Abstract
Background.
This phase I study evaluated the maximum tolerated dose (MTD), safety, pharmacokinetics (PK), and pharmacodynamics of pilaralisib (SAR245408), an oral pan-class I phosphoinositide 3-kinase (PI3K) inhibitor, in combination with erlotinib, an epidermal growth factor receptor (EGFR) inhibitor.
Methods.
In a 3 + 3 dose-escalation study, patients with advanced solid tumors received pilaralisib capsules once daily (21 days per 28-day cycle; 50–600 mg) plus erlotinib tablets once daily (28 days per 28-day cycle; 100 or 150 mg). An MTD expansion cohort of patients with non-small cell lung cancer who had previously received treatment with an EGFR inhibitor was included.
Results.
Thirty-five patients were enrolled. Only one patient had an EGFR activating mutation. One dose-limiting toxicity was reported (grade 4 drug reaction or rash with eosinophilia and systemic symptoms). MTD was pilaralisib 400 mg plus erlotinib 150 mg. The most commonly reported treatment-related adverse events were rash (62.9%), diarrhea (42.9%), and fatigue (40.0%). Pilaralisib PK findings were consistent with previous studies, suggesting erlotinib had no effect on pilaralisib pharmacokinetics. Pharmacodynamic analyses indicated moderate inhibition of PI3K, mitogen-activated protein kinase, and EGFR pathways. Of 27 evaluable patients, one had a partial response (3.7%) and 14 (51.9%) had stable disease. There was no association between molecular alterations of PI3K pathway components and clinical activity.
Conclusion.
Pilaralisib plus erlotinib had limited antitumor activity. Safety findings were similar to recent studies of single-agent pilaralisib or other PI3K inhibitors.
Abstract
摘要
背景. 本项I期研究评估了口服泛I型磷脂酰肌醇3-激酶(PI3K)抑制剂Pilaralisib(SAR245408)联合表皮生长因子受体(EGFR)抑制剂厄洛替尼的最大耐受剂量(MTD)、安全性、药代动力学(PK)以及药效动力学特征。
方法. 采取3 + 3剂量递增设计,对晚期实体瘤患者给予pilaralisib胶囊每日1次(用药21天,每28天为1周期;50 ∼ 600 mg)联合厄洛替尼片剂每日1次(用药28天,每28天为1周期;100或150 mg)治疗。既往接受过EGFR抑制剂的非小细胞肺癌患者纳入MTD扩大队列。
结果. 入组35例患者。仅1例患者携带EGFR激活突变。报告了1例剂量限制性毒性反应(4级;药物反应或皮疹伴嗜酸性粒细胞增多以及全身症状)。MTD为Pilaralisib 400 mg联合厄洛替尼150 mg。最常报告的治疗相关不良事件包括皮疹(62.9%)、腹泻(42.9%)以及乏力(40.0%)。Pilaralisib PK结果与既往研究一致,提示厄洛替尼对pilaralisib药代动力学无影响。药效动力学分析表明对PI3K、促丝裂原活化蛋白激酶以及EGFR通路有中度抑制。27例可评估患者中,1例(3.7%)部分缓解,14例(51.9%)疾病稳定。PI3K通路分子改变与临床活性之间无相关性。
结论. Pilaralisib联合厄洛替尼抗肿瘤活性有限。安全性结果与最近pilaralisib或其他PI3K抑制剂单药研究结果类似。The Oncologist 2015; 20:245–246
Author Summary
Discussion
In non-small cell lung cancer (NSCLC), resistance to EGFR inhibitors occurs through several mechanisms, including activation of parallel or downstream pathways, such as the PI3K and mammalian target of rapamycin (mTOR) pathway [1, 2]. In vitro studies suggest that PI3K pathway inhibition can overcome resistance to EGFR inhibition [3, 4]; therefore, combining PI3K and EGFR inhibitors is a rational therapeutic strategy.
Pilaralisib is a highly selective, reversible, pan-class I PI3K inhibitor. In a phase I dose-escalation study in patients with solid tumors, pilaralisib showed clinical activity, and the MTD was established as 600 mg once daily [5].
The current phase I dose-escalation study (ClinicalTrials.gov identifier NCT00692640) evaluated MTD, safety, PK, pharmacodynamics, and efficacy of pilaralisib in combination with the EGFR inhibitor erlotinib in patients with advanced solid tumors, including patients with NSCLC who had previously received an EGFR inhibitor.
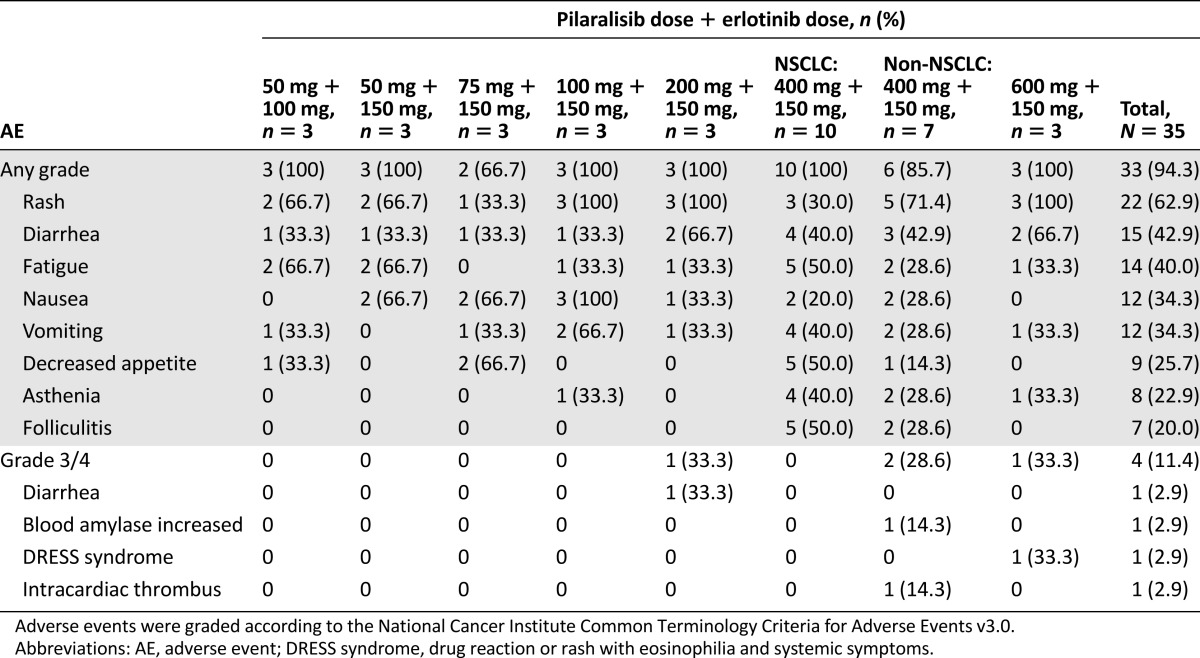
Thirty-five patients were enrolled; 57% had NSCLC. There was one dose-limiting toxicity: grade 4 DRESS syndrome (drug reaction or rash with eosinophilia and systemic symptoms) (Table 1). The MTD was determined to be pilaralisib 400 mg in combination with erlotinib 150 mg. Safety findings were similar to recent studies of single-agent pilaralisib or other PI3K inhibitors [5–9].
Table 1.
Treatment-related AEs occurring in ≥20% of patients and all treatment-related grade 3/4 AEs in patients treated with pilaralisib and erlotinib once daily
Day 21 PK parameters were consistent with previous findings for pilaralisib monotherapy at steady state [5] (Table 2), suggesting that erlotinib does not interact with pilaralisib pharmacokinetically. Exposure on day 21 increased in a less than dose-proportional manner; geometric mean maximum concentration and area under the concentration-time curve increased over the 12-fold dose range of pilaralisib (50–600 mg) by 6.91- and 7.91-fold, respectively.
Pharmacodynamic analyses in tumor and skin samples indicated moderate inhibition (61%–67% and 31%–66%, respectively) of PI3K, mitogen-activated protein kinase, and EGFR pathways. PIK3CA amplification or mutation was detected in three patients, phosphatase and tensin homolog protein deficiency was detected in three patients, and an EGFR activating mutation was detected in one patient.
In 27 evaluable patients, the best response was a partial response in one patient (3.7%) and stable disease in 14 patients (51.9%). Thirteen patients had progression-free survival for ≥90 days. The limited efficacy was consistent with the modest pharmacodynamic activity observed and with recent studies combining PI3K/mTOR pathway inhibitors and EGFR inhibitors [9, 10]. The combination of pilaralisib and erlotinib is no longer being investigated in solid tumors.
Supplementary Material
Footnotes
Access the full results at: Soria-14-449.theoncologist.com
ClinicalTrials.gov Identifier: NCT00692640
Sponsor(s): Sanofi and Exelixis
Principal Investigators: Jean-Charles Soria, Patricia LoRusso, Howard Burris
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


