFS among ever-smokers was 0.58 (95% confidence interval [CI]: 0.29–1.12) [1]. In EURTAC, the HR for PFS for current smokers was 0.56 (95% CI: 0.15–2.15), and that for former smokers was 1.05 (95% CI: 0.40–2.74) [4]. In LL3, the HR for PFS for current/ex-smokers was 1.04 (95% CI: 0.54–1.98), and that for recent light former smokers was 0.50 (95% CI: 0.19–1.34) (stopped >1 year ago and <15 pack years) [5]. On the other hand, exploratory univariate analyses in two of the six trials (OPTIMAL and LUX-Lung-6 [LL6]) did show statistical significant PFS benefit among former/current smoker from first-line EGFR TKIs. The HR for PFS among former/current smokers in OPTIMAL was 0.21 (95% CI: 0.09–0.49) [3]. The HR for PFS among current or ex smokers in LL6 was 0.46 (95% CI: 0.22–1.00) [6]. Two remaining trials (NEJ002 and ENSURE) have not reported univariate analysis by smoking status [2, 7]. Given that up to one-third of EGFRm patients had a previous smoking history [8], we performed a meta-analysis to analyze the role of smoking status and other potential predictive factors that may influence clinical outcome in EGFRm patients receiving first-line EGFR TKIs. In particular, we incorporated previously unpublished results of the univariate analysis of the NEJ002 trial outcome into this current meta-analysis.
Materials and Methods
Study Eligibility and Identification
All prospective randomized phase III trials enrolling EGFRm NSCLC patients comparing EGFR TKI and platinum doublet chemotherapy (chemotherapy) as first-line treatment for advanced NSCLC were eligible for inclusion. Trials were identified from the MEDLINE database using PubMed using the combination of the following terms (without the quotation marks): “non-small cell lung cancer,” “epidermal growth factor,” and “randomized controlled trial.” Abstracts from conference proceedings of the American Society of Clinical Oncology, the European Society for Medical Oncology, and the World Conference of Lung Cancer were reviewed to identify unpublished studies. All searches were limited to human studies and the English language.
Data Extraction
Information recorded from each trial including study name, year of publication or conference presentation, demographic area (age, gender, region of enrollment), methods of determining EGFR mutations, smoking status, type of platinum-doublet chemotherapy, and specific EGFR TKI were abstracted. All studies were retrieved independently by two investigators (Y.H. and S.Y.) to assess the reliability of data extraction. After selection of potential studies, the investigators reviewed each other’s selected studies and excluded inappropriate studies with the agreement of both. Disagreements were adjudicated by a third reviewer after referring to the original articles.
We extracted log-transformed HRs and corresponding 95% CI for PFS using a random-effect model to assess efficacy within several subgroups: smoking status (never-smokers versus ever-smokers [former and current smokers if the distinction is made in the trial]), age (<65 versus ≥65 years), gender (male versus female), EGFR mutation type (exon 19 deletion versus L858R substitution), ethnicity (Asians versus non-Asians), and EGFR TKI (gefitnib, erlotinib, and afatinib). Comparison of the pooled HRs was performed by metaregression analysis. HRs for former and current smokers were pooled as one HR for ever-smokers. A p < .05 was considered statistically significant, and all reported p values were two-sided. The I2 statistics were used to assess heterogeneity across studies, and I2 <25, 25 ≤ I2 < 50, and 50 ≤ I2 were interpreted as signifying low-level, intermediate-level, and high-level heterogeneity, respectively. The Egger’s test and Begg’s funnel plots were calculated using Comprehensive Meta-Analysis version 2 (Biostat Inc., Englewood, NJ, http://biostat.com). All other statistical analyses were performed with SPSS version 21 (SPSS, Chicago, IL, http://www-01.ibm.com/software/analytics/spss/) or SAS version 9.4 (SAS Inc., Cary, NC, http://www.sas.com).
Results
Clinical Trials
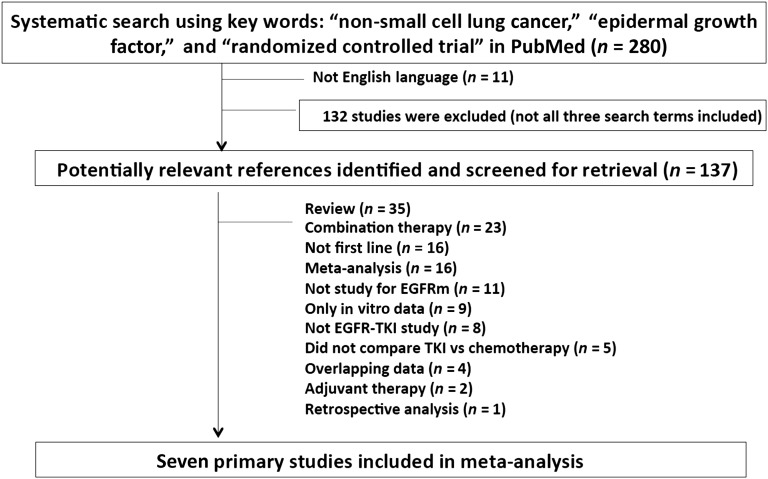
A total of 280 articles were identified, of which 132 articles were excluded primarily because only two of the three search criteria were present in the articles despite using the three combined search criteria (Fig. 1). We eventually identified seven (six published and one presented) (WJTOG3405, NEJ002, EURTAC, OPTIMAL, LL3, LL6, and ENSURE) eligible trials (Fig. 1). PFS was the primary endpoint for all seven trials, and assessment scans were performed every 6 weeks for 5 trials (EURTAC, OPTIMAL, LL3, LL6, and ENSURE) and 8 weeks for 2 trials (WJTOG3405 and NEJ002). The eligibility criteria were similar among all 7 trials with 3 trials (OPTIMAL, EURTAC, and ENSURE) allowing performance status up to 2. Gefitinib, erlotinib, and afatinib were investigated in two, three and two trials, respectively. The chemotherapy regimens investigated were platinum (carboplatin/cisplatin)-based with paclitaxel, docetaxel, gemcitabine, and pemetrexed. Five trials randomized patients 1:1 to EGFR TKIs and two trials (LL3 and LL6) randomized patients 2:1 to EGFR TKIs to chemotherapy. Five trials stratified the randomization by the type of EGFR mutations (OPTIMAL, EURTAC, ENSURE, LL3, and LL6), but only one trial stratified the randomization by smoking status (OPTIMAL). Three trials allowed (NEJ002, LL3, and LL6) enrollment of EGFRm patients with uncommon mutations in addition to the two common types of EGFR mutations (exon 19 deletion and L858R substitution). Details and primary results of all seven trials are summarized in Table 1.
Figure 1.
Trial selection process.
Abbreviations: EGFR, epidermal growth factor receptor; EGFRm, mutated EGFR; TKI, tyrosine kinase inhibitor.
Table 1.
List of the characteristics of the seven randomized trials

Patient Characteristics and Common EGFRm Types
Among the total of 1,649 EGFRm patients analyzed from the 7 prospective randomized phase III trials, 65.1% were female, 84.8% had stage 4 disease, 96.1% had adenocarcinoma histology, and 52.9% had exon 19 deletion (Table 2). Of the total 1,649 patients, 950 (57.6%) were randomized to EGFR TKIs, and 699 (42.4%) were randomized to platinum-doublet chemotherapy.
Table 2.
Clinicopathologic characteristics of the patients (total, EGFR TKI, and doublet chemotherapy) analyzed by the meta-analysis

Approximately 70.0% of the EGFRm patients were never-smokers. All the EGFRm patients were randomized in a similar proportion to EGFR TKIs (70.0% never-smokers) and chemotherapy (69.8% never-smokers) by smoking status (Table 2). Additionally, among never-smokers, 57.7% of them were randomized to EGFR TKI essentially equal to the 57.3% of ever-smokers, who were also randomized to EGFR TKI.
The vast majority of the patients enrolled in the 7 randomized trials were Asians (83.7%), and they were randomized to a similar proportion to EGFR TKIs (84.2%) and chemotherapy (83.0%) (Table 2). Among the common EGFRm mutations (exon 19 deletion and L858R substitution), 56.1% were exon 19 deletion, and 43.9% were L858R substitution. Among Asian EGFRm patients, 54.7% had exon 19 deletion, and 45.3% had L858R substitution. Among non-Asian EGFRm patients, 63.0% had exon 19 deletion, and 37.0% had L858R substitution. Among EGFRm patients with exon 19 deletions, 57.4% were randomized to EGFR TKI, and among EGFRm patients with L858R substitution, 56.6% were randomized to EGFR TKI. Among EGFRm patients with common EGFR mutation randomized to EGFR TKI, 56.4% had exon 19 deletion. In a similar proportion, among EGFRm patients with common EGFR mutations randomized to platinum-doublet chemotherapy, 56.6% had exon 19 deletion.
Among the patients randomized to EGFR TKI, 49.7% of the patients were randomized to receive afatinib, 29.3% were randomized to receive erlotinib, and 21.1% were randomized to receive gefitinib. Among the patients randomized to receive platinum-doublet chemotherapy, 37.8% were randomized to receive cisplatin/gemcitabine, 16.5% were randomized to receive cisplatin/pemetrexed, 15.8% were randomized to receive carboplatin/paclitaxel, 14.3% were randomized to receive carboplatin/gemcitabine, 13.2% were randomized to receive cisplatin/docetaxel, and 2.4% were randomized to receive carboplatin/docetaxel.
All seven randomized trials demonstrated significant PFS improvement of EGFR TKIs over platinum-doublet chemotherapy. The median PFS in patients who received EGFR TKI ranged from 9.2 to 13.1 months, whereas the range of median PFS in patients who received platinum-doublet chemotherapy was 4.6 to 6.9 months (Table 1).
PFS Benefits of EGFR TKIs by Smoking Status
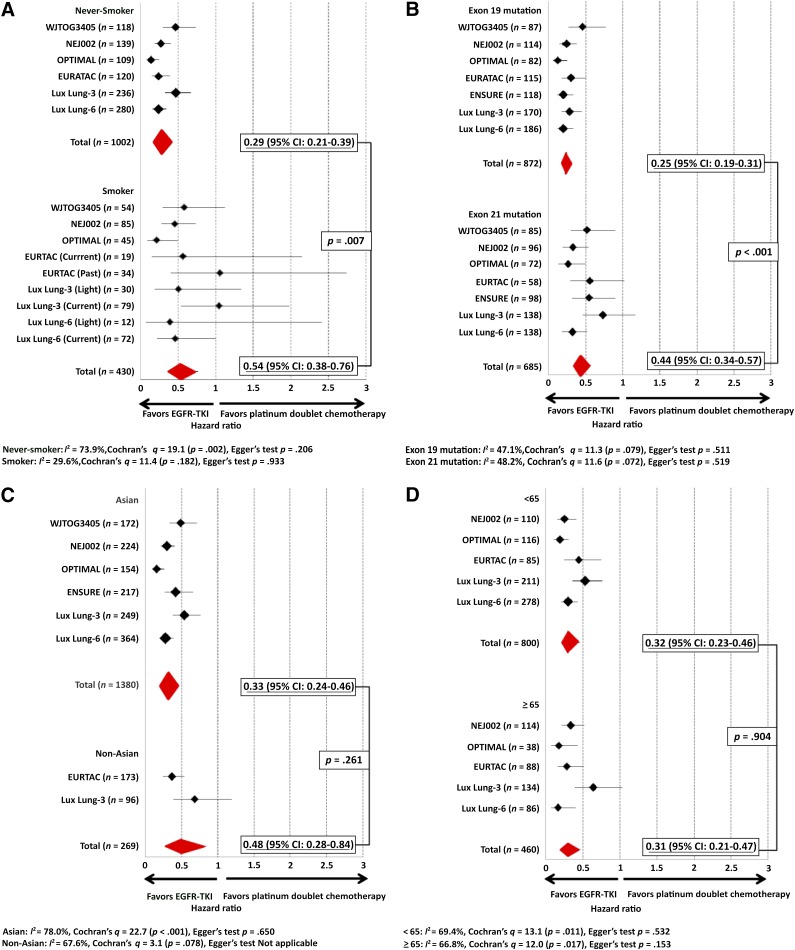
The PFS HRs by smoking status for NEJ002 [2, 9–10] and ENSURE have not been previously presented or published, but we were able to obtain the individual NEJ002 patient data (smoking status, gender, type of EGFR mutation, age) from the North East Japan study group but not the data from ENSURE. Hence, the meta-analysis on smoking status was based on 86.8% of the total population (excluding the ENSURE patient population). The PFS HR for never-smokers in the NEJ002 trial was 0.27 (95% CI: 0.18–0.41), whereas the PFS HR for ever-smokers in NEJ002 was 0.46 (95% CI: 0.28–0.74). Therefore, the meta-analysis was based on 86.8% of the total patient population. The pooled PFS HR for never-smokers was 0.29 (95% CI: 0.21–0.39), whereas the pooled PFS HR for ever-smokers was 0.54 (95% CI: 0.38–0.76). Metaregression analysis of the HRs was significant, with a p value of .007 (Fig. 2A).
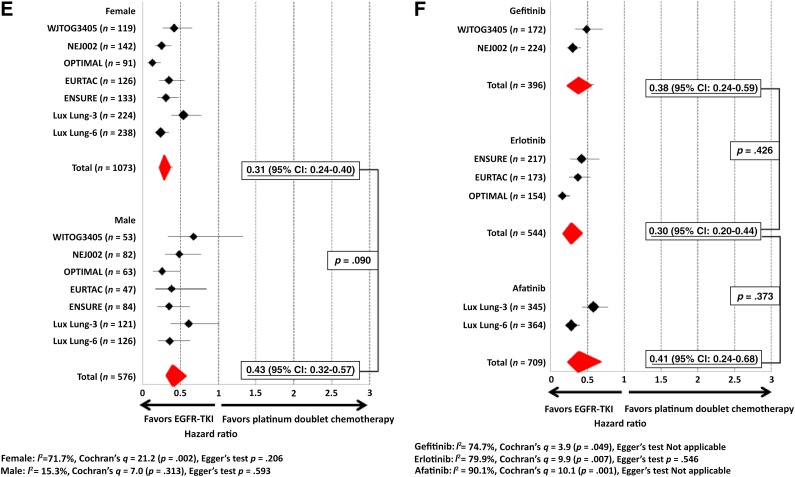
Figure 2.
Pooled hazard ratios (HRs) and metaregression analysis of pooled HRs of EGFR TKI compared with platinum-doublet chemotherapy. (A): HRs and metaregression analysis according to smoking status. (B): HRs and metaregression analysis according to two common types of EGFR mutation. (C): HRs and metaregression analysis according to ethnicity. (D): HRs and metaregression analysis according to age. (E): HRs and metaregression analysis according to gender. (F): HRs and metaregression analysis according to type of EGFR TKI.
Abbreviations: CI, confidence interval; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
PFS Benefits of EGFR TKIs by the Two Common EGFR Mutations
The PFS HR for patients with exon 19 deletion in the NEJ002 trial was 0.24 (95% CI: 0.15–0.38), whereas the PFS HR for patients with L858R in NEJ002 was 0.32 (95% CI: 0.20–0.53). The pooled PFS HR for EGFR exon 19 deletion was 0.25 (95% CI: 0.19–0.31), whereas the pooled PFS HR for L858R substitution was 0.44 (95% CI: 0.34–0.57). Metaregression analysis of the HRs was significant, with a p value of <.001 (Fig. 2B).
PFS Benefits of EGFR TKIs by Ethnicity
The pooled PFS HR for Asians was 0.33 (95% CI: 0.24–0.46), whereas the pooled PFS HR for non-Asians was 0.48 (95% CI: 0.28–0.84). Metaregression analysis of the HRs was not significant (p = .261) (Fig. 2C).
PFS Benefits of EGFR TKIs by Age
Although the breakdown by patients’ age was presented in ENSURE, the PFS HR by age for ENSURE has not been presented. The PFS HR for patients less than 65 years old in the NEJ002 trial was 0.25 (95% CI: 0.15–0.41), whereas the PFS HR for patients aged 65 and older in NEJ002 was 0.34 (95% CI: 0.22–0.52). The pooled PFS HR for patients less than 65 years old was 0.32 (95% CI: 0.23–0.46), whereas the pooled PFS HR for patients aged 65 and older was 0.31 (95% CI: 0.21–0.47). Metaregression analysis of the HRs was not significant (p = .904) (Fig. 2D).
PFS Benefits of EGFR TKIs by Gender
The PFS HR for female patients in the NEJ002 trial was 0.25 (95% CI: 0.17–0.38), whereas the PFS HR for male patients in NEJ002 was 0.48 (95% CI: 0.30–0.77). The pooled PFS HR for female patients was 0.31 (95% CI: 0.23–0.40), whereas the pooled PFS HR for male patients was 0.43 (95% CI: 0.32–0.57). Metaregression analysis of the HRs was not significant (p = .090) (Fig. 2E).
PFS Benefits of EGFR TKIs by EGFR TKI
The pooled PFS HR for gefitnib over platinum-doublet chemotherapy was 0.38 (95% CI: 0.24–0.59), the pooled PFS HR for erlotinib over chemotherapy was 0.30 (95% CI: 0.20–0.44), and the pooled PFS HR for afatinib was 0.41 (95% CI: 0.24–0.68). Metaregression analysis showed the p value between erlotinib and gefitinib to be 0.43, whereas the p value between erlotinib and afatinib was .37 (Fig. 2F).
Publication Bias
Potential publication bias was evaluated using the Egger’s test and Begg’s funnel plots with log-transformed hazards calculated from prevalence rate as the outcome and their standard errors as the index for accuracy. The funnel plots were symmetrical, and the Egger’s tests for all study were shown in Figure 2A–2F. These data indicate that there is little evidence of publication bias.
Discussion
In this meta-analysis, we have shown that patients with advanced EGFRm NSCLC benefited in terms of PFS from first-line EGFR TKI when compared with platinum-doublet chemotherapy regardless of smoking status, although there was a significant difference in the HRs for PFS benefit favoring patients without a smoking history. Although activating EGFR mutations are very common among NSCLC patients who were never-smokers, it is important to note that approximately 30% of the EGFRm patients in this meta-analysis had a history of tobacco use. Our results indicated that the efficacy of EGFR TKI may be less efficacious in EGFRm patients who had a smoking history. This is likely due to the difference in the genetic background of EGFR mutated NSCLC between never-smokers and ever-smokers. It has been demonstrated from comprehensive genomic profiling in adenocarcinoma between never-smokers and ever-smokers that the mutation burden (including point mutations) is at least 10-fold higher among adenocarcinoma patients who were ever-smokers [11, 12]. Furthermore, these point mutations in ever-smokers tend to occur in DNA mismatch repair genes, likely leading to secondary resistance to EGFR TKI or activation of bypass pathways [12]. Finally, the frequency of transversion increased with increasing tobacco smoke exposure. Transversion involves a purine to pyrimidine mutation or vice versa and is more likely to lead to structure changes in protein that harbors the transversion. Another potential mechanistic explanation to our observation of better PFS achieved with EGFR TKI in never-smokers compared with ever-smokers is that cigarette smokes have been shown in vitro to activate bypass signaling pathways that overcome the blockade of activated EGFRm by EGFR TKIs [13, 14]. Furthermore, active smoking has been shown to decrease the bioavailability of erlotinib by 50% [15, 16]. Thus active cigarette smoking during EGFR TKI treatment may directly and indirectly reduce the efficacy of EGFR TKI. Although we cannot rule out the less likely interpretation of the results of this meta-analysis is that platinum-doublet chemotherapy may be more efficacious in EGFRm patients with a history of smoking, the narrow range of median PFS from platinum-doublet chemotherapy indicated that the difference, if present, is very subtle.
Kim et al. [17] have also recently reported that smoking history is detrimental to NSCLC patients with EGFRm receiving EGFR TKIs. They showed that PFS was significantly shorter among EGFRm NSCLC patients receiving EGFR TKIs who were ever-smokers than never-smokers primarily from EGFRm patients with a ≥30-pack year smoking habit [18]. The disease control rate and overall response rate (ORR) to EGFR TKIs were also significantly lower among EGFRm patients with a ≥30-pack year smoking history [17]. The advantage of our meta-analysis was that all EGFRm patients were treated with first-line EGFR TKIs, whereas the patients in Kim et al. received EGFR TKIs as first to fourth lines of therapy. Additionally, our meta-analysis included previously unpublished predictive factor analysis from NEJ002. Furthermore, the patients in this meta-analysis were well balanced by gender, ethnicity, and type of EGFR mutation. Given that ORR was not the primary endpoint of any of the seven trials and not reported according to smoking status, we could not analyze any potential difference in ORR among EGFRm patients receiving EGFR TKIs by smoking status. We could also not analyze PFS outcome by the amount of tobacco smoke exposure because none of the seven trials systematically reported outcome according to exposure by pack years. We did not include IPASS [18] or First-SIGNAL [19] trials because both trials mainly enrolled never-smokers, the analysis of the EGFRm subgroup was retrospective, and a significant amount of patients had unknown EGFR mutation status. Although three of the seven trials did not show that the PFS HRs by smoking were positive, as shown in Figure 2A almost all the HRs by smoking status were in the left of the Forest plot (HR < 1), with only former smokers from EURTAC and current smokers from LUX-Lung 3 lying just to the right of the Forest plot. Thus our results are consistent with what has been observed in individual trials and indicate the importance of performing this meta-analysis.
Finally, this meta-analysis also demonstrates that EGFR TKI is significantly more effective in conferring PFS benefit against exon 19 deletion than against L858R substitution when compared with platinum-doublet chemotherapy. In vitro data have demonstrated that gefitinib and erlotinib both have a higher affinity for the exon 19 deletion than L858R mutation [20], resulting in inhibition of the kinase activity of mutated exon 19 deletion EGFR much faster and tighter with both EGFR TKIs [21]. As early as in 2006, clinical observations have reported that exon 19 deletion seems to derive longer PFS from EGFR TKI than L858R substitution [22, 23]. Indeed five of the seven randomized trials in this meta-analysis had already been stratified for the type of EGFR mutation, whereas only one trial was stratified for smoking status. Liang et al. [24] performed a similar metaregression analysis on the two common EGFR mutations and demonstrated that exon 19 deletion conferred significant longer PFS than L858R substitution when treated with EGFR TKIs. Recently a pooled analysis of LL3 and LL6 demonstrated significant overall survival benefit of afatinib over platinum-doublet chemotherapy among EGFRm patients with exon 19 deletions [25], providing further strengthening evidence that the two common activating EGFRm mutations should be treated differently. Similar proportions of EGFRm patients with exon 19 deletion and L858R mutation received EGFR TKI and platinum-doublet chemotherapy, respectively, in this meta-analysis. However, we could not analyze the role of smoking status in determining the PFS outcome by EGFR TKI according to the type of EGFRm because the breakdown of the types of EGFRm by smoking status was not presented in any of the seven randomized trials.
The incidence of NSCLC patients with EGFRm is highest among Asians [26] and could be as high as 62% in one molecular epidemiology study among newly diagnosed treatment-naïve advanced adenocarcinoma in seven Southeast Asian regions including mainland China [27]. More importantly, the percentage of EGFRm among heavy Asian smokers (>50 pack years) in the same study was as high as 31.4% [27]. Furthermore 20.7% of the EGFRm patients were active smokers [27]. It is unlikely that these EGFRm patients with >50 pack years of smoking had the same genetic background in their tumors as EGFRm patients who were never-smokers. Thus EGFRm NSCLC patients represent a diverse group of patients with both intrinsic different genetic and environmental exposure. While the presence of activating EGFR mutations defines a unique molecular subtype of lung cancer, EGFRm lung cancer is likely to be a fairly heterogeneous disease in terms of underlying genomic alterations. Next generation sequencing techniques such as targeted paralleling sequencing, whole exome sequencing, and whole genome sequencing will reveal much more genetic heterogeneity between never-smokers and ever-smokers, potentially allowing better fine-tuning of personalized therapy with EGFR TKIs.
Author Contributions
Conception/Design: Hideo Saka, Akihito Kubo, Tomoya Kawaguchi, Minoru Takada, Takayasu Kurata, Sai-Hong Ignatius Ou
Provision of study material or patients: Yoshikazu Hasegawa, Masahiko Ando, Makoto Maemondo, Satomi Yamamoto, Shun-ichi Isa, Rafael Rosell
Collection and/or assembly of data: Yoshikazu Hasegawa, Masahiko Ando, Satomi Yamamoto, Shun-ichi Isa, Hideo Saka, Rafael Rosell, Sai-Hong Ignatius Ou
Data analysis and interpretation: Yoshikazu Hasegawa, Masahiko Ando, Makoto Maemondo, Satomi Yamamoto, Shun-ichi Isa, Akihito Kubo, Tomoya Kawaguchi, Rafael Rosell, Takayasu Kurata, Sai-Hong Ignatius Ou
Manuscript writing: Yoshikazu Hasegawa, Masahiko Ando, Makoto Maemondo, Shun-ichi Isa, Akihito Kubo, Tomoya Kawaguchi, Minoru Takada, Rafael Rosell, Takayasu Kurata, Sai-Hong Ignatius Ou
Final approval of manuscript: Masahiko Ando, Makoto Maemondo, Satomi Yamamoto, Shun-ichi Isa, Hideo Saka, Akihito Kubo, Tomoya Kawaguchi, Minoru Takada, Rafael Rosell, Takayasu Kurata, Sai-Hong Ignatius Ou
Disclosures
Akihito Kubo: Chugai (H); Takayasu Kurata: AstraZenaca, Eli Lilly, Boehringer Ingelheim, Taiho, Pfizer (H); Makoto Maemondo: AstraZeneca, Chugai, Boehringer (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Liam CK, Zhou C, et al. First-line erlotinib versus cisplatin/gemcitabine (GP) in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC): Interim analysis from the phase 3, open-label, ENSURE study. J Thorac Oncol. 2013;8(suppl 2):S603. [Google Scholar]
- 8.Ou SH. Lung cancer in never-smokers: Does smoking history matter in the era of molecular diagnostics and targeted therapy? J Clin Pathol. 2013;66:839–846. doi: 10.1136/jclinpath-2012-201296. [DOI] [PubMed] [Google Scholar]
- 9.Oizumi S, Kobayashi K, Inoue A, et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: Quality of life analysis of North East Japan Study Group 002 Trial. The Oncologist. 2012;17:863–870. doi: 10.1634/theoncologist.2011-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 11.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filosto S, Baston DS, Chung S, et al. Src mediates cigarette smoke-induced resistance to tyrosine kinase inhibitors in NSCLC cells. Mol Cancer Ther. 2013;12:1579–1590. doi: 10.1158/1535-7163.MCT-12-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Takayama K, Tanaka K, et al. Nicotine induces resistance to epidermal growth factor receptor tyrosine kinase inhibitor by α1 nicotinic acetylcholine receptor-mediated activation in PC9 cells. J Thorac Oncol. 2013;8:719–725. doi: 10.1097/JTO.0b013e31828b51d4. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 16.Hughes AN, O’Brien MER, Petty WJ, et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J Clin Oncol. 2009;27:1220–1226. doi: 10.1200/JCO.2008.19.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer. 2014;84:196–202. doi: 10.1016/j.lungcan.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 19.Han JY, Park K, Kim SW, et al. First-SIGNAL: First-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 20.Mulloy R, Ferrand A, Kim Y, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 21.Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 22.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 23.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 24.Liang W, Sheng J, Wu X, et al. Exon 19 deletion association with progression-free survival compared to L858R mutation at exon 21 in treatment with first-line EGFR-TKIs: A meta-analysis of subgroup data from eight phase III randomized controlled trials. J Clin Oncol. 2014;32(suppl):8107a. [Google Scholar]
- 25.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015 [Epub ahead of print] doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 26.Liam CK, Wahid MIA, Rajadurai P, et al. Epidermal growth factor receptor mutations in lung adenocarcinoma in Malaysian patients. J Thorac Oncol. 2013;8:766–772. doi: 10.1097/JTO.0b013e31828b5228. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]