On August 5, 2013, a marketing authorization valid throughout the European Union was issued for pomalidomide in combination with dexamethasone for the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy. This paper summarizes the scientific review of the application leading to that approval.
Keywords: Pomalidomide, Imnovid, Multiple myeloma, EMA, European Medicines Agency
Abstract
On August 5, 2013, a marketing authorization valid throughout the European Union (EU) was issued for pomalidomide in combination with dexamethasone for the treatment of adult patients with relapsed and refractory multiple myeloma (MM) who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy. Pomalidomide is an immunomodulating agent. The recommended starting dose of pomalidomide is 4 mg once daily taken on days 1–21 of repeated 28-day cycles. The main evidence of efficacy for pomalidomide in MM was based on a phase III multicenter, randomized, open-label study (CC-4047-MM-003) in which pomalidomide plus low-dose dexamethasone therapy (POM+LoDEX) was compared with high-dose dexamethasone alone (HiDEX) in previously treated adult patients with relapsed and refractory multiple myeloma who had received at least two prior treatment regimens, including both lenalidomide and bortezomib, and had demonstrated disease progression on the last therapy. For the intent-to-treat population, median progression-free survival based on International Myeloma Working Group criteria was 15.7 weeks (95% confidence interval [CI]: 13.0–20.1) in the POM+LoDEX group versus 8.0 weeks (95% CI: 7.0–9.0) in the HiDEX group (log-rank p value <.001). Overall survival (secondary endpoint) was also different in the two treatment groups (hazard ratio 0.53 [95% CI: 0.37–0.74]). The most commonly reported adverse reactions to pomalidomide in clinical studies were anemia (45.7%), neutropenia (45.3%) and thrombocytopenia (27%), fatigue (28.3%), pyrexia (21%), peripheral edema (13%), and infections including pneumonia (10.7%). Peripheral neuropathy adverse reactions were reported in 12.3% of patients, and venous embolic or thrombotic (VTE) adverse reactions were reported in 3.3% of patients. Pomalidomide is expected to be teratogenic. This paper summarizes the scientific review of the application leading to approval in the EU. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the EMA website (http://www.ema.europa.eu).
Implications for Practice:
Pomalidomide in combination with low-dose dexamethasone has been approved in the European Union to treat adult patients with relapsed and refractory multiple myeloma. The approval is based on efficacy data in 455 adults with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy (Study CC-4047-MM-003). In this study, pomalidomide/low-dose dexamethasone was associated with a median progression-free survival of 16 weeks compared to 8 weeks for high-dose dexamethasone alone. The most common side effects associated with pomalidomide/low-dose dexamethasone were anemia, neutropenia, thrombocytopenia, fatigue and pyrexia. A teratogenic effect of pomalidomide in humans is expected.
Introduction
Multiple myeloma (MM) is a rare and incurable disease that is characterized by the accumulation of clonal plasma cells in the bone marrow and accounts for 10% of all hematological malignancies [1].
Bortezomib- and lenalidomide-based regimens are the most commonly used agents in the treatment of relapsed or refractory MM in combination with corticosteroids and sometimes an alkylating agent or with an anthracycline [2].
Patients whose disease is relapsed or refractory after treatment with conventional agents have limited treatment options and short median survival of about 9 months [3].
Pomalidomide (CC-4047; Imnovid; Celgene Europe Ltd., Uxbridge, U.K., http://www.celgene.co.uk) is a structural analog of lenalidomide and thalidomide, which belong to the class of immunomodulating agents (Fig. 1). The precise molecular mechanism of action and the targets through which pomalidomide and similar agents exert their antitumor effect are unclear. The applicant company, Celgene Ltd., submitted a new drug application for pomalidomide (hard capsules) for the treatment of MM to the European Medicines Agency (EMA). The review was conducted by the Committee for Medicinal Products for Human Use. Following the review, a marketing authorization was granted in the European Union (EU) for pomalidomide in combination with dexamethasone for the treatment of adult patients with relapsed and refractory MM who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy.
Figure 1.
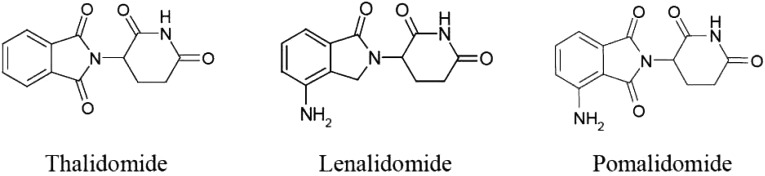
Structural formulae of thalidomide, lenalidomide, and pomalidomide. Pomalidomide chemical name: (RS)4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione; molecular formula: C13H11N3O4.
This paper summarizes the scientific review of the application leading to approval of pomalidomide in the EU. The detailed scientific assessment report and product information are available on the EMA website (http://www.ema.europa.eu).
Mechanism of Action
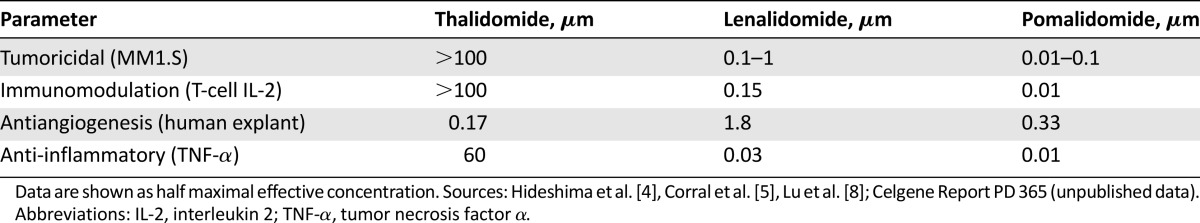
In vitro, pomalidomide appeared to have a dual mechanism of action, being directly tumoricidal for MM cells [4] and having immunomodulatory activity with direct effects on T-cell- and natural killer-cell-mediated immunity [5, 6]. In vitro, cereblon, a primary teratogenic target of thalidomide, was an essential requirement for antimyeloma immunomodulatory activity of lenalidomide and pomalidomide and was suggested as a possible biomarker for the clinical assessment of antimyeloma efficacy [7]. Antiangiogenic and anti-inflammatory effects have also been reported [5, 8, 9]. Pomalidomide shares a number of potentially therapeutic pharmacological properties with thalidomide and lenalidomide and has different activity and potency profiles (Table 1).
Table 1.
Pharmacological properties of pomalidomide, lenalidomide, and thalidomide and relative potency (EC50)
Pathway analysis in lenalidomide-resistant cell lines revealed a number of biogroups differentially modulated by pomalidomide. The biological significance of these pathways is still being explored. It is expected that the analysis and experimental verification of any identified gene or protein will be completed postapproval. Celgene will submit a report on completion.
In vitro, the combination of pomalidomide plus dexamethasone was synergistic in both H929 lenalidomide-sensitive and -resistant cells, inhibiting cell proliferation and inducing apoptosis.
Nonclinical Toxicology and Clinical Pharmacology
In the rat, pomalidomide was well tolerated up to the top dose tested in all studies. In the monkey, the main toxic effects were associated with the hematopoietic and lymphoreticular systems. In the 9-month repeated-dose toxicity study in monkeys, acute myelogenous leukemia was reported in one female. This finding may be a secondary effect of the product due to immunosuppression.
Pomalidomide had adverse effects on fertility and early embryonic development and was teratogenic in rat and rabbit.
Pomalidomide is absorbed with a maximum plasma concentration occurring between 2 and 3 hours and is at least 73% absorbed following administration of a single oral dose. The predominant metabolic pathways are hydroxylation with subsequent glucuronidation or hydrolysis. In vitro, CYP1A2 and CYP3A4 were identified as the primary enzymes involved in the CYP-mediated hydroxylation of pomalidomide, with additional minor contributions from CYP2C19 and CYP2D6.
Clinical Efficacy and Safety
This application was initially supported by two phase II studies, CC-4047-MM-002 (phase II segment) and IFM-2009-02. These studies were not considered sufficient for approval, but during evaluation, the final study report from the pivotal phase III study CC-4047-MM-003 was submitted [10]. This phase III, multicenter, randomized, open-label study was designed to compare the efficacy and safety of pomalidomide plus low-dose dexamethasone (POM+LoDEX) with high-dose dexamethasone (HiDEX) in previously treated adult patients with relapsed and refractory multiple myeloma who had received at least two prior treatment regimens, including both lenalidomide and bortezomib, and had demonstrated disease progression on the last therapy. The rationale for the 4-mg starting dose was based primarily on one phase I dose-escalating study and study CC-4047-MM-002 [11, 12].
Patients in the POM+LoDEX group received 4 mg pomalidomide orally on days 1–21 of each 28-day cycle. LoDEX (40 mg) was administered once per day on days 1, 8, 15, and 22 of a 28-day cycle. In the HiDEX group, dexamethasone (40 mg) was administered once per day on days 1–4, 9–12, and 17–20 of a 28-day cycle. Patients aged >75 years started treatment with 20 mg dexamethasone. Treatment continued until disease progression. One-way cross-over was not permitted.
The primary efficacy endpoint was progression-free survival (PFS) by International Myeloma Working Group criteria [13] reviewed by an independent response adjudication committee in a blinded manner.
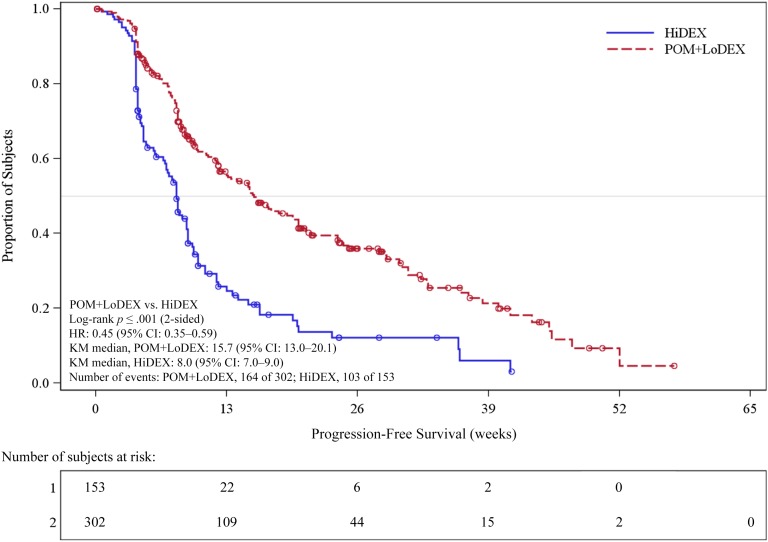
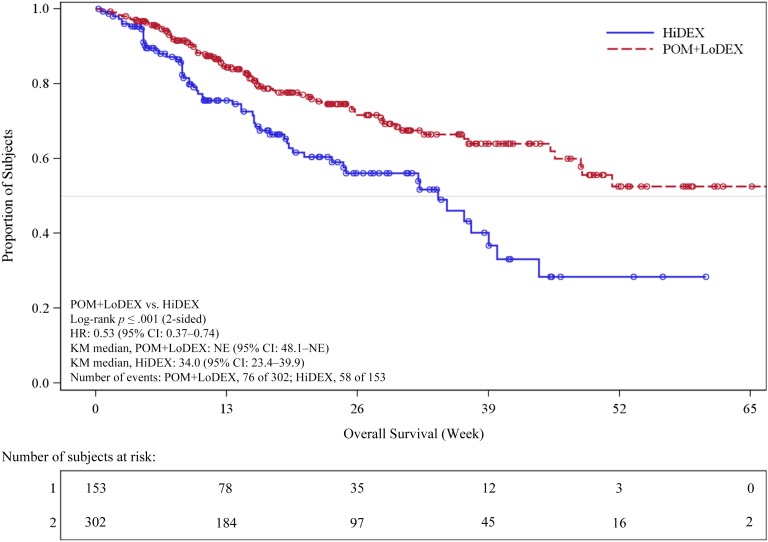
A total of 455 patients were enrolled in the study. Refractoriness to selected prior antimyeloma drugs by treatment group is summarized in Table 2. Efficacy results on the intent-to treat population as of September 7, 2012, are summarized in Table 3 and Figure 2. The study met its primary efficacy endpoint (PFS), and a statistically significant difference was observed for overall survival (OS; secondary endpoint) (Fig. 3). In patients aged >75 years (24 with POM+LoDEX and 12 with HiDEX), the hazard ratios were 0.34 (95% confidence interval [CI]: 0.13–0.88) for PFS and 0.39 (95% CI: 0.11; 1.38) for OS.
Table 2.
Refractoriness to selected prior antimyeloma drugs by treatment group (intent-to-treat population)
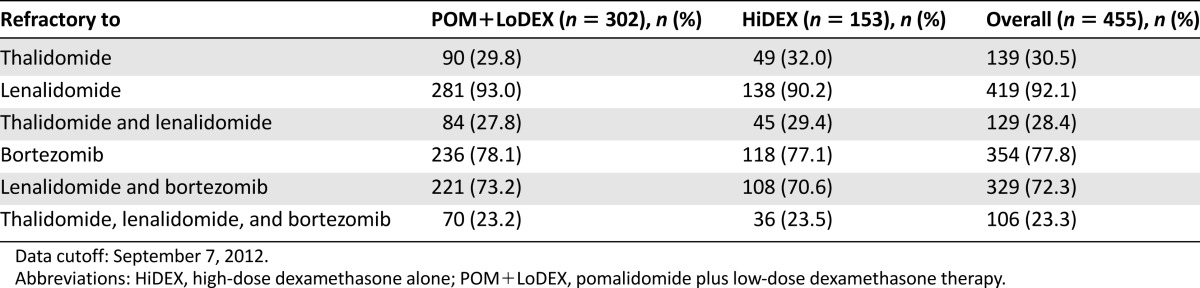
Table 3.
Efficacy endpoints by independent response adjudication committee assessment, International Myeloma Working Group criteria (intent-to-treat population)
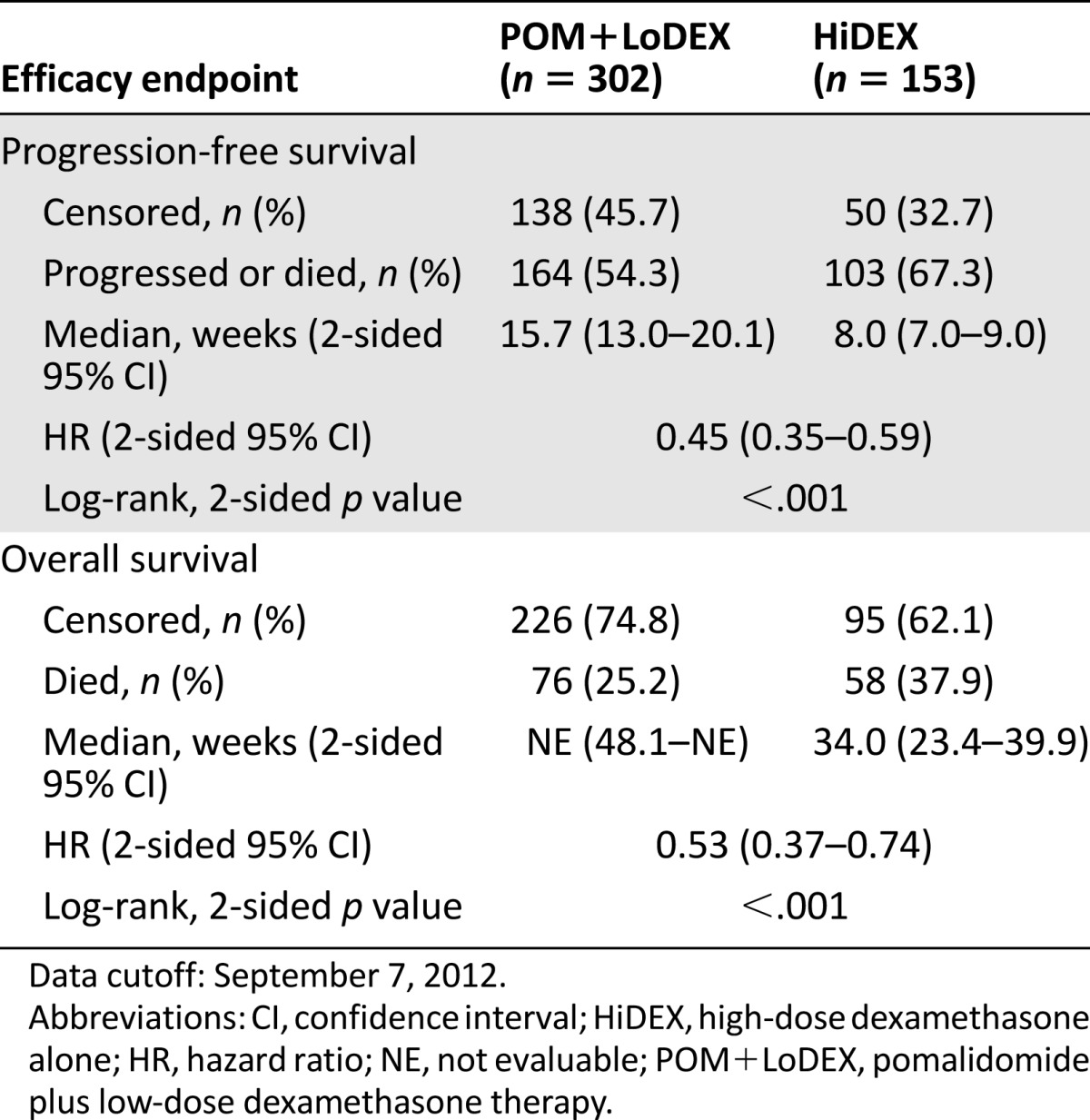
Figure 2.
Progression-free survival based on independent response adjudication committee review of responses by International Myeloma Working Group criteria (stratified log-rank test; intent-to-treat population). Data cutoff: September 7, 2012.
Abbreviations: CI, confidence interval; HiDEX, high-dose dexamethasone alone; HR, hazard ratio; KM, Kaplan-Meier; POM+LoDEX, pomalidomide plus low-dose dexamethasone therapy.
Figure 3.
Kaplan-Meier curve, overall survival (intent-to-treat population). Data cutoff: September 7, 2012.
Abbreviations: CI, confidence interval; HiDEX, high-dose dexamethasone alone; HR, hazard ratio; KM, Kaplan-Meier; NE, not evaluable; POM+LoDEX, pomalidomide plus low-dose dexamethasone therapy.
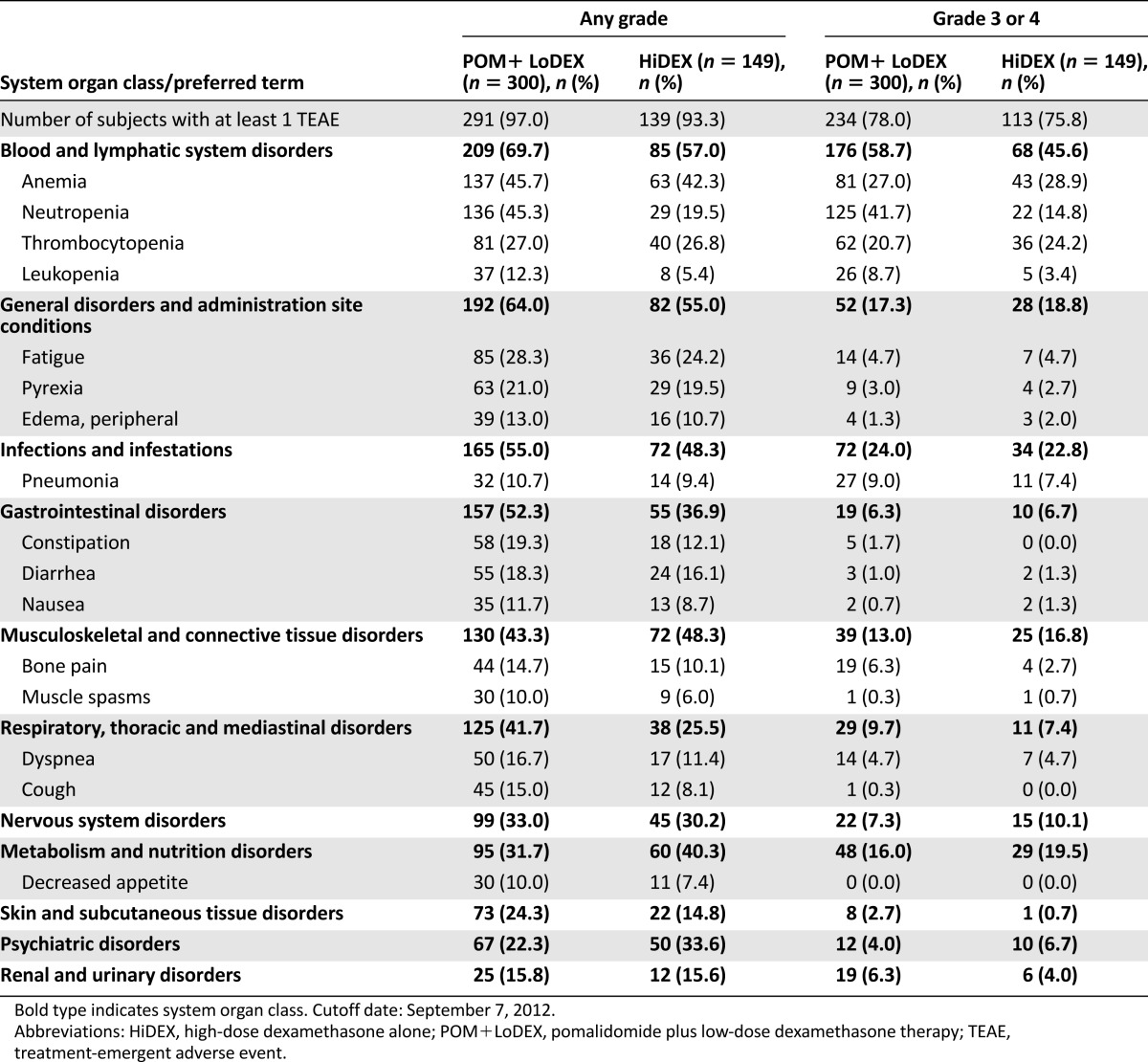
The most common adverse event (AE) was a noncumulative reversible hematology toxicity with neutropenia (the most frequent grade 3 or 4 event), anemia, and, to a lesser extent, thrombocytopenia (Table 4). Infection was the most commonly reported nonhematology toxicity and serious AE, especially pneumonia and upper respiratory tract infections. The most commonly reported serious adverse reaction was pneumonia (9.3%). Other serious adverse reactions reported included febrile neutropenia (4.0%), neutropenia (2.0%), thrombocytopenia (1.7%), and VTE (1.7%).
Table 4.
Very common treatment-emergent adverse events by any National Cancer Institute Common Toxicity Criteria of Adverse Events grade and treatment group
Important potential risks that are part of the risk management program include second primary malignancies, thyroid disorders, renal failure, QT prolongation, severe skin reactions, cardiac failure, cardiac arrhythmia, and off-label use.
Benefit-Risk Assessment
Pomalidomide used in combination with low-dose dexamethasone in the relapsed and refractory MM population at the proposed posology has been shown to be superior to the acceptable comparator high-dose dexamethasone in terms of PFS and overall survival. A benefit was also seen in patients who were refractory to several lines of therapy or who had received prior stem cell transplant; there was no apparent cross-resistance between pomalidomide and other immunomodulatory agents (82% of patients had progressed at or within 60 days of both lenalidomide- and bortezomib-based treatments). POM+LoDEX has also shown relevant efficacy in patients aged >75 years despite adjusting the dose of dexamethasone.
Pomalidomide used in combination with low-dose dexamethasone in the relapsed and refractory MM population at the proposed posology has been shown to be superior to the acceptable comparator high-dose dexamethasone in terms of PFS and overall survival.
Expected toxicity for this class of drugs such as neuropathy, thromboembolism, or constipation were also seen with pomalidomide. Pomalidomide showed greater myelosuppression compared with thalidomide or lenalidomide but had a lower risk for neuropathy than thalidomide and a lower thromboembolic risk than lenalidomide.
The safety profile of pomalidomide was considered acceptable, especially considering the relevance of the observed effects of this combination and the few therapeutic alternatives for these patients.
Additional safety data will be provided after approval through a noninterventional registry of patients treated with pomalidomide for relapsed and refractory multiple myeloma to monitor incidence of adverse reactions and implementation of and compliance with the Celgene pregnancy prevention program, off-label use, and the controlled distribution system in agreement with national competent authorities.
The pregnancy prevention program is part of the risk management plan of the medicinal product. This plan describes the set of pharmacovigilance activities and interventions designed to identify, characterize, prevent, or minimize risks relating to the medicinal product, including the assessment of the effectiveness of those interventions. Further to the risk of teratogenicity of pomalidomide, additional risk-minimization activities are being put in place including a controlled distribution system and physician and patient information packs. For further information, please refer to the product information available on the EMA website (http://www.ema.europa.eu).
Overall, the benefit-risk balance was considered positive, and a marketing authorization valid throughout the EU was granted for pomalidomide (Imnovid) in combination with low-dose dexamethasone for the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy.
Discussion
Multiple myeloma remains an incurable cancer of the bone marrow plasma cells. The overall survival of patients with multiple myeloma has increased dramatically within the past decade [14]. This is due, in part, to newer agents such as immunomodulatory drugs and proteasome inhibitors. However, once patients present with relapsed or refractory disease, treatment options have historically been very limited [15].
Pomalidomide has shown a positive benefit-risk balance in patients who have been shown to be refractory to lenalidomide and bortezomib. It is worth mentioning that carfilzomib, a second-generation proteasome inhibitor, has shown high response rates as a single agent in the relapsed and refractory setting, leading to an accelerated approval by the U.S. Food and Drug Administration for the treatment of patients with multiple myeloma who have received at least two prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression at or within 60 days of the completion of the last therapy. Carfilzomib is claimed to be associated with reversible hematological toxicity and minimal neurotoxicity [16]. This product is not authorized in the EU for the treatment of patients previously exposed to bortezomib and lenalidomide.
A discussion of pomalidomide’s place among the different treatments of multiple myeloma goes beyond the scope of the regulatory review; however, hematologists expect that pomalidomide might be a good candidate for combination therapy and for development in earlier lines of treatment [15]. Inclusion of patients in clinical trials and the use of new drug combinations have been advocated including pomalidomide; new proteasome inhibitors, such as carfilzomib, ixazomib, or oprozomib; antibodies, such as elotuzumab, daratumumab or SAR650984, siltuximab, tabalumab, denosumab, or romosozumab; Bruton tyrosine kinase and heat shock protein inhibitors; and other innovative phase I/II agents [17].
Acknowledgments
The scientific assessment as summarized in this report is based on the marketing authorization application submitted by the applicant company and on important contributions from, among others, the rapporteur and corapporteur assessment teams, Committee for Medicinal Products for Human Use members, and additional experts. This publication is a summary of the European Public Assessment Report, the summary of product characteristics, and other product information available on the EMA website. Christian Gisselbrecht and Edward Laane are members of the EMA Scientific Advisory Group on Oncology and did not participate in the EMA review of pomalidomide. Health care professionals and interested readers are referred to the EMA website for up-to-date information on this marketing authorization (http://www.ema.europa.eu). The authors remain solely responsible for the opinions expressed in this publication.
Author Contributions
Data analysis and interpretation: Beatriz Flores, Robert Hemmings, Jorge Camarero, Arantxa Sancho-Lopez
Manuscript writing: Zahra Hanaizi, Francesco Pignatti
Final approval of manuscript: Zahra Hanaizi, Beatriz Flores, Robert Hemmings, Jorge Camarero, Arantxa Sancho-Lopez, Tomas Salmonson, Christian Gisselbrecht, Edward Laane, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig H, Beksac M, Blade J, et al. Multiple myeloma treatment strategies with novel agents in 2011: A European perspective. The Oncologist. 2011;16:388–403. doi: 10.1634/theoncologist.2010-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 5.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 6.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 10.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 11.Schey SA, Fields P, Bartlett JB, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22:3269–3276. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–1967. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 14.Sant M, Minicozzi P, Mounier M, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: Results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15:931–942. doi: 10.1016/S1470-2045(14)70282-7. [DOI] [PubMed] [Google Scholar]
- 15.Faiman B, Richards T. Innovative agents in multiple myeloma. J Adv Pract Oncol. 2014;5:193–202. [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Diefenbach C, Zain J, et al. Emerging role of carfilzomib in treatment of relapsed and refractory lymphoid neoplasms and multiple myeloma. Core Evid. 2011;6:43–57. doi: 10.2147/CE.S13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Wolf IG, Straka C, Scheid C, et al. State of the art treatment of progressive or refractory multiple myeloma. Dtsch Med Wochenschr. 2014;139:2091–2095. doi: 10.1055/s-0034-1387268. [in German] [DOI] [PubMed] [Google Scholar]