The aim of this study was to retrospectively evaluate the efficacy and safety of brentuximab vedotin (BV) in a multicenter setting of classical Hodgkin lymphoma relapsing or progressing after allogeneic stem cell transplant. Results showed that BV therapy is an effective and safe approach for achieving transient disease control in these patients. To improve disease control, future studies should explore the combination of BV with targeted agents.
Keywords: Brentuximab vedotin, Relapsed or refractory Hodgkin lymphoma, Allogeneic stem cell transplantation, CD30, Targeted therapy
Abstract
Background.
Brentuximab vedotin (BV) has demonstrated an extraordinary efficacy in heavily pretreated classical Hodgkin lymphoma (cHL) patients, targeting CD30-positive cells; however, limited data have been reported on the efficacy of BV in cHL patients failing allogeneic stem cell transplantation (allo-SCT). The aim of this study was to retrospectively evaluate the efficacy and safety of BV in a multicenter setting of cHL relapsing or progressing after allo-SCT.
Methods.
Sixteen BV-naïve patients with recurrent cHL after allo-SCT were included in a compassionate use program and treated with intravenous BV at the dose of 1.8 mg/kg of body weight every 3 weeks for a maximum of 16 cycles.
Results.
The objective response rate was 69%. Five patients (31%) had complete remission, and 6 (37%) had partial remission. Stable disease was observed in 4 patients (25%), and progressive disease was observed in 1 (6%). After median follow-up of 26 months (range: 5–30 months), median progression-free survival (PFS), overall survival (OS), and duration of response were 7, 25, and 5 months, respectively. The 2-year PFS and OS were 20% and 61%, respectively. Grade 3–4 hematological adverse events included anemia (15%), thrombocytopenia (12%), and neutropenia (18%). Grade 3 peripheral sensory neuropathy occurred in 2 patients (12%).
Conclusion.
BV therapy is an effective and safe approach for achieving transient disease control in cHL patients with failed allo-SCT. To improve disease control, future studies should explore the combination of BV with targeted agents.
Implications for Practice:
Brentuximab vedotin (BV) is an effective and safe therapy to achieve disease control in classical Hodgkin lymphoma (cHL) relapsing or progressing after allogeneic stem cell transplantation. Our results confirm previously reported data and strongly suggest that single-agent BV is an effective and manageable treatment in patients who have failed allogeneic transplant. However, to improve disease control, future studies should explore the combination of BV with molecularly targeted agents.
Introduction
Modern chemoradiotherapy programs for advanced-stage classical Hodgkin lymphoma (cHL) induce cure rates approaching 80% [1]; however, refractoriness to first-line treatment or disease relapse occurs in 25%–30% of patients after achieving an initial complete remission (CR). High-dose chemotherapy followed by autologous stem cell transplantation (auto-SCT) has become the standard of care for refractory or relapsed cHL, leading to durable responses in ∼50% of relapsed and a minority of refractory patients [2]. Failure of auto-SCT is associated with median survival ranging from 12 to 29 months in different series [2–5]. Various therapeutic options are currently available for relapsed or refractory cHL patients with failed auto-SCT [6–8]. Among others, brentuximab vedotin (BV) demonstrated extraordinary efficacy in patients who have failed auto-SCT, mainly by selectively targeting CD30-positive cells [9, 10]. In the pivotal, phase II, open-label, single-arm, multicenter trial involving 102 patients with cHL who relapsed after auto-SCT, BV as a single agent led to an overall response rate (ORR) of 75% (CR: 34%) and a median duration of response (DoR) of 6.7 months (range: 3.6–14.8 months) [10]. The long-term efficacy of BV remains controversial, and allogeneic SCT (allo-SCT) still represents the only strategy with curative potential for relapsed or refractory cHL patients with failed auto-SCT [11, 12]. Nevertheless, among patients who received allo-SCT, long-term progression-free survival (PFS) did not exceed 25%–35% in most series, and disease relapse was associated with an exceedingly poor outcome, with less than half of the patients surviving for 3 years [11–16]. Thus far, there is no general consensus regarding the optimal therapy to be offered to cHL patients with disease recurrence following allo-SCT.
The treatment of patients with cHL who have relapsed after allo-SCT remains challenging, and there is a clear unmet medical need for drug development for these patients. Whereas various conventional cytotoxic drugs used as single agents or in combinations failed to provide substantial evidence of efficacy, bendamustine has recently been shown to be an effective drug for relapsed or refractory cHL patients with failed allo-SCT [17, 18]. Several molecularly targeted agents, including histone deacetylase inhibitors [19], mammalian target of rapamycin inhibitors [20], and immunomodulatory drugs [21], have been tested in phase I/II trials. Most of the targeted drugs used as single agents have shown limited antitumor activity, whereas encouraging results have been reported recently for BV [22]. In addition to the CD30-targeted cytotoxicity of the antimicrotubule agent monomethyl auristatin E (MMAE), the mechanism of action of BV might also involve a cytokine-mediated antitumor immune response, further supporting the rationale for using this molecule in cHL patients who have recurred after allo-SCT [23, 24]. The largest retrospective study of BV in allo-SCT failure involved 24 patients who showed ORR and CR rates of 50% and 38%, respectively, and median PFS of 7.8 months; however, the median overall survival (OS) was not reached [22].
To extend the efficacy and toxicity evaluation of BV in cHL progression after allo-SCT, we retrospectively analyzed 16 cHL patients enrolled at four Italian centers in a named patient program.
Methods
Patient Eligibility
Between June 2011 and January 2014, 16 cHL patients who relapsed or progressed after allo-SCT were enrolled in a single-agent BV named patient program approved by the ethics committees of four different institutions. The need for treatment was determined by the treating physician. Patients in this series had not received prior therapy with BV. Cases had to fulfill the following criteria: age ≥18 years, at least 1 target lesion ≥2 cm, availability of clinical documentation, computed tomography (CT) scan and 18F-fluorodeoxyglucose-positron emission tomography (PET) scan at staging and throughout the study. Bulky disease was defined as a mediastinal mass larger than one-third of the maximum thoracic diameter and/or any node >10 cm. Retrospectively collected patient data and clinical outcomes included the number of prior regimens, stage and extranodal involvement, response to prior therapies, details of allo-SCT (disease status before allo-SCT, donor type, conditioning regimen), a history of acute and chronic graft-versus-host disease (GVHD), and a history of clinically significant infections between allo-SCT and the first dose of BV. The present study was conducted in agreement with the Italian privacy laws and the Declaration of Helsinki, and written informed consent was obtained from all patients prior to any study-related procedure.
Treatment Plan
Patients received BV (1.8 mg/kg, intravenously) in 3-week cycles until unacceptable toxicity, disease progression, or achievement of complete remission. A maximum of 16 cycles was allowed.
Study Assessments
Disease assessment by PET and CT scans was performed at baseline, during therapy, and at the end of therapy. Tumor response was assessed according to the revised response criteria for malignant lymphoma of the International Working Group [25]. Responding patients were followed until disease progression, initiation of subsequent therapy, or death. Patients were monitored prior to and after each cycle for adverse events, clinical status, vital signs, and critical laboratory data. The National Cancer Institute Common Toxicity Criteria of Adverse Events (CTCAE) version 3.0 was used for the classification of adverse events.
Statistical Analysis
The activity and efficacy of BV were evaluated in terms of ORR, safety, PFS, and OS. ORR included CR and partial remission (PR). PFS was defined as the time from the date of treatment initiation to the date of progression or death from any cause. Data from patients alive without tumor progression were censored at the time of their last visit. OS was defined as the time from the date of treatment initiation to the date of death resulting from any cause or to the date of the last contact for patients who were still alive. DoR was defined for responder patients from the date of the best response to the date of the last observation or relapse. Time-to-event endpoint distributions were estimated using the Kaplan-Meier method [26]. All p values were two-sided and were considered significant for values <.05. All statistical analyses were conducted using the R statistical package (R Foundation, Vienna, Austria, http://www.r-project.org).
Results
Demographics and Clinical Characteristics
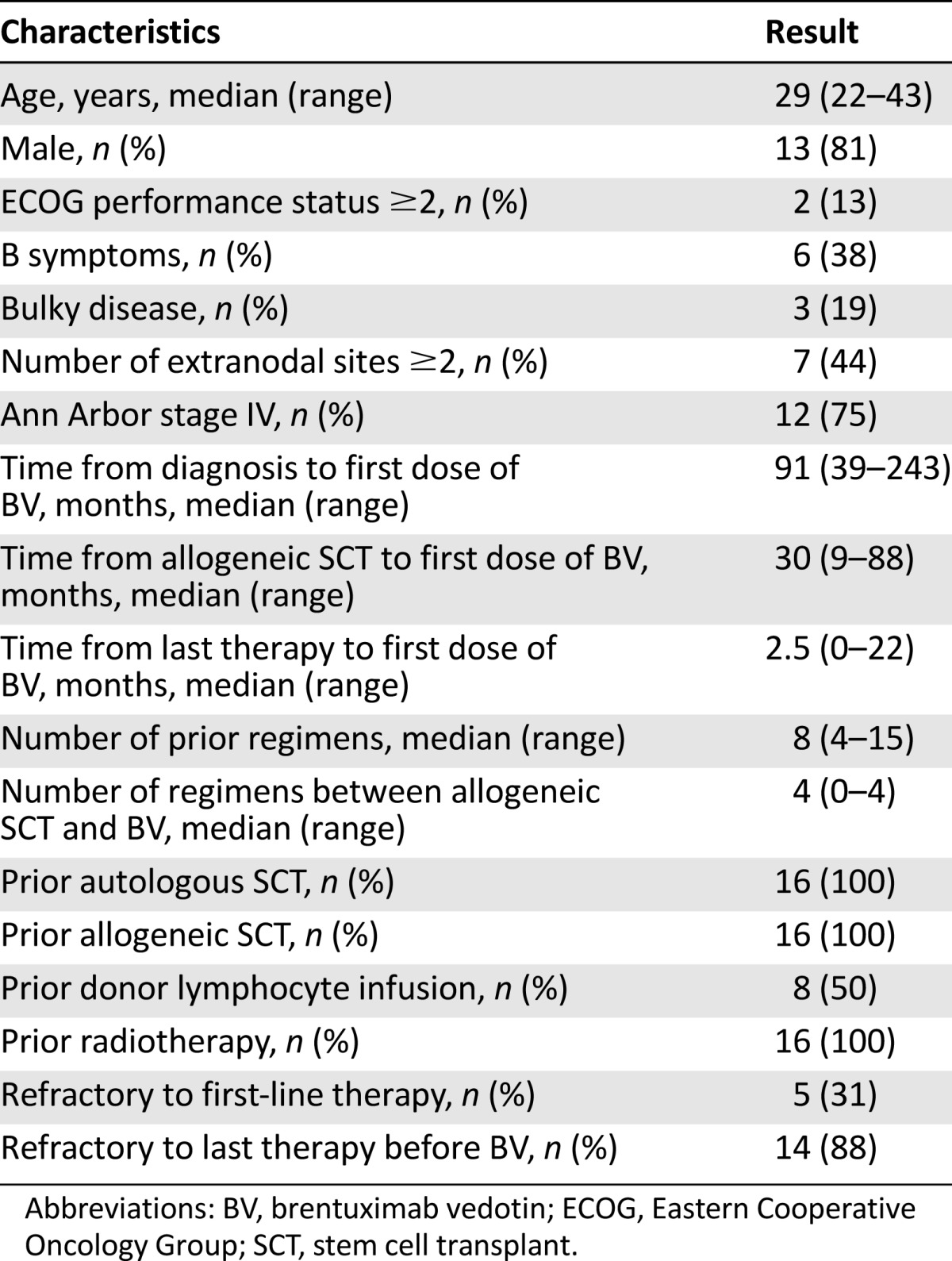
Between June 2011 and January 2014, 16 heavily pretreated cHL patients (13 men and 3 women) with a median age of 29 years (range: 22–43 years) received BV after failing allo-SCT (Table 1). At study entry, patients had received a median of 8 lines of treatment (range: 4–15). Poor prognostic features included B symptoms (n = 6, 37%), bulky disease (n = 3, 19%), and extranodal involvement (n = 7, 44%). Two patients (12%) had an Eastern Cooperative Group performance status ≥2. All patients were allografted using reduced-intensity conditioning regimens. Three of 16 patients received a second allo-SCT procedure prior to BV. Donor stem cell sources included matched related donors (n = 13) and matched unrelated donors (n = 3). At allo-SCT, 8 patients (50%) showed chemorefractory disease. Allo-SCT induced a response in 11 patients (69%), including 7 cases of CR, whereas 5 patients showed disease persistence. Prior to BV therapy, 7 patients (43%) showed GVHD, including chronic GVHD (n = 4), acute GVHD (n = 1), and both chronic and acute GVHD (n = 2). GVHD occurrence was associated in 5 patients (31%) with a history of clinically significant infections, including asymptomatic cytomegalovirus (CMV) reactivation and multifocal pneumonia (n = 1), pneumonia (n = 3) associated with Gram-positive sepsis (n = 2), and a post-transplant Epstein-Barr virus-related lymphoproliferative disorder (n = 1). At a median of 5 months (range: 1–22 months) after allografting, all of the responding patients relapsed or progressed. BV was administered after a median of 30 months from allo-SCT (range: 9–87 months). During this interval, patients received a median of 4 further treatment regimens (range: 0–6 regimens), and eight patients were treated with at least one donor lymphocyte infusion (DLI). The last assessment prior to BV therapy revealed refractory disease in all but two patients.
Table 1.
Clinical characteristics of the patients prior to enrollment in the brentuximab vedotin named patient program
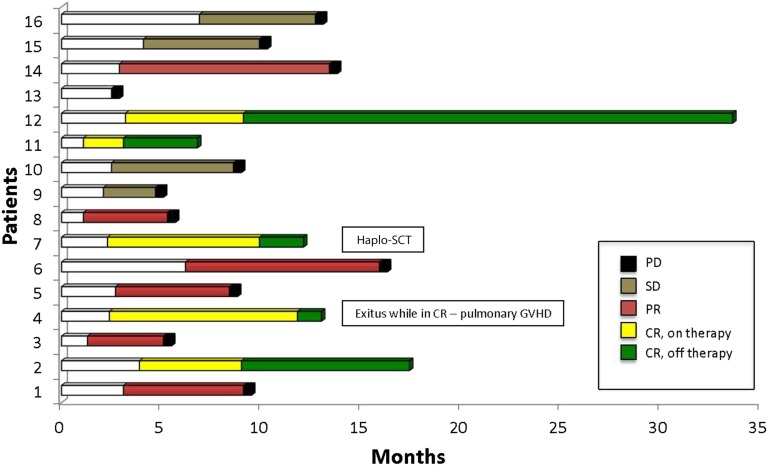
Treatment Efficacy
Patients received a median of 8 BV cycles (range: 1–17 cycles) over a median treatment duration of 6 months (range: 2–14 months). The best response to BV was achieved after a median of 4 cycles (range: 2–12 cycles) and included 5 CRs (31%) and 6 PRs (37%), for an ORR of 69% (Fig. 1). Stable disease (SD) was observed in 4 cases (25%), and progressive disease (PD) was observed in 1 patient (6%). Patients achieving CR (n = 5) discontinued BV treatment after a median of 7 cycles (range: 4–10 cycles). Reasons for discontinuation included donor availability for a second allo-SCT (n = 1), toxicity (n = 2), and treatment completion on physician decision (n = 2). After BV discontinuation, CR was maintained for a median of 4 months (range: 3 to ≥24 months), and none of the 5 patients had PD. One patient who received DLI while on BV therapy developed pulmonary GVHD while in CR (Fig. 1). All patients who achieved PR (n = 6) as the best response showed disease progression while on BV therapy after a median of 8 cycles (range: 5–17 cycles). Similarly, patients who achieved SD (n = 4) and PD (n = 1) progressed after a median of 6 cycles (range: 4–10 cycles) from the initiation of BV therapy.
Figure 1.
Duration of response in 16 patients with relapsed or refractory classical Hodgkin lymphoma who received brentuximab vedotin.
Abbreviations: CR, complete remission; GVHD, graft-versus-host disease; haplo-SCT, haploidentical stem cell transplant; PD, progressive disease; PR, partial remission; SD, stable disease.
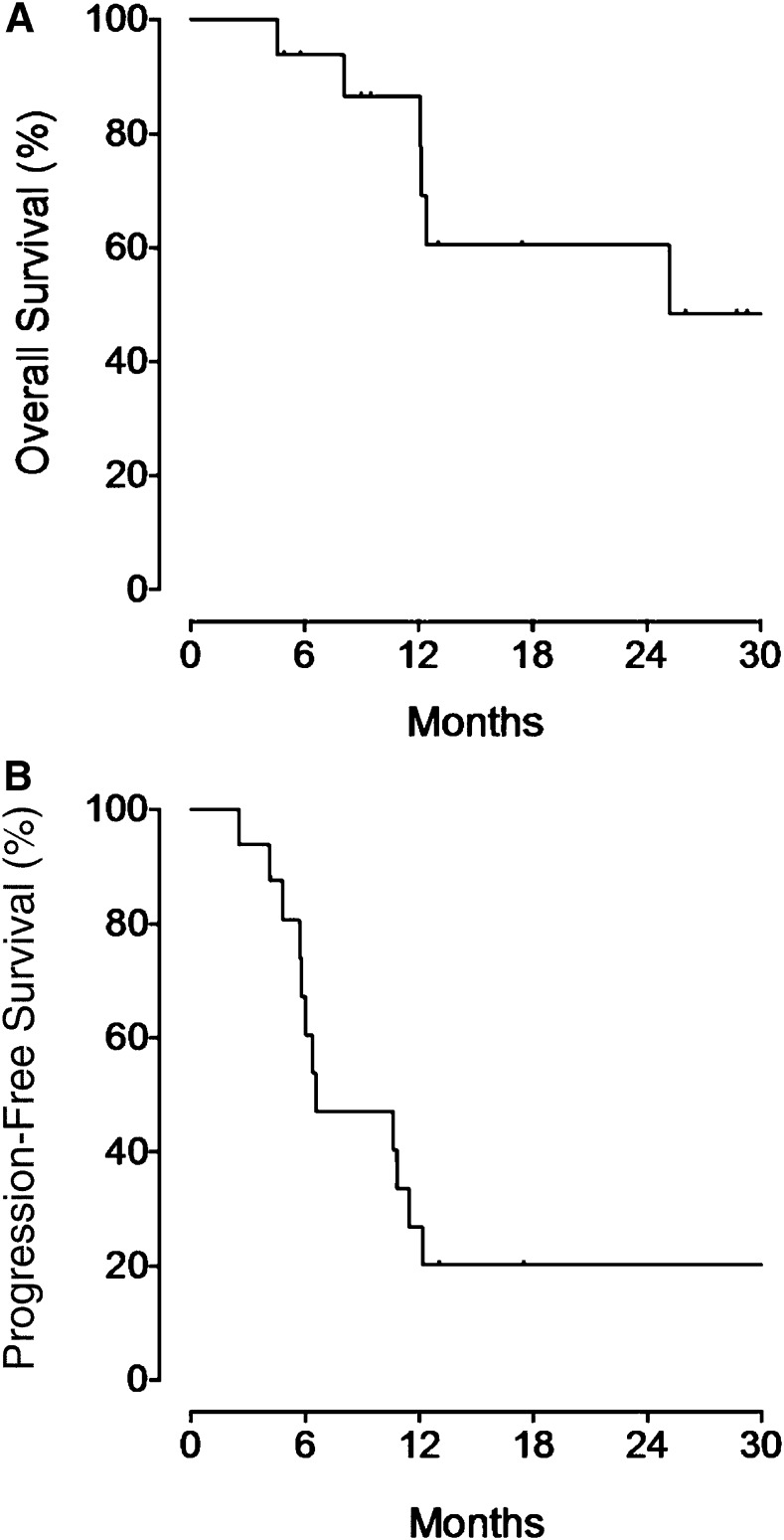
After median follow-up of 26 months (range: 5–30 months), the median DoR was 5 months (range: 3–27 months) for the entire population, 11 months (range: 5–27 months) for CR patients, and 3 months (range: 3–8 months) for PR patients. The median OS (Fig. 2A) was 25 months, and the median PFS (Fig. 2B) was 7 months. The 2-yr PFS and OS were 20% and 61%, respectively. All patients with disease progression received further treatments. Among those patients, five died from uncontrolled disease.
Figure 2.
Kaplan-Meier survival curves. Overall survival curve (A) and progression-free survival curve (B) in 16 patients with relapsed or refractory classical Hodgkin lymphoma who received brentuximab vedotin.
Safety
Treatment was well tolerated, confirming a manageable safety profile for BV. No treatment-related deaths were recorded. The most common adverse event was neurological toxicity. Four patients developed grade 1 reversible peripheral sensory neuropathy, whereas two patients developed grade 3 neurotoxicity (one with peripheral sensory neuropathy, one with Guillain-Barré syndrome) requiring BV discontinuation. No grade 3 or 4 renal, hepatic, or myocardial toxicity was observed. After 125 cycles administered to 16 patients, 3 cases of infections, including 2 cases of sepsis sustained by Gram-positive bacteria and 1 case of pneumonia (which were empirically and successfully treated with high-dose cotrimoxazole), were documented. Two patients developed fever of unknown origin, and both recovered quickly. No CMV reactivation was reported after the initiation of BV therapy. One patient who received DLI while on BV therapy died after the development of grade IV pulmonary GVHD. The most common grade ≥3 hematological adverse events were neutropenia (18%), anemia (15%), and thrombocytopenia (12%).
Discussion
Recent data have strongly supported the role of BV as a potent, quick-acting, “bridge” therapy to auto-SCT [9, 10, 27–30] or allo-SCT [22]. In patients with recurrent disease after allo-SCT, the potential ability of single-agent BV to induce a long-lasting complete response remains debatable. In the present report, BV therapy was administered to a cohort of 16 patients with cHL that relapsed or progressed after allo-SCT. All patients were heavily pretreated, and 87% had chemorefractory disease prior to BV. Treatment with BV resulted in an ORR of 69% and a CR rate of 31%. After median follow-up of 26 months, median PFS was 7 months, and median OS was 25 months. Although it is well known that each subsequent line of treatment for multirelapsed lymphoma results in progressive PFS reduction, the PFS achieved with BV was significantly longer than that achieved with the most recent prior therapy. Our results are consistent with those reported by Gopal et al., who published the largest report on the use of BV in patients with recurrent or progressive cHL after allo-SCT [22]. Those data are particularly interesting if compared with those reported in the recent literature concerning the use of BV in less heavily pretreated patients who did not undergo an allo-SCT procedure. In the pivotal phase II trial of 102 patients with relapsed or refractory cHL after auto-SCT, BV monotherapy resulted in an ORR of 75% with a CR rate of 34% [10]. Superimposable results have been achieved in the more recent Italian series of 65 heavily pretreated cHL patients in which 79% of patients had received a previous auto-SCT but only 5% had a previous allo-SCT [28].
In our series of cHL patients with very poor prognosis who progressed after allo-SCT, BV showed clinically relevant activity that compared favorably with the response rate of 35% reported with the most common salvage strategies, such as the reduction of immunosuppression or chemoimmunotherapy [31]. Among cytotoxic drugs, only bendamustine has been shown to have efficacy comparable to that of BV when used either as a single agent or in combination with DLI [17, 18, 32, 33].
In terms of ORR and CR rates, our data also remain encouraging if compared with the results reported for DLI administered with or without chemotherapy [34–36]. In fact, DLI yielded a response rate of 43%–56%, with a grade II–IV GVHD rate of 32%–38% [34–36]. In contrast to the general feasibility of BV, the feasibility of DLI was restricted to a minority of patients [34]. Recently, a single-center experience combining BV with DLI has been reported in four cHL patients with early relapse after allo-SCT [24]. The working hypothesis supporting the combined BV-DLI treatment suggests that selective targeting of lymphoma cells could enhance the graft versus leukemia response by inducing immunogenic cell death [24]. In addition, BV might potentially reduce GVHD by targeting CD30-positive T-lymphocytes [37]. Our study confirms that BV treatment seems to be more effective than other targeted agents tested as single agents, such as panobinostat (ORR: 27%), lenalidomide (ORR: 19%), and everolimus (ORR: 47%) [19–21].
Adverse events were generally manageable and were not worse than expected in heavily pretreated patients. The most common events were typically grade 1 or 2. Based on the hypothesis that targeting an antigen on activated T cells could further impair cell-mediated immunity in this high-risk population, particular attention was paid to the close monitoring of clinical infection: no grade III or IV infections were recorded, and no CMV reactivation occurred. Consequently, our data suggest that BV, as a single agent, is safe and highly effective in inducing short-term disease control in a very high-risk population. In contrast, our study failed to demonstrate the potential ability of this drug to induce long-lasting disease control, even if, as expected, the quality of the response enhanced the long-term disease outcome.
Conclusion
BV is a highly effective therapy with a good toxicity profile that can be offered to cHL patients with relapse or progression after allo-SCT to achieve effective but transient disease control. Future studies should explore the combination of BV with DLI, conventional chemotherapy (e.g., bendamustine), or targeted agents (e.g., PI3K inhibitors or anti-PD1 agents) to enhance tumor burden reduction and increase the CR rate, thereby improving disease control. BV therapy with or without DLI could also be considered a prophylaxis strategy in patients at high risk for recurrence after allo-SCT. Recent data concerning BV retreatment support this therapeutic approach in patients who, in the near future, will have received BV-based regimens earlier during the disease course [38]. Future studies may be warranted to explore these new BV-based strategies in BV-naïve and BV-sensitive patients.
Acknowledgments
We thank the patients and their families as well as the study sites and staff. Carmelo Carlo-Stella is supported by the Ministry of Health (RF 2010-2313979) and the Italian Association for Cancer Research (Grant 15835).
Author Contributions
Conception/Design: Carmelo Carlo-Stella, Laura Giordano
Provision of study material or patients: Carmelo Carlo-Stella
Collection and/or assembly of data: Carmelo Carlo-Stella, Francesca Ricci, Serena Dalto, Rita Mazza, Michele Malagola, Francesca Patriarca, Simonetta Viviani, Domenico Russo, Paolo Corradini
Data analysis and interpretation: Carmelo Carlo-Stella, Francesca Ricci, Serena Dalto, Rita Mazza, Michele Malagola, Francesca Patriarca, Simonetta Viviani, Domenico Russo, Laura Giordano, Luca Castagna, Paolo Corradini, Armando Santoro
Manuscript writing: Carmelo Carlo-Stella, Francesca Ricci, Domenico Russo, Laura Giordano, Luca Castagna, Paolo Corradini, Armando Santoro
Final approval of manuscript: Carmelo Carlo-Stella
Disclosures
Simonetta Viviani: Takeda Pharmaceuticals International GmbH (C/A); Armando Santoro: Takeda (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Canellos GP, Rosenberg SA, Friedberg JW, et al. Treatment of Hodgkin lymphoma: A 50-year perspective. J Clin Oncol. 2014;32:163–168. doi: 10.1200/JCO.2013.53.1194. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz AJ, Perales M-A, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146:158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology (Am Soc Hematol Educ Program) 2008;1:326–333. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
- 4.Martínez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24:2430–2434. doi: 10.1093/annonc/mdt206. [DOI] [PubMed] [Google Scholar]
- 5.Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533. doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- 6.Canellos GP. Brentuximab vedotin and panobinostat: New drugs for Hodgkin’s lymphoma—can they make one of medical oncology’s chemotherapy success stories more successful? J Clin Oncol. 2012;30:2171–2172. doi: 10.1200/JCO.2011.39.6416. [DOI] [PubMed] [Google Scholar]
- 7.Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology (Am Soc Hematol Educ Program) 2009;1:507–519. doi: 10.1182/asheducation-2009.1.507. [DOI] [PubMed] [Google Scholar]
- 8.Younes A. Beyond chemotherapy: New agents for targeted treatment of lymphoma. Nat Rev Clin Oncol. 2011;8:85–96. doi: 10.1038/nrclinonc.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 10.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: A retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 12.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SP, Sureda A, Canals C, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: Identification of prognostic factors predicting outcome. Haematologica. 2009;94:230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: Importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14:418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corradini P, Dodero A, Farina L, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: Pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21:2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 16.Ram R, Gooley TA, Maloney DG, et al. Histology and time to progression predict survival for lymphoma recurring after reduced-intensity conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1537–1545. doi: 10.1016/j.bbmt.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corazzelli G, Angrilli F, D’Arco A, et al. Efficacy and safety of bendamustine for the treatment of patients with recurring Hodgkin lymphoma. Br J Haematol. 2013;160:207–215. doi: 10.1111/bjh.12120. [DOI] [PubMed] [Google Scholar]
- 18.Anastasia A, Carlo-Stella C, Corradini P, et al. Bendamustine for Hodgkin lymphoma patients failing autologous or autologous and allogeneic stem cell transplantation: A retrospective study of the Fondazione Italiana Linfomi. Br J Haematol. 2014;166:140–142. doi: 10.1111/bjh.12821. [DOI] [PubMed] [Google Scholar]
- 19.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: Results of a phase II study. J Clin Oncol. 2012;30:2197–2203. doi: 10.1200/JCO.2011.38.1350. [DOI] [PubMed] [Google Scholar]
- 20.Johnston PB, Inwards DJ, Colgan JP, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118:5119–5125. doi: 10.1182/blood-2011-07-362475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal AK, Ramchandren R, O’Connor OA, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120:560–568. doi: 10.1182/blood-2011-12-397893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35) Clin Cancer Res. 2011;17:6428–6436. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 24.Theurich S, Malcher J, Wennhold K, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013;31:e59–e63. doi: 10.1200/JCO.2012.43.6832. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Rothe A, Sasse S, Goergen H, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: The German Hodgkin Study Group experience. Blood. 2012;120:1470–1472. doi: 10.1182/blood-2012-05-430918. [DOI] [PubMed] [Google Scholar]
- 28.Zinzani PL, Viviani S, Anastasia A, et al. Brentuximab vedotin in relapsed/refractory Hodgkin’s lymphoma: The Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica. 2013;98:1232–1236. doi: 10.3324/haematol.2012.083048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: A bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98:611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illidge T, Bouabdallah R, Chen R, et al. Allogeneic transplant following brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2014:1–34. doi: 10.3109/10428194.2014.930852. [DOI] [PubMed] [Google Scholar]
- 31.Wudhikarn K, Brunstein CG, Bachanova V, et al. Relapse of lymphoma after allogeneic hematopoietic cell transplantation: Management strategies and outcome. Biol Blood Marrow Transplant. 2011;17:1497–1504. doi: 10.1016/j.bbmt.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–460. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sala E, Crocchiolo R, Gandolfi S, et al. Bendamustine combined with donor lymphocytes infusion in Hodgkin’s lymphoma relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1444–1447. doi: 10.1016/j.bbmt.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365:1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 35.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: The updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YB, McDonough S, Hasserjian R, et al. Expression of CD30 in patients with acute graft-versus-host disease. Blood. 2012;120:691–696. doi: 10.1182/blood-2012-03-415422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. doi: 10.1186/1756-8722-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]