Assays for mutations in the TERT promoter and the FGFR3 gene have been shown to be promising biomarkers for UBC diagnosis and surveillance in Western patients. Surprisingly, results of this study showed that the frequency of FGFR3 mutations was very low in Han Chinese patients with UBC and was unlikely to be a diagnostic marker for them. These patients also had a relatively low rate of TERT promoter mutations.
Keywords: FGFR3, Telomerase, TERT, Urinary markers, Urothelial bladder cancer
Abstract
The TERT promoter and FGFR3 gene mutations are two of the most common genetic events in urothelial bladder cancer (UBC), and these mutation assays in patient urine have been shown to be promising biomarkers for UBC diagnosis and surveillance. These results were obtained mainly from studies of patients with UBC in Western countries, and little is known about such information in Han Chinese patients with UBC. In the present study, we addressed this issue by analyzing tumors from 182 Han Chinese patients with UBC and urine samples from 102 patients for mutations in the TERT promoter and FGFR3 and TERT mRNA expression in tumors and/or urine. TERT promoter and FGFR3 mutations were identified in 87 of 182 (47.8%) and 7 of 102 (6.7%) UBC cases, respectively. In 46 urine samples from patients with TERT promoter mutation-carrying tumors, the mutant promoter was detected in 24 (52%) prior to operation and disappeared in most examined urine samples (80%) taken 1 week after operation. TERT mRNA was detected in urine derived from 46 of 49 patients (94%) that was analyzed before operation independently of the presence of TERT promoter mutations. Collectively, FGFR3 mutations occur at a very low rate in Han Chinese UBC and cannot serve as diagnostic markers for Chinese patients. Han Chinese patients with UBC have relatively low TERT promoter mutation frequency compared with patients in Western countries, and simultaneous detection of both mutant TERT promoter and TERT mRNA improves sensitivity and specificity of urine-based diagnosis.
Abstract
摘要
TERT 启动子和 FGFR3 基因突变是膀胱尿路上皮癌 (UBC) 中两个最常见的遗传事件,并且已经显示患者尿中的这些突变分析是用于 UBC 诊断和监测的有前景的生物标志物。这些结果主要从西方国家 UBC 患者的研究中获得,但关于中国汉族 UBC 患者的这类信息知之甚少。在本研究中,通过对源于 182 位中国汉族 UBC 患者的肿瘤和源于 102 位患者的尿样分析 TERT 启动子和 FGFR3 中的突变以及肿瘤和/或尿中的 TERT mRNA 表达,我们解决了这个问题。分别在 87/182 (47.8%) 的 UBC 病例和 7/102 (6.7%) 的 UBC 病例中鉴定出 TERT 启动子和 FGFR3 突变。在源自 TERT 启动子突变携带型肿瘤患者的 46 份尿样中,手术前的 24 (52%) 份尿样中检出突变启动子并且该启动子在手术后 1 周取得的大部分所查尿样 (80%) 中消失。在 46/49 (94%) 位患者于手术前分析的尿中检出 TERT mRNA,这与 TERT 启动子突变的存在无关。总体而言,FGFR3 突变以很低比率出现在中国汉族 UBC 患者中且不能充当中国患者的诊断标志物。与西方国家的患者相比,中国汉族 UBC 患者具有相对低的 TERT 启动子突变频率,并且同时检测突变 TERT 启动子和 TERT mRNA 改善了基于尿液诊断的灵敏性和特异性。The Oncologist 2015; 20:263–269
Implications for Practice:
The TERT promoter and FGFR3 gene mutations are two of the most common genetic events in urothelial bladder cancer (UBC), and these mutation assays in patient urine have been shown to be promising biomarkers for UBC diagnosis and surveillance, based on the study of patients from Western countries. Surprisingly, we found that the frequency of FGFR3 mutations was very low in Han Chinese patients with UBC and was unlikely to be a diagnostic marker for them. These patients also had a relatively low rate of TERT promoter mutations, and simultaneous detection of both mutant TERT promoter and TERT mRNA improves sensitivity and specificity of urine-based diagnosis.
Introduction
Urothelial bladder cancer (UBC), derived from the urothelium, is one of the most common urological malignancies in both Eastern and Western countries, with a global annual incidence rate of 350,000 [1]. Because the pathogenesis of UBC is a multistep process that involves multiple genetic changes including loss of tumor suppressor genes and activation of oncogenes, the molecular dissection of UBC has been one of the most intensively studied areas. The identification of new molecular markers not only allows more complete characterization of UBC but also provides potential tools for UBC diagnosis, surveillance, monitoring of treatment response, and prognosis.
Telomerase is an RNA-dependent DNA polymerase responsible for synthesizing telomeric DNA at the ends of chromosomes, and telomerase-mediated lengthening of telomeres is required for sustained cellular proliferation [2–4]. Consistent with its biological activity, telomerase is observed to be silent in most normal somatic cells, with a limited life span while activated in up to 90% of human malignancies, acquiring an infinite proliferation potential [2, 4]. Although telomerase is a huge ribonucleoprotein complex, its catalytic component—telomerase reverse transcriptase (TERT)—is the determinant for controlling telomerase activity [2–4]. Consequently, telomerase silence and activation in normal and malignant cells results generally from the repression and derepression, respectively, of the TERT gene. Numerous studies have evaluated telomerase activity and TERT as cancer diagnostic markers and therapeutic targets, given their cancer-specific expression or activation [3, 5–7]. Widespread telomerase activation and TERT expression, for example, occur in UBC, and detection of telomerase activity and/or TERT mRNA in voided urine has been successfully applied to urine-based diagnostics for and recurrence surveillance of UBC [8–18]; however, non-tumor-derived TERT expression poses a potential drawback. Inflammatory lymphocytes, for example, may contain substantial amounts of TERT transcripts or telomerase activity and may cause a false-positive result when exfoliated into urine [8]. Consequently, more specific telomerase-based diagnostic markers are required.
Hotspot mutations in the TERT core promoter region (C228T and C250T) were recently identified in human malignancies [13, 19–30], and up to 84% of UBC tumors harbor TERT promoter mutations [27, 28]. These novel genetic events create ETS/TCF binding motifs in the TERT promoter and stimulate TERT transcription, thereby activating telomerase in UBC cells [20, 23]. More important, because these mutations are absent in normal cells, mutation detection in voided urine from patients with UBC has been tested as a biomarker for urine-based diagnostics and recurrence surveillance [21, 23, 28].
Fibroblast growth factor receptor 3 (FGFR3) is a tyrosine kinase receptor that mediates the effects of fibroblast growth factors and stimulates the RAS-mitogen-activated protein kinase and phosphatidylinositide-3 kinase-AKT pathway, triggering a variety of cellular processes [31]. Earlier experimental studies established a causal link between the presence of FGFR3-activating mutations and tumorigenesis. Indeed, the activating mutation of the FGFR3 gene is one of the most frequently mutated genes in patients with UBC [31]. In particular, 60%–80% of non-muscle-invasive UBC has been observed to harbor FGFR3 mutations [32–40]. The mutations have also been used as urinary biomarkers for UBC diagnostics and recurrence monitoring. Interestingly, the occurrence of TERT promoter and FGFR3 mutations is highly correlated; moreover, the combined detection of both TERT promoter and FGFR3 mutations in the urine of patients with UBC has been shown to increase sensitivity and specificity of UBC diagnosis [28].
Wu et al. [23] reported a TERT promoter mutation (C228T/A and C250T) rate of 54.2% in a cohort of Han Chinese patients with UBC, indicating relatively fewer mutations compared with Western patients; however, little is known about the FGFR3 mutation in Han Chinese patients with UBC. Because this information is important to the development of mutant TERT promoter- and FGFR3-based UBC diagnostics and disease surveillance, we addressed this issue by sequencing the proximal TERT promoter region and FGFR3 (exons 7, 10, and 15) in UBC tumors derived from 182 Han Chinese patients at diagnosis. Our results revealed that 47% of these patients carried C228T/A or C250T TERT promoter mutations, and surprisingly, only 6.7% had FGFR3 gene mutations. Given such a low frequency of FGFR3 mutations, their detection is unlikely to be a useful biomarker for Chinese UBC diagnostics. We further explored the combined assay of the mutant TERT promoter and TERT mRNA in voided urine from patients with UBC, and our findings demonstrate the feasibility and accuracy of their simultaneous detection for urine-based UBC diagnostics.
Patients and Methods
Patients and Tumor Specimens
The study was conducted with 182 patients with UBC who underwent surgery at Shandong University Qilu Hospital and Second Hospital in Jinan, People’s Republic of China. UBC tumors from 181 patients were transitional cell carcinoma, and 2 were adenocarcinoma. Tumors were also obtained at recurrence from 3 of 182 patients, for a total of 185 tumor specimens. Tumor grading and staging were performed according to the criteria of the World Health Organization and the TNM classification of the International Union Against Cancer (seventh edition). Patient clinical characteristics including: sex, age at diagnosis, tumor size and other histopathological characteristics, and metastases and recurrence are summarized in Table 1 and supplemental online Table 1. The specimens were collected after surgical treatment and kept frozen at −70°C or paraffin-embedded until use. All samples were collected with informed consent and approval by the institutional ethics committee.
Table 1.
Comparison of TERT promoter mutations with clinical characteristics in patients with urothelial bladder cancer

Voided Urine Samples From UBC Patients
Urine was collected from 102 patients with UBC before surgical treatment (supplemental online Table 2) and from 10 patients without cancer who were recruited as controls. Fifty mL of urine was centrifuged, and the pellet was kept at −70°C until use.
DNA Extraction and Sequencing
Genomic DNA was extracted from frozen and/or paraffin-embedded tumor tissue samples and urine pellets using Qiagen DNA extraction kits (Qiagen, Venlo, The Netherlands, http://www.qiagen.com). The two mutations defined as C228T and C250T in the TERT core promoter correspond to positions 124 and 146 bp upstream of the ATG site (Fig. 1A). The target region was amplified using polymerase chain reaction (PCR) followed by Sanger sequencing, as described previously [13]. PCR was performed with the following primer pairs: 5′-CAC CCG TCC TGC CCC TTC ACC TT-3′ (forward) and 5′-GGC TTC CCA CGT GCG CAG CAG GA-3′ (reverse). The C228T and C250T mutations were verified by sequencing from both directions. The same set of DNA from these specimens was also analyzed for the alterations of the FGFR3 gene by Sanger sequencing, and the specific PCR primers are as follows [40–43]: exon 7, 5′-AGT GGC GGT GGT GGT GAG GGA G-3′ (forward) and 5′- AGC ACC GCC GTC TGG TTG GC-3′ (reverse); exon 10, 5′-CAA CGC CCA TGT CTT TGC AG-3′ (forward) and 5′-CAA GAT CTC CCG CTT CCC G-3′ (reverse); and exon 15, 5′-GAG AGG TGG AGA GGC TTC AG-3′ (forward) and 5′- TCA TGC CAG TAG GAC GCC T-3′ (reverse).
Figure 1.
TERT promoter and FGFR3 gene mutations detected in urothelial bladder cancer (UBC) tumors. (A): Location of C228T, CC242-243TT, and C250T (in red) in the TERT core promoter. TSS and ATG indicate transcription and translation start sites, respectively. The mutations create de novo binding motifs (GGAA) for the transcription factor ETS1. (B): Sequencing chromatographs of the TERT promoter locus in tumor genomic DNA obtained by Sanger sequencing from three patients with UBC. C to T transition at C250, CC242-243TT, and C228 in the TERT promoter locus were identified in three tumors, respectively. (C): The schematic expression of frequently mutated positions at the FGFR3 gene. (D): Sequencing chromatographs of the FGFR3 locus in tumor genomic DNA obtained by Sanger sequencing from three patients with UBC. Shown are R248C, S249C, G372C, and Y375C mutations identified in four tumors.
RNA Extraction and Quantitative PCR for TERT mRNA Determination
Total cellular RNA in UBC tumors and exfoliated cells from urine was extracted using Trizol (Life Technologies; Thermo Fisher Scientific, Paisley, U.K., http://www.lifetechnologies.com). cDNA was synthesized using random primers (N6) (Amersham; GE Healthcare, Buckinghamshire, U.K., http://www3.gehealthcare.com) and M-MLV reverse transcriptase. Quantitative PCR was carried out in an ABI7700 sequence detector (Applied Biosystems; Thermo Fisher Scientific) using the SYBR Green kit (Applied Biosystems) and the specific primer pair for TERT transcripts, as described previously [13]. β2-microglobulin (β2-M) was PCR-amplified as an internal control. Levels of TERT mRNA were calculated based on the threshold values and normalization of β2-M expression.
Statistical Analyses
Differences in the TERT promoter mutation frequency between tumors with FGFR3 alteration, sex, clinical stage, and metastasis were determined using Fisher’s exact test. Student’s t test was used to analyze differences in age and tumor size between the TERT promoter mutation-positive and -negative groups. Differences in TERT mRNA levels were examined using the Mann-Whitney rank sum test. All tests were two-tailed and computed using SigmaStat3.1 software (Systat Software, Inc., Richmond, CA, http://www.systat.com). p values of <.05 were considered statistically significant.
Results
A Relatively Lower Frequency of TERT Promoter Mutations in Han Chinese Patients With UBC
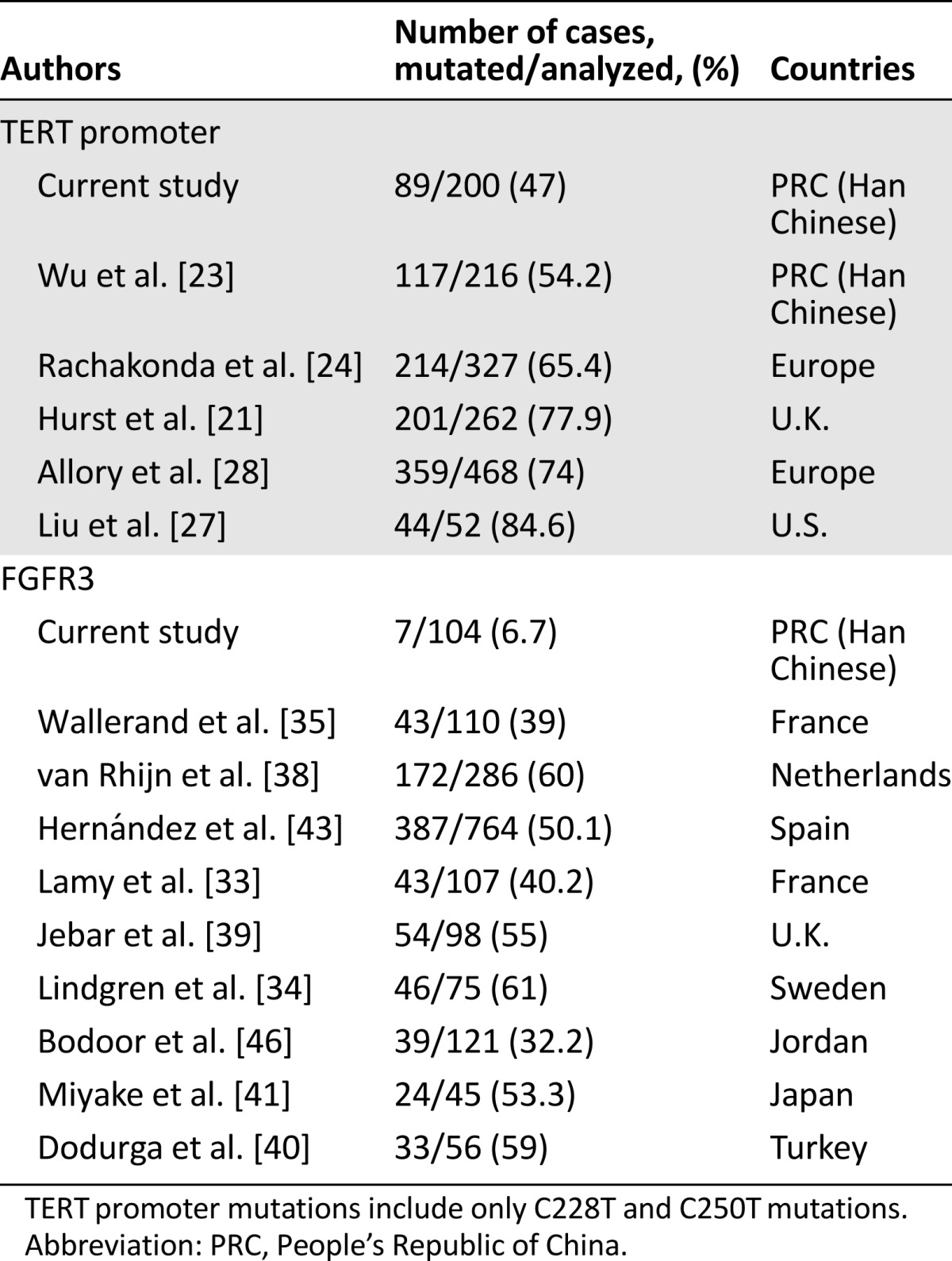
Sanger sequencing was used to determine TERT promoter mutations in tumor DNA derived from 182 patients with UBC. A total of 87 UBC tumors (87 of 182, 47.8%) were found to carry TERT promoter mutations, 38 in C228T, 15 in C250T, 1 in C228A, and 1 in CC242-243TT (Fig. 1A and B). Four different mutations were mutually exclusive, consistent with published observations [19, 20]. Altogether, C228T, C228A, and C250T comprised 47% of the mutation frequency in 182 Han Chinese UBCs, lower than 65%–84% observed in patients from Western countries (Table 2).
Table 2.
Reports of TERT promoter and FGFR3 mutations in urothelial bladder cancer
In addition, we analyzed three tumors at recurrence from three patients with C228T-harboring UBC tumors at diagnosis and observed the presence of the same mutation in these consecutive samples.
Because TERT promoter mutations facilitate gene transcription by creating de novo ETS binding motifs [20, 23], we were interested in the relationship between the presence of the mutation and the abundance of TERT transcripts. Total RNA from 46 patients’ tumors was available and analyzed for TERT mRNA expression using quantitative PCR. TERT transcripts were detected in 42 of 46 tumors (91%), and 3 of 4 tumors lacking TERT mRNA were TERT promoter mutation negative. The tumors carrying TERT promoter mutations tended to express higher TERT mRNA, but the difference between mutation-positive and -negative tumors was not statistically significant (mutation vs. wild type: 0.555 ± 0.473 vs. 0.148 ± 0.057; p = .438) (Table 1).
The Relationship Between TERT Promoter Mutations and Clinical Variables in Patients With UBC
We then determined the potential association of the presence of TERT promoter mutations with clinical variables in this cohort of patients. Male patients tended to have a higher rate of TERT promoter mutations than did female patients, but the difference did not reach statistical significance (51% vs. 23%; p = .068) (Table 1). Patient age, tumor size and number, and TNM and pathological stages also were not correlated with the TERT promoter mutation status (supplemental online Table 1; Table 1).
Very Low Incidence of FGFR3 Gene Mutations in Han Chinese Patients With UBC
We next analyzed the FGFR3 gene by sequencing the hotspot mutation-localized regions including exon 7, 10, and 15 (Fig. 1C). A total of 104 tumors were screened, and 7 were observed to carry mutations (2 with R248C/C742T, 2 with Y375C/A1124G, 2 with G372C/G1114T, and 1 with both S249C/C476G and G372C/G1114T) (supplemental online Table 1; Fig. 1C, 1D). The FGFR3 mutation was not associated with grade, stage, invasiveness, or TERT promoter mutations (supplemental online Table 1; Table 1). The mutation frequency was much lower than that in non-Chinese patients (Table 2).
Detectable Mutant TERT Promoters in Urine Derived From Patients With UBC
Having demonstrated the presence of TERT promoter mutations in UBC tumors from this cohort of patients, we sought to determine whether the mutant sequence was detectable in patients’ voided urine, using Sanger sequencing. Urine samples were available from 102 patients prior to surgical treatment. Of these 102 patients, 46 had mutation-carrying tumors, whereas the remaining 56 lacked the mutation in their tumors (supplemental online Table 2; Table 3). The mutant sequence was detected in 24 of 46 urine samples (52%) derived from patients with tumors harboring TERT promoter mutations (supplemental online Table 2; Table 3). There was no association between positive urine detection and tumor size or disease stage and grade. In urine specimens from 56 mutation-negative patients with UBC, the mutation was undetectable in the vast majority (53 of 56, 95%); however, 3 of these patients (5%) were C228T or C250T positive, as determined using Sanger sequencing, and thus inconsistent with tumor tissue results (supplemental online Table 2; Table 3). Taken together, in TERT promoter mutation-positive tumors, the sensitivity for urinary assay using Sanger sequencing is 52%, whereas the specificity was 95% (53 of 56) when all analyzed urine samples were counted together.
Table 3.
Concordance of TERT promoter mutations between tumor tissues and urine samples in urothelial bladder cancer patients, as determined using Sanger sequencing

To determine whether the mutant TERT promoter present in patients’ urine would disappear following a surgical treatment, we analyzed consecutive urine specimens from 15 originally urine-positive patients at 1 week after operation (supplemental online Table 2). TERT promoter mutations remained detectable in 3 of 15 urine samples and became undetectable in the rest, indicating that most patients had fast clearance of the urinary mutant promoter once tumors were removed. One patient with a C228T-negative tumor had detectable urinary C228T prior to operation and exhibited a wild-type TERT promoter in his urine specimen after the surgical operation.
TERT mRNA Present in Urine From Patients With UBC
TERT mRNA was previously observed to be detected in urine from patients with UBC. To probe whether a relationship exists between TERT promoter mutations in tumors and presence of TERT mRNA in patient urine and whether the combined assay of both TERT promoter mutations and TERT mRNA is capable of increasing the sensitivity and specificity for UBC diagnostics, we determined urinary TERT mRNA in 49 patients. As controls, urine specimens from 10 healthy adults were assessed, and TERT mRNA was undetectable in all of them. Urine specimens from 47 of 49 patients (96%) contained TERT mRNA at different levels (supplemental online Table 2). Urinary TERT mRNA tended to be more abundant in patients with TERT promoter mutation-carrying tumors than in those without the mutation (1.31 ± 0.668 vs. 0.498 ± 0.184), but the difference was not statistically significant (p = .496). Of note, the combination of both mutation and mRNA results further increased sensitivity to 98%.
Discussion
Recent evidence has shown that UBC is very heterogeneous clinically, pathologically, and genetically. Because of a high risk of recurrence, life-long surveillance is recommended [1]. Cystoscopy and urinary cytology are useful tools for UBC diagnostics and disease monitoring; however, the former is invasive and expensive, whereas the later may be insufficiently sensitive, particularly to low-grade disease, and prone to interobserver and intraobserver variability [44]. Consequently, accurate, noninvasive tests are needed for initial diagnosis, surveillance, monitoring of treatment response, and prognosis. The identification of prevalent TERT promoter and FGFR3 gene mutations in UBC, together with their absence in normal tissues and cells, makes them potential urinary markers for disease diagnostics [21, 23, 28, 31].
To determine the sensitivity and specificity of TERT promoter mutations in urine-based tests, it is essential to define the prevalence of these genetic events in UBC tumors. Analyses of European and American patients with UBC demonstrated 65%–84% mutation frequencies (Table 2) [21, 23, 27, 28]; however, a relatively lower mutation rate (54%) was found in a cohort of 216 Chinese patients with UBC [23]. In the present study, we showed an even lower occurrence of the mutation (47%) in Han Chinese patients with UBC. These results indicate that TERT promoter mutations occur less frequently in Han Chinese patients with UBC than in those from Western countries. This information must be taken into consideration when urine examination of the mutant TERT promoter is performed on Han Chinese patients with UBC for diagnostic and monitoring purposes.
It is currently unclear what causes different TERT promoter mutation frequencies between Han Chinese and Western patients with UBC. Potential reasons include differences in ethnicity, in oncogenic factors or pathways, and in carcinogen exposure. We also noted that renal pelvic and ureter carcinomas have very different occurrences of TERT promoter mutations, despite originating from the same urothelium or belonging to transitional cell carcinoma as UBC [25]. The mutation frequency in renal pelvic carcinoma is approximately 50% and is very similar between Chinese and U.S. patients [22, 23, 25]. Based on our analyses of small numbers of ureter carcinoma samples, only 11% of these tumors harbored the mutation [25].
Although a relatively lower frequency of TERT promoter mutation occurs in Han Chinese patients with UBC, the mutation assay may still be a urinary marker for those patients with mutation-carrying tumors. Sanger sequencing is specific, but its sensitivity is not high enough. In the present study, we demonstrated 52% sensitivity for the Sanger sequencing assay in tumors with mutations. A more sensitive approach is required to reliably detect the mutant promoter present in patients’ voided urine. In contrast, despite 95% specificity with Sanger sequencing, it remains unclear where the mutant promoter sequence detected in 3 urine samples (5%) came from, given the lack of TERT promoter mutations in these patients’ tumors, or whether they were simply false positive due to contamination. Dissociation between tumor and urine tests was also reported by Allory et al. [28]. Using Sanger sequencing and SNaPshot to examine TERT promoter mutations in bladder cancer, they found that the mutant TERT promoter was present in urine but not in tumors from 4 of their 39 bladder cancer patients [28]. During a 3.5-year follow-up period, these 4 patients did not have recurrent disease.
Because TERT and telomerase activity is widely present in UBC, its detection in patient urine has been tested as molecular markers for UBC diagnostic and follow-up [8]. The present results demonstrated high sensitivity for urinary detection of TERT transcripts. Despite this, non-tumor-derived TERT expression poses potential drawbacks. Inflammatory lymphocytes may contain substantial amounts of TERT transcripts or telomerase activity and may cause a false-positive result when exfoliated into urine [8]. This scenario may occur more frequently in women [45]. Because TERT promoter mutations are exclusively absent in normal cells and tissues, the combined detection of both TERT transcripts and mutant promoter may solve this problem in TERT promoter mutation-carrying UBCs by increasing detection specificity. Our present results support simultaneous detection of both TERT promoter mutations and TERT mRNA as urinary markers for UBC.
Given the observed prevalence of the FGFR3 mutation in UBC [32–40], the detection of the mutant FGFR3 has been widely investigated for UBC diagnostic and prognostic purposes. It is surprising to observe an extremely lower frequency of FGFR3 mutations in our cohort of patients than in Western patients. The analyzed patients were randomly recruited with different stages and grades, and hence the difference is unlikely to be the result of bias in patient selection. Of note, patients with UBC from other Asian countries such as Japan and Jordan exhibit higher frequency of the FGFR3 mutation [41, 46]. It is unclear what causes such a dramatic difference in the FGFR3 mutation between patients from China and other countries. More investigation is needed.
Conclusion
We identified frequencies of 47% for TERT promoter mutations and 6.7% for FGFR3 mutations in Han Chinese patients with UBC, lower than rates reported for patients with UBC from Western countries. This finding should be considered when these mutations are used as biomarkers for urine-based UBC diagnosis and recurrence surveillance. FGFR3 mutation detection is not suitable for diagnosis and outcome prediction in Han Chinese patients with UBC, whereas urine-based TERT promoter mutation assays are specific to patients with the mutant promoter-carrying UBC, and the combined TERT mRNA and mutant promoter detection may increase sensitivity and specificity for UBC.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
This study was supported by the National Basic Research Program of China (Grant No. 973 Program 2012CB911202), the Adolf H. Lundin Charitable Foundation, the Swedish Cancer Society, the Swedish Research Council, the Cancer Society in Stockholm, the Stockholm County Council and the Karolinska Institutet, the National Natural Science Foundation of China (NO: 81372765), and the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet.
Author Contributions
Conception/Design: Kun Wang, Tiantian Liu, Cheng Liu, Yan Meng, Yidong Fan, Dawei Xu
Provision of study material or patients: Kun Wang, Cheng Liu, Yan Meng, Xiaotian Yuan, Li Liu, Nan Ge, Jikai Liu, Hongbo Ren, Keqiang Yan, Zhonghua Xu
Collection and/or assembly of data: Kun Wang, Cheng Liu, Yan Meng, Xiaotian Yuan, Li Liu, Nan Ge, Jikai Liu, Chang Wang, Hongbo Ren, Keqiang Yan, Sanyuan Hu, Zhonghua Xu, Yidong Fan
Data analysis and interpretation: Kun Wang, Tiantian Liu, Cheng Liu, Yan Meng, Xiaotian Yuan, Li Liu, Nan Ge, Jikai Liu, Chang Wang, Sanyuan Hu, Zhonghua Xu, Yidong Fan, Dawei Xu
Manuscript writing: Kun Wang, Tiantian Liu, Cheng Liu, Yan Yidong Fan, Dawei Xu
Final approval of manuscript: Kun Wang, Tiantian Liu, Cheng Liu, Yan Meng, Xiaotian Yuan, Li Liu, Nan Ge, Jikai Liu, Chang Wang, Hongbo Ren, Keqiang Yan, Sanyuan Hu, Zhonghua Xu, Yidong Fan, Dawei Xu
Disclosures
The authors indicated no financial relationships.
References
- 1.Griffiths TR. Current perspectives in bladder cancer management. Int J Clin Pract. 2013;67:435–448. doi: 10.1111/ijcp.12075. [DOI] [PubMed] [Google Scholar]
- 2.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT) Gene. 2012;498:135–146. doi: 10.1016/j.gene.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong F, Zheng C, Xu D. Telomerase as a “stemness” enzyme. Sci China Life Sci. 2014;57:564–570. doi: 10.1007/s11427-014-4666-6. [DOI] [PubMed] [Google Scholar]
- 5.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 6.Hiyama E, Hiyama K. Telomerase as tumor marker. Cancer Lett. 2003;194:221–233. doi: 10.1016/s0304-3835(02)00709-7. [DOI] [PubMed] [Google Scholar]
- 7.Chiu JW, Wong H, Leung R, et al. Advanced pancreatic cancer: Flourishing novel approaches in the era of biological therapy. The Oncologist. 2014;19:937–950. doi: 10.1634/theoncologist.2012-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamarca A, Barriuso J. Urine telomerase for diagnosis and surveillance of bladder cancer. Adv Urol. 2012;693631 doi: 10.1155/2012/693631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchini MA, Gunelli R, Nanni O, et al. Relevance of urine telomerase in the diagnosis of bladder cancer. JAMA. 2005;294:2052–2056. doi: 10.1001/jama.294.16.2052. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Suzuki Y, Fujioka T. Expression of the catalytic subunit associated with telomerase gene in human urinary bladder cancer. J Urol. 1999;162:2217–2220. doi: 10.1016/S0022-5347(05)68162-1. [DOI] [PubMed] [Google Scholar]
- 11.Weikert S, Krause H, Wolff I, et al. Quantitative evaluation of telomerase subunits in urine as biomarkers for noninvasive detection of bladder cancer. Int J Cancer. 2005;117:274–280. doi: 10.1002/ijc.21168. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Sugino T, Tahara H, et al. Telomerase activity in bladder carcinoma and its implication for noninvasive diagnosis by detection of exfoliated cancer cells in urine. Cancer. 1997;79:362–369. doi: 10.1002/(sici)1097-0142(19970115)79:2<362::aid-cncr20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014;33:4978–4984. doi: 10.1038/onc.2013.446. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita H, Ogawa O, Kakehi Y, et al. Detection of telomerase activity in exfoliated cells in urine from patients with bladder cancer. J Natl Cancer Inst. 1997;89:724–730. doi: 10.1093/jnci/89.10.724. [DOI] [PubMed] [Google Scholar]
- 15.Neves M, Ciofu C, Larousserie F, et al. Prospective evaluation of genetic abnormalities and telomerase expression in exfoliated urinary cells for bladder cancer detection. J Urol. 2002;167:1276–1281. [PubMed] [Google Scholar]
- 16.Heine B, Hummel M, Müller M, et al. Non-radioactive measurement of telomerase activity in human bladder cancer, bladder washings, and in urine. J Pathol. 1998;184:71–76. doi: 10.1002/(SICI)1096-9896(199801)184:1<71::AID-PATH988>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Kyo S, Kanaya T, et al. Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res. 1998;4:1603–1608. [PubMed] [Google Scholar]
- 18.Kavaler E, Landman J, Chang Y, et al. Detecting human bladder carcinoma cells in voided urine samples by assaying for the presence of telomerase activity. Cancer. 1998;82:708–714. doi: 10.1002/(sici)1097-0142(19980215)82:4<708::aid-cncr14>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 20.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–369. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 22.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Huang P, Li C, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: A genomic and molecular study. Eur Urol. 2014;65:274–277. doi: 10.1016/j.eururo.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Liu T, Liu L, et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget. 2014;5:1829–1836. doi: 10.18632/oncotarget.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang N, Liu T, Sofiadis A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120:2965–2979. doi: 10.1002/cncr.28800. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Wu G, Shan Y, et al. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle. 2013;12:1637–1638. doi: 10.4161/cc.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Brown TC, Juhlin CC, et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr Relat Cancer. 2014;21:427–434. doi: 10.1530/ERC-14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie H, Liu T, Wang N, et al. TERT promoter mutations and gene amplification: Promoting TERT expression in Merkel cell carcinoma. Oncotarget. 2014;5:10048–10057. doi: 10.18632/oncotarget.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knowles MA. Role of FGFR3 in urothelial cell carcinoma: Biomarker and potential therapeutic target. World J Urol. 2007;25:581–593. doi: 10.1007/s00345-007-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kompier LC, van der Aa MN, Lurkin I, et al. The development of multiple bladder tumour recurrences in relation to the FGFR3 mutation status of the primary tumour. J Pathol. 2009;218:104–112. doi: 10.1002/path.2507. [DOI] [PubMed] [Google Scholar]
- 33.Lamy A, Gobet F, Laurent M, et al. Molecular profiling of bladder tumors based on the detection of FGFR3 and TP53 mutations. J Urol. 2006;176:2686–2689. doi: 10.1016/j.juro.2006.07.132. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren D, Gudjonsson S, Jee KJ, et al. Recurrent and multiple bladder tumors show conserved expression profiles. BMC Cancer. 2008;8:183. doi: 10.1186/1471-2407-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallerand H, Bakkar AA, de Medina SG, et al. Mutations in TP53, but not FGFR3, in urothelial cell carcinoma of the bladder are influenced by smoking: Contribution of exogenous versus endogenous carcinogens. Carcinogenesis. 2005;26:177–184. doi: 10.1093/carcin/bgh275. [DOI] [PubMed] [Google Scholar]
- 36.van Kessel KE, Kompier LC, de Bekker-Grob EW, et al. FGFR3 mutation analysis in voided urine samples to decrease cystoscopies and cost in nonmuscle invasive bladder cancer surveillance: A comparison of 3 strategies. J Urol. 2013;189:1676–1681. doi: 10.1016/j.juro.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 37.van Oers JM, Zwarthoff EC, Rehman I, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol. 2009;55:650–657. doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 38.van Rhijn BW, Lurkin I, Radvanyi F, et al. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001;61:1265–1268. [PubMed] [Google Scholar]
- 39.Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 40.Dodurga Y, Tataroglu C, Kesen Z, et al. Incidence of fibroblast growth factor receptor 3 gene (FGFR3) A248C, S249C, G372C, and T375C mutations in bladder cancer. Genet Mol Res. 2011;10:86–95. doi: 10.4238/vol10-1gmr923. [DOI] [PubMed] [Google Scholar]
- 41.Miyake M, Sugano K, Sugino H, et al. Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non-muscle invasive bladder cancer. Cancer Sci. 2010;101:250–258. doi: 10.1111/j.1349-7006.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieger-Christ KM, Mourtzinos A, Lee PJ, et al. Identification of fibroblast growth factor receptor 3 mutations in urine sediment DNA samples complements cytology in bladder tumor detection. Cancer. 2003;98:737–744. doi: 10.1002/cncr.11536. [DOI] [PubMed] [Google Scholar]
- 43.Hernández S, López-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 44.Frantzi M, Makridakis M, Vlahou A. Biomarkers for bladder cancer aggressiveness. Curr Opin Urol. 2012;22:390–396. doi: 10.1097/MOU.0b013e328356ad0e. [DOI] [PubMed] [Google Scholar]
- 45.Bravaccini S, Sanchini MA, Granato AM, et al. Urine telomerase activity for the detection of bladder cancer in females. J Urol. 2007;178:57–61. doi: 10.1016/j.juro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Bodoor K, Ghabkari A, Jaradat Z, et al. FGFR3 mutational status and protein expression in patients with bladder cancer in a Jordanian population. Cancer Epidemiol. 2010;34:724–732. doi: 10.1016/j.canep.2010.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



