Abstract
This case report discusses a patient with sickle cell disease who presented with fungemia from Pichia anomala (teleomorph: Candida pelliculosa). The organism was identified as P. anomala by MALDI-TOF VITEK mass spectrometry and VITEK 2 yeast identification card. Pichia anomala should be considered in sickle cell patients with recurrent fungemia.
Keywords: Pichia anomala, Candida pelliculosa, Wickerhamomyces, Hansenula, Sickle cell, Fungemia
Brief Report
In June 2012, a 21-year-old man presented to Grady Memorial Hospital Sickle Cell Center with fever, shortness of breath at rest, and back pain. The patient was known to have sickle cell disease with a history of priapism and avascular necrosis of the right hip. The patient had been recently admitted on multiple occasions to the Sickle Cell Center for pain crises of increasing severity and was seen regularly by the Sickle Cell Clinic for typical maintenance and outpatient titration of his pain regimen. Four months prior to this admission, the patient had a permanent intravenous access device placed. Two months prior to this admission, the patient had been admitted to the medical intensive care unit at Grady Memorial Hospital for acute chest syndrome. One month prior to admission, the patient had been admitted at this hospital for sickle cell pain crisis, and blood cultures were obtained. One of two set of blood culture from BacT/ALERT 3D system (bioMerieux, Durham, NC) was positive. The organism isolated from FAN aerobic blood culture bottle was identified as Candida pelliculosa but reported as Candida non-albicans in the laboratory information system (LIS) at Grady Memorial Hospital. The identification by VITEK2 yeast identification card (bioMerieux) was very good at 95 %. The isolate was further identified as Pichia anomala/ciferrii by using Matrix-assisted laser desorption/ionization time-offlight mass spectrometry (MALDI-TOF MS) VITEK system by bioMerieux. The confidence level was at 81.1 %. P. anomala is a teleomorph of Candida pelliculosa. Susceptibility testing was not performed on this isolate. The permanent catheter was removed and multiple diagnostic studies were performed including transesophageal echocardiogram, but no clear source of infection was identified. The patient returned home to complete a 14-day course of oral fluconazole.
Three days before admission to the hospital, the patient began experiencing recurrent low back pain which was typical of his sickle cell pain crises. Two days prior to admission, the patient developed shortness of breath, fever, and shaking chills. Finally, he came to the Grady Memorial Hospital emergency department because of worsening pain and respiratory distress. The patient also reported a 2-week history of night sweats but noted compliance with his earlier outpatient regimen of fluconazole.
On physical exam, the patient had a 3/6 systolic ejection murmur loudest over the left upper sternal border and also had bilateral crackles in the lower lung fields with egophony noted in the right lower lobe. Laboratory data were as follows: white blood cell count 11,200/μL, hemoglobin 6.8 gm/dL, platelet count 345,000/μL, sodium 136 meq/L, potassium 3.7 meq/L, glucose 98 mg/dL, creatinine 1.0 mg/dL, absolute reticulocyte count 225,000/μL, and reticulocyte 10.4 %. A portable one view chest X-ray revealed a hazy density in the left mid-lung zone, consistent with pulmonary infection. Blood cultures were obtained. The patient was started on vancomycin and piperacillin–tazobactam.
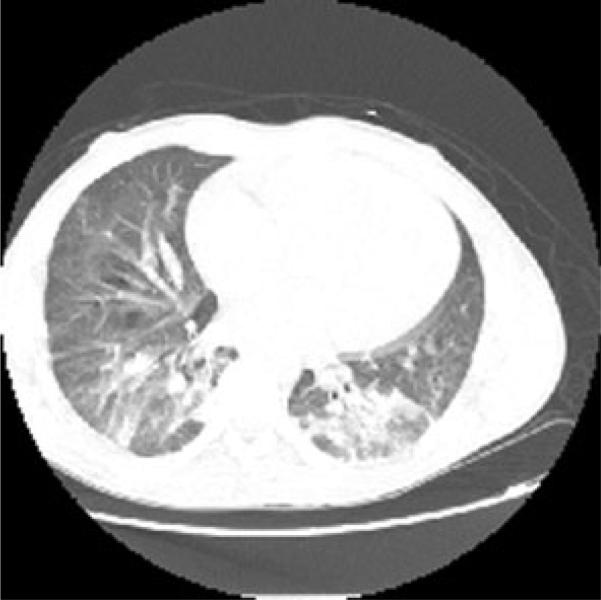
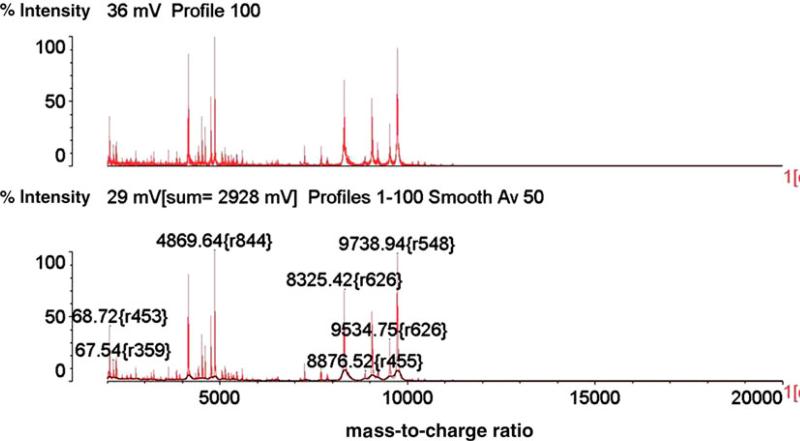
On the second day of hospitalization, a computed tomography (CT) scan was performed revealing bibasilar bronchocentric consolidative opacities with diffuse ground glass concerning for bronchopulmo-nary pneumonia/acute chest syndrome (Fig. 1). On the fifth day of hospitalization, the blood cultures were positive for yeast. The Infectious Disease service was consulted, and the patient was started on micafungin. Blood cultures obtained on therapy were positive for yeast; a transesophageal echocardiogram revealed no evidence of valvular vegetations. Only the FAN aerobic bottles cultured in the BacT/ALERT 3D system were positive. Dilated fundoscopic exam, performed on the eighth day of hospitalization, did not demonstrate any evidence of ocular involvement of the fungemia, and colonoscopy performed during hospitalization did not reveal any inflammation or masses. Isolates from positive blood cultures were identified by using the MALDI-TOF VITEK MS (VMS) as P. anomala/ciferrii with confidence level at 79.2–99.9 %. As MALDI-TOF VMS testing has been used routinely in the clinical laboratory as reported as preliminary result, speciation on the yeast revealed that it was reported as Pichia species in LIS, as opposed to Candida non-albican. The identification of the isolate was confirmed by outside laboratory (Quest Diagnostic/Nichols, Chantilly, VA) as well. By using the broth microdilution method for antifungal susceptibility testing, specifically by using the YeastOne (#YO-9) panel (Thermo Fisher Scientific TREK Diagnostic Systems, Cleveland, OH), the isolate was found to have a minimum inhibitory concentration (MIC) to micafungin of 0.12 μg/ml, caspofungin at 0.25 lg/ml, and an MIC to fluconazole of 8 lg/ml (Fig. 2).
Fig. 1.
Computed tomography scan of the chest with contrast from the second day of admission showing bibasilar bronchocentric consolidative opacities with diffuse ground glass concerning for bronchopulmonary pneumonia or acute chest syndrome and a prominent main pulmonary artery which may be seen with sickle cell disease secondary to high flow state
Fig. 2.
MALDI-TOF VITEK MS Spectrum analysis of the organism identified P. anomala/ciferrii with confidence level at from 79.2 to 99.9 %. %Int % Intensity, m/z mass-to-charge ratio
Blood cultures from the seventh day of hospitalization did not reveal any further microorganism growth. Further questioning of the patient revealed that he regularly consumed organic yogurt smoothies. A peripherally inserted central catheter was placed on the sixteenth day of admission, and the patient was returned home to complete 6 weeks of micafungin therapy given concerns for occult endovascular infection given that his blood cultures had been persistently positive initially while on treatment. Although Pichia species are often found in raw milk and cheese, we are not aware of any cases of P. anomala fungemia from ingestion of raw milk and yogurt.
Pichia anomala and its teleomorph C. pelliculosa has been documented as a pathogen in pediatric patients. P. anomala has been well described as causing serious nosocomial infections in newborn and severely immunocompromised children [1–3]. Risk factors associated with infection from P. anomala include central venous catheter placement and total parenteral nutrition [1, 3]. P. anomala fungemia has also been described in immunocompromised adult patient population including patients receiving chemotherapy [4, 5] and bone marrow transplant patients [5, 6]. All of these adult cases were associated with a chronic indwelling catheter and/ or central venous catheter use [4, 6, 7]. There are also isolated case reports of P. anomala causing fungal arthritis in a diabetic patient [8], fungal keratitis in a patient with systemic lupus erythematosus [9], and a urinary tract infection following cadaveric kidney transplant [10]. Candida pelliculosa has been described in isolated case reports as causing dacryocystitis after penetrating keratoplasty [11], fungemia following cardiac surgery [12], and fungemia in the setting of necrotizing pancreatitis [13].
The literature on the susceptibilities of P. anomala are limited, though based on the susceptibility profiles of most isolates, it appears to be most similar to that of Candida glabrata [14]. The initial reports by Klein et al. [5] noted that P. anomala was susceptible to Amphotericin B. A series of case reports by Chitasombat describes 1 isolates of P. anomala that was susceptible to the azoles, amphotericin B, and echinocandins [4]. Finally, da Matta et al. [14] were able to publish the largest susceptibility profile of P. anomala isolates from patients presenting with nosocomial fungemia. They reported MICs of 16, 1, 0.5, 0.25, and 1 to fluconazole, itraconazole, voriconazole, caspofungin, and Amphotericin B, respectively. An additional case report by Krcmery et al. described 3 C. pelliculosa isolates following cardiac surgery with MICs of>4 and>32 to voriconazole and fluconazole, respectively [12]. The P. anomala isolate appeared to be more susceptible than those noted in the literature with a slightly lower MIC to fluconazole and caspofungin.
Pichia (Hansenula) anomala recently underwent a renaming to Wickerhamomyces anomalus [15]. Under the traditional classification system of glucose fermentation, nitrate assimilation, and ascospore morphology, the genus Pichia included nearly 100 species which represented about 20 % of known ascomycetous yeasts [15]. The traditional diagnosis of the genus Pichia, based on phenotype, included the following: (1) multilateral budding on a narrow base, (2) presence or absence of hyphae and pseudohyphae, (3) ascospores that may be hat shaped, hemispheroidal, or spherical with or without a ledge, (4) sugars may be fermented and (5) nitrate is utilized by some species as a sole source of nitrogen [15]. Following the advent of genomic sequencing, there have been major changes in the classification of yeasts which has allowed mycologists to group yeasts based on their phylogenetic relatedness. The decision was made to conserve the name ‘anomalus’ based on the fact that it was so widely used. However, justification for the conservation of the genus Hansenula was less robust as many of the species formerly classified in Hansenula and Pichia were now resolved into four major clades from multigene phylo-genetic analysis, one of which is Wickerhamomyces. The teleomorph form C. pelliculosa is also known under the heterotypic synonym of Candida beverwijkiae [16], having undergone a renaming of its own in 1965; however, the current medical literature prefers the 1925 name of C. pelliculosa as all current case reports and reports of outbreak are listed under that nomenclature.
After searching the literature, there are currently no documented cases of P. anomala causing fungemia in the sickle cell population. Although no source was identified, we postulate that the patient's fungemia at the time of admission represented the same entity that was previously grown on the prior admission given the dosage of the fluconazole therapy and the relatively high fluconazole MIC (8 μg/ml) of the organism grown on the current admission. The patient possessed the risk factor of an indwelling intravenous catheter at the time of the initial fungemia. For the 1-month period following the removal of the catheter, it is likely that the patient had a persistent, subclinical fungemia. Alternatively, if this episode of fungemia represented an entirely new infection, then it would represent a case of P. anomala fungemia in the absence of a chronic indwelling intravenous catheter with no source other than yogurt ingestion. Our case report demonstrates the continued importance of organism identification and differentiation of ascomycetous yeast species, specifically in high risk patient populations such as those with total parenteral nutrition, chronic indwelling intravenous access, and compromised immune systems. With a growing number of fungal pathogens in these patient populations that are not reliably sensitive to azoles [5], it is increasingly necessary to cultivate a healthy suspicion for azole-resistant Candida species and non-Candida organisms that require further susceptibility testing.
Acknowledgments
The authors received financial support from CSK—NIH/NCRR KL2 TR000455.
Footnotes
Conflict of interest None.
Contributor Information
Austin W. Chan, Department of Medicine, Emory University, Atlanta, GA, USA
Emily J. Cartwright, Division of Infectious Diseases, Emory University, Atlanta, GA, USA
Sujan C. Reddy, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, USA
Colleen S. Kraft, Division of Infectious Diseases, Emory University, Atlanta, GA, USA Department of Pathology and Laboratory Medicine, Emory University, Atlanta, GA, USA.
Yun F. Wang, Department of Pathology and Laboratory Medicine, Emory University, Atlanta, GA, USA Clinical Laboratory, Grady Memorial Hospital, Room 1C049C, 80 Jesse Hill Dr. SE, P.O. Box 26248, Atlanta, GA 30303, USA.
References
- 1.Aragao PA, Oshiro IC, Manrique EI, Gomes CC, Matsuo LL, Leone C, et al. Pichia anomala outbreak in a nursery: exogenous source? Pediatr Infect Dis J. 2001;20(9):843–8. doi: 10.1097/00006454-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bakir M, Cerikcioglu N, Tirtir A, Berrak S, Ozek E, Canpolat C. Pichia anomala fungaemia in immunocompromised children. Mycoses. 2004;47(5–6):231–5. doi: 10.1111/j.1439-0507.2004.00962.x. doi:10.1111/j.1439-0507.2004.00962.x. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Singh K, Narang A, Singhi S, Batra R, Rao KL, et al. Outbreak of Pichia anomala infection in the pediatric service of a tertiary-care center in Northern India. J Clin Microbiol. 2001;39(5):1702–6. doi: 10.1128/JCM.39.5.1702-1706.2001. doi:10.1128/JCM.39.5.1702-1706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitasombat MN, Kofteridis DP, Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Infect. 2012;64(1):68–75. doi: 10.1016/j.jinf.2011.11.002. doi:10.1016/j.jinf.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein AS, Tortora GT, Malowitz R, Greene WH. Hansenula anomala: a new fungal pathogen. Two case reports and a review of the literature. Arch Intern Med. 1988;148(5):1210–3. doi: 10.1001/archinte.148.5.1210. [DOI] [PubMed] [Google Scholar]
- 6.Goss G, Grigg A, Rathbone P, Slavin M. Hansenula anomala infection after bone marrow transplantation. Bone Marrow Transplant. 1994;14(6):995–7. [PubMed] [Google Scholar]
- 7.Haron E, Anaissie E, Dumphy F, McCredie K, Fainstein V. Hansenula anomala fungemia. Rev Infect Dis. 1988;10(6):1182–6. doi: 10.1093/clinids/10.6.1182. [DOI] [PubMed] [Google Scholar]
- 8.Choi SW, Lee TJ, Kim MK, Lee M, Jung JH. A case of fungal arthritis caused by Hansenula anomala. Clin Orthop Surg. 2010;2(1):59–62. doi: 10.4055/cios.2010.2.1.59. doi:10.4055/cios.2010.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KA, Ahn K, Chung ES, Chung TY. Pichia anomala fungal keratitis. Cornea. 2008;27(5):619–20. doi: 10.1097/ICO.0b013e318166c442. doi:10.1097/ICO.0b013e318166c442. [DOI] [PubMed] [Google Scholar]
- 10.Qadri SM, Al Dayel F, Strampfer MJ, Cunha BA. Urinary tract infection caused by Hansenula anomala. Mycopathologia. 1988;104(2):99–101. doi: 10.1007/BF00436934. [DOI] [PubMed] [Google Scholar]
- 11.Hanada K, Miyokawa N, Sano A, Igarashi S, Yoshida A. Fungal dacryocystitis with cacosmia after penetrating keratoplasty–taxonomy and identification of pathogenic fungi based on DNA sequence analysis. Nippon Ganka Gakkai zasshi. 2012;116(12):1144–9. [PubMed] [Google Scholar]
- 12.Krcmery V, Kisac P, Liskova A. Voriconazole and posaconazole resistant Candida pelliculosa fungemia after cardiac surgery. Pediatr Infect Dis J. 2009;28(1):75–6. doi: 10.1097/INF.0b013e31818edde1. doi:10.1097/INF.0b013e31818edde1. [DOI] [PubMed] [Google Scholar]
- 13.Neumeister B, Rockemann M, Marre R. Fungaemia due to Candida pelliculosa in a case of acute pancreatitis. Mycoses. 1992;35(11–12):309–10. doi: 10.1111/j.1439-0507.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 14.da Matta VL, de Melhem MSC, Colombo AL, Moretti ML, Rodero L, Duboc de Almeida GM, et al. Antifungal drug susceptibility profile of Pichia anomala isolates from patients presenting with nosocomial fungemia. Antimicrob Agents Chemother. 2007;51(4):1573–6. doi: 10.1128/AAC.01038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtzman CP. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to Wickerhamomyces anomalus. Antonie Van Leeuwenhoek. 2011;99(1):13–23. doi: 10.1007/s10482-010-9505-6. doi:10.1007/s10482-010-9505-6. [DOI] [PubMed] [Google Scholar]
- 16.Novak EK, Vitez I. Mycological investigations on clinical materials. II. Description of new yeasts. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene 1 Abt Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie Originale. 1964;193(1):127–33. [PubMed] [Google Scholar]