Abstract
Sepsis is a systemic, deleterious inflammatory host response triggered by an infective agent leading to severe sepsis, septic shock and multi-organ failure. The host response to infection involves a complex, organized and coherent interaction between immune, autonomic, neuroendocrine and behavioral systems. Recent data have confirmed that disturbances of the autonomic nervous and neuroendocrine systems could contribute to sepsis-induced organ dysfunction.
Through this review, we aimed to summarize the current knowledge about the endocrine dysfunction as response to sepsis, specifically addressed to vasopressin, copeptin, cortisol, insulin and leptin. We searched the following readily accessible, clinically relevant databases: PubMed, UpToDate, BioMed Central.
The immune system could be regarded as a “diffuse sensory organ” that signals the presence of pathogens to the brain through different pathways, such as the vagus nerve, endothelial activation/dysfunction, cytokines and neurotoxic mediators and the circumventricular organs, especially the neurohypophysis. The hormonal profile changes substantially as a consequence of inflammatory mediators and microorganism products leading to inappropriately low levels of vasopressin, sick euthyroid syndrome, reduced adrenal responsiveness to ACTH, insulin resistance, hyperglycemia as well as hyperleptinemia.
In conclusion, clinical diagnosis of this “pan-endocrine illness” is frequently challenging due to the many limiting factors. The most important benefits of endocrine markers in the management of sepsis may be reflected by their potential to be used as biomarkers in different scoring systems to estimate the severity of the disease and the risk of death.
Keywords: Sepsis, endocrine dysfunction, hormones, inflammation, host response
Introduction
Sepsis is a systemic, deleterious inflammatory host response triggered by an infective agent leading to severe sepsis, septic shock and multi-organ failure.1,2 With an increasing incidence, severe sepsis and septic shock are major health care problems worldwide affecting millions of people each year.1 There are 750,000 cases of severe sepsis diagnosed every year in the United States, representing the main reason for intensive care unit admission with a mortality rate reported as high as 30–50% in developed countries.2 The pathogenesis of severe sepsis and septic shock has been explained by the excessive production of cytokines, endothelial activation and microvascular thrombosis.3 The host response to infection involves a complex, organized and coherent interaction between immune, autonomic, neuroendocrine and behavioral systems.4 Recent data have confirmed that disturbances of the autonomic nervous and neuroendocrine systems could contribute to sepsis-induced organ dysfunction, and several studies have correlated the degree of neuroendocrine dysfunction with severity of illness.4,5 The clinical features of the endocrine disorder in sepsis are variable, making it difficult to distinguish between a beneficial and a deleterious response to the aggression of pathogens.5 Moreover, the accuracy of laboratory diagnosis has several limitations5 related to circadian secretion and pharmacokinetic properties of different hormones as well as specific requirements of sampling, processing and storing blood specimens.
Through this review, we aimed to summarize the current knowledge about the endocrine dysfunction as response to sepsis. We focused on the pathophysiology of endocrine dysfunction during sepsis, specifically addressed to vasopressin, copeptin, cortisol, insulin and leptin.
Search criteria
For the preparation of this review we searched through the following readily accessible, clinically relevant databases: PubMed, UpToDate, BioMed Central, using different search keywords as neuroendocrine dysfunction in sepsis, copeptin, leptin, insulin and thyroid dysfunction in sepsis. We selected the publications (original or review articles, book chapters and abstracts) which matched our review objectives.
Literature review
Interrelation between the central nervous system and the immune system during sepsis
Sepsis is one of the most stressful conditions encountered by humans and animals.6 Stress is defined as anything that disrupts the body homeostasis and comprises a set of complex biological disturbances induced by aggression on the host defense systems.6,7 Vertebrates are endowed with two systems for the rapid recognition of threats and mobilization of host defenses.3 The central nervous system (CNS) recognizes macroscopic threats to survival and activates physiologic responses.3 The innate immune system identifies the microscopic aggressors, such as pathogenic microorganisms, and activates cellular and humoral mechanisms to counteract the germs invasion.3 Reciprocal interactions between the CNS and the immune system are considered essential parts of the host response during septic shock.4 The immune system could be regarded as a “diffuse sensory organ” that signals the presence of pathogens to the brain through different pathways, such as the vagus nerve, endothelial activation/dysfunction which leads to release or passive diffusions of cytokines and neurotoxic mediators, and the circumventricular organs, especially the neurohypophysis.4 These afferent signals trigger efferent CNS responses leading to the activation of the autonomic nervous system and, most importantly, the hypothalamic-pituitary-adrenal (HPA) axis.7 The hormonal cascade involves the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the hypothalamic paraventricular and supraoptic nuclei.4,7 It is suggested that AVP potentiates the action of CRH at the level of the pituitary gland. Thus, both AVP and CRH stimulate the release of adrenocorticotropic hormone (ACTH), which in turn is responsible for the secretion of cortisol from the adrenal cortex.7
The neuroendocrine response to a stressor is heterogeneous due to the various factors, such as the type of stressor, the known or unknown threat, short or persistent exposure and the characteristics of the host (the most relevant being age and health status).7
Hypothalamic-pituitary-adrenal axis in sepsis
The HPA axis is the main neuroendocrine structure involved in modulating the adaptive response to different stressors. It works through the interconnection of the sympathoadrenal and neurohypophyseal systems, which in turn are responsible for catecholamine secretion, cytokines activation and vasopressin release, respectively.5
The final step of the HPA axis activation is characterized by an increase in cortisol secretion from the adrenal cortex, usually proportional to the severity of stress.6 Two different patterns of anterior pituitary gland response have been observed during severe sepsis. Initially, an acute, adaptive and presumed beneficial response is generated, most probably explained by the release of hormones from storages. Subsequently, due to the persistence of microbial aggression, a chronic response is elicited, which ultimately leads to the exhaustion of the neuroendocrine system, which could have a deleterious effect on the host.8 A prolonged septic condition causes a blunting of HPA axis activation, leading to transient or permanent adrenal insufficiency in critically ill patients.9
The HPA axis dysfunction in sepsis is generated by complex and multifactorial mechanisms, resulting in either an absolute deficiency of serum hormones levels (decreased production of CRH, ACTH and cortisol), or a relative hormonal deficiency caused by dysfunction of their receptors.5 The final outcome of the HPA axis dysfunction is a syndrome of adrenal insufficiency. Recently, the term “critical illness-related corticosteroid insufficiency” (CIRCI) has been adopted, to replace absolute or relative adrenal deficiency.10 The definition of CIRCI comprises the discrepancy between the low level of corticosteroid activity compared to the severity of illness.5 In field literature, the observed incidence of CIRCI varies from one study to another, between 10% and 70% in patients with severe sepsis.5 The laboratory diagnosis of CIRCI is reflected by the increase of the cortisol level less than 9 µg/dL at 30–60 minutes after stimulation test with a lower dose of ACTH (1 µg) instead of the high classical dose (250 µg).5,10–12 The clinical diagnosis of CIRCI has benefits for clinical practice as it seems to be associated with more frequent use of vasopressors and a worse prognosis.13
To assess the integrity of the HPA axis, an ideal approach is based on CRH, AVP and cortisol measurements, but the accuracy of the results is questionable because of confounding factors. Both CRH and AVP are released in a pulsatile pattern, are unstable especially at room temperature, and have a very short plasmatic half time.7
Vasopressin
One of the major hypothalamic stress hormones, which is stimulated by different stressors, is AVP.7 AVP, a nonapeptide, also known as antidiuretic hormone, derives from a larger 164-amino acid precursor peptide (preprovasopressin) consisting of a signal peptide, AVP, neurophysin II and copeptin (Figure).14–17
Figure. The structure of the preprovasopressin with the main constituent domains: Signal peptide, AVP, neurophysin II and copeptin (aa, aminoacid position; AVP, arginine vasopressin).
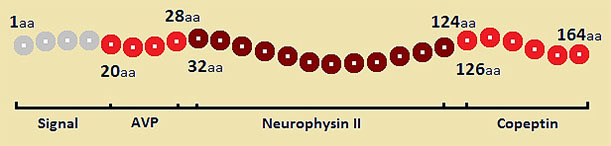
AVP synthesis comprises several steps of enzymatic cleavage of the precursor preprovasopressin synthetized in the magnocellular neurons of the hypothalamus. The process starts with the removal of the signal peptide resulting provasopressin, which in turn is packaged into neurosecretory vesicles and the enzymatic cleavage continues during axonal transport to the posterior pituitary.16,17 This process is usually completed at the level of the neurohypophysis, and it occurs in two steps: a first cleavage splits off AVP, and a second cleavage separates neurophysin II from copeptin.17
Synthesis of AVP is mainly mediated by variations in blood volume, blood pressure and osmolality, thereby contributing to the regulation of osmotic and cardiovascular homeostasis.15 Once released into the circulation, AVP exerts its peripheral effects through three different receptors: V1a, V1b and V2.17 V1a receptor is responsible for arteriolar vasoconstriction induced by AVP and is expressed on vascular smooth muscle, hepatocytes and platelets.16 V1b receptor, mainly central, is expressed in the anterior pituitary gland and hippocampus, and its activation by AVP releases ACTH. Thereby, AVP interacts with the corticosteroid axis in response to different stressors.16 V2 receptor, expressed in the renal collecting duct, mediates AVP's antidiuretic effect by increasing water retention through aquaporin-2 water channels.16,17
It has been noted that in the very early stages of sepsis (within 15 minutes of initiation of sepsis) a massive release of AVP, to supraphysiologic levels, occurs.16,18 Several mechanisms have been postulated to explain the AVP release so quickly after the onset of sepsis, such as the existence of stimuli other than hypotension, like proinflammatory cytokines and bacterial products (lipopolysaccharides).16 Following the early release of AVP in septic shock, the serum levels of AVP become inadequate compared to the illness severity due to the depletion of deposits along with the sustained decreasing of AVP synthesis.16,19 In sepsis, one of the main patterns of endocrine response is defined by the rapidity of activation followed by the exhaustion with the same rapidity.20
The measurement of endogenous AVP plasmatic levels could be a useful tool to guide therapy in pathologies where osmotic and cardiovascular homeostasis is disturbed, such as severe sepsis and septic shock.5,14 The detection of endogenous AVP deficiency is limited by the fact that the mature hormone is unstable in isolated plasma and serum, it has a short half-life (24 minutes in vivo) and more than 90% of AVP circulates attached to platelets.5,14 There are currently no recommendations for routine testing for endogenous AVP levels in the setting of sepsis.5
Crystalloids are indicated as the initial fluid of choice in the resuscitation of severe sepsis and septic shock, and catecholamine infusion, especially norepinephrine (NE), remains the first line vasopressor agent of choice in adults with septic shock to target a mean arterial pressure (MAP) of 65 mmHg.1,5 The current guidelines for sepsis make clear recommendations regarding the use of AVP: it should be used in combination with NE in order to raise the MAP or to reduce the NE dosage; low doses of AVP should not be used as the single initial vasopressor for treatment of hypotension; higher doses, over 0.03–0.04 units/minute, should be reserved for salvage therapy.1
Copeptin
Copeptin, a 39-amino-acid glycopeptide, represents the C-terminal fragment of the provasopressin peptide (CTproAVP).14,20 Copeptin is released in an equimolar ratio to AVP and is more stable in the blood and easy to determine.7,14,20 The exact role of copeptin has not been elucidated yet. However, it has been suggested that copeptin may play a role in the process of AVP precursor production.21 There are some relevant advantages of copeptin, as it reliably mirrors the production of AVP, it closely reflects the individual stress level compared to cortisol and, unlike AVP, it is extremely stable in plasma or serum ex vivo.7,14,17 Due to the positive association of copeptin with the severity of illness and outcome, copeptin has been proposed as a more sensitive and potential prognostic biomarker in sepsis.17 The role of copeptin as a prognostic biomarker has been investigated in various infectious conditions, such as sepsis, pneumonia, lower respiratory tract infections, and it was found to accurately discriminate between patients with favorable and unfavorable outcomes.7
In patients with sepsis, copeptin concentration increases in parallel with the severity of the disease, reaching levels more than 30-fold higher in septic shock than in healthy individuals.22
In this context, copeptin appears to have an interesting potential as a new prognostic biomarker.7 However, there are some limitations of copeptin use as a single biomarker.7 Copeptin levels may be decreased by exogenous corticosteroid usage in a dose-dependent way, and, on the other hand copeptin levels are higher in patients with renal insufficiency.7
Cortisol
The normal response of the HPA axis to septic aggression consists in the release of cortisol from the adrenal cortex.5
Cortisol is important in maintaining vascular reactivity to circulating catecholamines through up-regulation of adrenergic receptors and inhibition of nitric oxide synthase, prostaglandin E1 and prostacyclin, thus preserving perfusion of vital organs.6,7 It is already known that, in sepsis, adrenal insufficiency is partly responsible for reduction of vascular reactivity to vasopressors and is associated with an increased risk of death.4 In the setting of severe sepsis and septic shock, defining adrenal function based on cortisol serum levels is challenging due to the confounding factors.7 An accurate cortisol level is dependent on the integrity of the anterior pituitary and adrenal gland.7 In addition, it is well known that existing commercially assays are able to quantify only the total level of serum cortisol.23 On the other hand, the serum cortisol is largely (90%) bound to albumin and cortisol-binding globulin, underscoring the difficulties of interpreting the results especially in hypoalbuminemic patients.24 As adrenal insufficiency can occur in severe sepsis, there are some expectations related to steroid use in this setting such as attenuation of the systemic inflammation and cytokine activation and improvement of the hemodynamic function.7 The main issues of the exogenous steroids usage in sepsis are related to an increased risk of morbidity secondary to superinfection, myopathy, hyperglycemia and hypernatremia.1,7 More data support the role of corticosteroids in reversing shock and decreasing mortality mainly in patients with sepsis-induced hypotension refractory to fluid replacement and vasopressor therapy.25 The 2012 Surviving Sepsis Campaign recommended administration of intravenous hydrocortisone alone at a dose of 200 mg/day as a treatment of adult septic shock patients only if adequate fluid resuscitation and vasopressor therapy are not able to restore hemodynamic stability.1 Another controversy is related to the use of ACTH stimulation test to identify the subset of adults with septic shock who should receive hydrocortisone. Currently, its use is not recommended for the purposes of identifying patients who may benefit from steroid therapy.1 The response of septic shock patients to fluid and vasopressor therapy seems to be an important factor in selection of patients for optional hydrocortisone therapy.1 Although the clinical impact is not clear, it is now recognized that etomidate or ketoconazole are potential suppressors of the HPA axis, and their use in septic patients should be avoided.1,7
Glucose metabolism in sepsis
Patients with diabetes mellitus have an increased risk of developing infections and sepsis, and constitute 20.1–22.7% of all sepsis patients.26 Hyperglycemia, glucose intolerance and insulin resistance are common features of patients with sepsis or septic shock. There are various mechanisms involved in this setting such as cytokine-related insulin resistance, action of circulating counter-regulatory hormones, activation of hepatic glycogenolysis and gluconeogenesis, hepatic clearance of serum lactate in the Cori cycle and medications (glucocorticoids).5,6,27 Hyperglycemia is a marker of severity of illness and is a predictor of poor outcome.6 It has been observed that septic diabetic patients have a better prognosis compared to patients without known diabetes and hyperglycemia. This is referred to as paradox diabetes phenomenon.28 Also, hyperglycemia has been independently associated with mortality in non-diabetic septic individuals.29 Proposed mechanisms by which hyperglycemia increases morbidity and mortality in sepsis include abnormalities of the host response, especially reduced chemotaxis and phagocytosis, increased concentration of pro-inflammatory cytokines (interleukin(IL)-1, IL-6, and tumor necrosis factor (TNF)α), formation of oxygen free radicals, prothrombotic effects, and impairment in the generation of endothelial nitric oxide.5,6,26
The rationale for insulin use in hyperglycemic septic states is based mainly on its direct anti-inflammatory role and its inhibitory effect on glycogen synthase kinase 3β.5,6 However, current evidence states that intensive insulin therapy (IIT) with target glucose between 80–110 mg/dL compared to a moderately hyperglycemic goal (140–180 mg/dL) offered no apparent benefits, but instead increased the risk of hypoglycemia.30–32 The latest Surviving Sepsis Campaign recommended commencing insulin dosing when two consecutive blood glucose levels are >180 mg/dL.1 Less restrictive upper target glucose values (≤180 mg/dL) appear safer than an upper target blood glucose ≤110 mg/dL (grade 1A).1
Leptin
Although initially considered as a simple storage of fat, increasing evidence drew attention to fat as an immune-endocrine organ that interacts with other physiological systems.33 Leptin is a hormone/cytokine synthetized by the white adipose tissue, with various functions such as regulation of appetite and energy expenditure via the hypothalamic effects on satiety, in addition to regulation of the immune system and systemic inflammatory response, reproduction, glucose homeostasis, hematopoiesis, angiogenesis and wound healing.33–35 During states of normal nutrition, the circulating leptin levels are directly correlated with the adipose tissue mass.36 The biological effects of leptin are mediated through the activation of its various receptors expressed in the brain and peripheral tissues such as pancreas, liver, adipose tissue and immune cells (neutrophils, monocytes, macrophages, subpopulations of T and B cells, mast cells, dendritic cells and natural killer cells).33,36 One of the six isoforms of the leptin receptor (Ob-R) is secreted in plasma and serves as a leptin-binding protein.33 Leptin circulates both as a biologically active free form and a presumably inactive form bound to plasma proteins and secreted receptor isoform (Ob-Re).37 Leptin levels correlate closely with body-mass index. This is observed in obese individuals who are leptin-resistant and have high serum levels of free active leptin.37
It was observed that levels of leptin increase early during sepsis, consistent with the stimulation of the leptin messenger RNA expression triggered by lipopolysaccharides and cytokines such as TNF-α, IL-6, IL-1β.37 Moreover, the increased serum leptin concentration correlates with sepsis severity, and it is thought that leptin could play a role in the pathogenesis of sepsis-associated multi-organ failure.34 On the other hand, the absence of leptin appears to be associated with an impaired immune response which leads to an increasing mortality due to infections.33 There is strong evidence that leptin is also involved in cell-mediated immunity and cytokine crosstalk.38 Furthermore, leptin has been shown to modulate innate and adaptive immune response towards the Th1 phenotype characterized by the secretion of type I cytokines (TNF-α, IL-2, interferon-γ).37 In 2010, Yousef et al. published a prospective observational study which confirmed a positive correlation between leptin and IL-6 and TNF-α in patients with sepsis.39 The same study concluded that elevated leptin levels can distinguish between sepsis and non-infectious systemic inflammatory response syndrome with a sensitivity of 91.2% and a specificity of 85% for a threshold of 38 µg/L.39
Hypothalamo-pituitary-thyroid axis in sepsis
Critical illness, such as sepsis, is often associated with thyroid hormone abnormalities.6,40 It is believed that the thyroid dysfunction observed during sepsis constitutes part of an adaptive metabolic response in an attempt to increase resistance to different stressors by lowering the cellular metabolic activity.41 The prevalence of thyroid abnormalities in the general population varies between 1% and 10%, which could explain the presence of various forms of thyroid dysfunction in some patients with sepsis.41 Thyroid dysfunction in sepsis is often defined by the low T3 syndrome, euthyroid sick syndrome or nonthyroidal illness syndrome.6 The initial abnormality is a decrease in total T3 concentration secondary to a decrease of peripheral conversion of thyroxine (T4) to T3 by reducing 5′-deiodinase activity.6 Typically, an initially low level of total T3 is followed by a decline in total T4 caused by a decrease in thyroxin-binding globulin as well as a reduction in its binding affinity.6 With increasing severity of illness the levels of total T4, free T4 and TSH may also decrease.40 The hypothalamic-pituitary-thyroid axis malfunction could be attributed to various cytokines.40 Several inflammatory cytokines (IL-1β, IL-6, and TNF-α) can directly or indirectly suppress thyroid function at different levels, leading to a reduction in thyrotropin-releasing hormone (TRH) secretion at the level of the hypothalamus or a direct suppression of TSH release.6,41
The majority of existing data suggest a correlation between lower levels of baseline T3 or T4 and worse outcome.40,41 There is little evidence regarding thyroid hormone substitution in human critical illness.41 Some studies have shown that T4 substitution can result in reduction in the need for vasopressors in circulatory shock.42 Follow-up thyroid function tests are recommended if abnormal levels are detected during sepsis, as usually most of these abnormalities are transient and do not represent a true underlying thyroid disease.5,41
Conclusion
In summary, these data have clearly shown that endocrine dysfunction is a common feature in septic patients (Table).
Table. Summary data on the different endocrine responses during sepsis and potential implications for sepsis management.
| Hormone | The type of endocrine response in sepsis and specific requirements |
| Vasopressin | Activation and depression of hypothalamic-pituitary-adrenal (HPA) axis with biphasic vasopressin secretory response and inappropriately low levels compared to the severity of illness; it is not recommended as first line vasopressor and should be used in low dose in addition to vasopressors (norepinephrine) |
| Copeptin | Copeptin concentration increases in parallel with the severity of the disease and accurately discriminates between patients with favorable and unfavorable outcomes; it reliably mirrors the production of arginine vasopressin, closely reflects the individual stress level compared to cortisol and is extremely stable in plasma or serum ex vivo |
| Cortisol | Activation and depression of HPA axis which leads to critical illness-related corticosteroid insufficiency; it is associated with poor outcome in sepsis; it is recommended to use intravenous hydrocortisone alone in patients with sepsis-induced hypotension refractory to fluid replacement and vasopressor therapy |
| Insulin | There is a tendency to hyperglycemia; it is a marker of severity of illness and a predictor of poor outcome in patients without previous diabetes; a tight glycemic control is not recommended |
| Leptin | It increases early during sepsis and correlates with sepsis severity; it could play a role in the pathogenesis of sepsis-associated multi-organ failure through pro-inflammatory actions |
| Thyroid hormones | Depression of thyroid function is generally a transient event, but it seems to be associated with a worse prognosis; it does not require specific treatment |
The endocrine system acts as an innate defense mechanism against threats, such as invasion of microorganisms, being timely activated in individuals with severe sepsis and septic shock. Thereafter, with the same quickness, certain endocrine systems are exhausted resulting in absolute or relative hormonal deficiency corresponding to the severity of illness. The hormonal profile changes substantially as a consequence of inflammatory mediators and microorganism products leading to inappropriately low vasopressin levels, sick euthyroid syndrome, reduced adrenal responsiveness to ACTH, insulin resistance, hyperglycemia as well as hyperleptinemia. Although it seems that endocrine dysfunction has a deleterious effect on the host and contributes to the pathogenesis of sepsis-associated organ dysfunction, there are only few therapeutic recommendations widely accepted. Moreover, clinical diagnosis of this “pan-endocrine illness” is frequently challenging due to the confounders related to pharmacokinetics of hormones, sample variability, drug interactions, circadian rhythm and different laboratory assays. The most important benefits of endocrine markers in the management of sepsis may be reflected by their potential to be used as biomarkers in different scoring systems to estimate the severity of the disease and the risk of death.
Acknowledgment
This work received financial support through the project entitled “CERO–career profile: Romanian Researcher”, grant number POSDRU/159/1.5/S/135760, cofinanced by the European Social Fund for Sectorial Operational Programme Human Resources Development 2007-2013.
Footnotes
References
- 1.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Wang B, Xu Y, et al. Diagnostic value of serum leptin and a promising novel diagnostic model for sepsis. Exp Ther Med. 2014;7:881–6. doi: 10.3892/etm.2014.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munford RS, Tracey KJ. Is severe sepsis a neuroendocrine disease? Mol Med. 2002;8:437–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Sharshar T, Hopkinson NS, Orlikowski D, Annane D. Science review: The brain in sepsis – culprit and victim. Crit Care. 2005;9:37–44. doi: 10.1186/cc2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choong K. Hormonal therapies in severe sepsis. In: Fernandez R, editor. Severe sepsis and septic shock - understanding a serious killer. InTech. pp. 359–78. [Google Scholar]
- 6.Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96:1095–105. doi: 10.1016/j.mcna.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. doi: 10.4414/smw.2010.13101. [DOI] [PubMed] [Google Scholar]
- 8.Van der Berghe G, de Zegher F, Bouillon R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83:1827–34. doi: 10.1210/jcem.83.6.4763. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–34. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–49. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 11.Menon K, Ward RE, Lawson ML, et al. A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med. 2010;182:246–51. doi: 10.1164/rccm.200911-1738OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tordman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf) 2000;52:633–40. doi: 10.1046/j.1365-2265.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 13.Lipiner-Frieman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective Corticus study. Crit Care Med. 2007;35:1012–8. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 14.Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26:2500–04. doi: 10.1016/j.peptides.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Palmiere C, Augsburger M. Copeptin as a diagnostic biomarker for sepsis-related deaths. Peptides. 2014;59:75–8. doi: 10.1016/j.peptides.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Russell JA. Bench-to-bedside review: Vasopressin in the management of septic shock. Crit Care. 2011;15:226. doi: 10.1186/cc8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–9. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Wilson MF, Brackett DJ, Tompkins P, Benjamin B, Archer LT, Hinshaw LB. Elevated plasma vasopressin concentrations during endotoxin and E. coli shock. Adv Shock Res. 1981;6:15–26. [PubMed] [Google Scholar]
- 19.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–87. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 20.Latronico N, Castioni CA. Copeptin in critical illness. Clin Chem Lab Med. 2014;52:1391–93. doi: 10.1515/cclm-2014-0529. [DOI] [PubMed] [Google Scholar]
- 21.Barat C, Simpson L, Breslow E. Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry. 2004;43:8191–203. doi: 10.1021/bi0400094. [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28:219–26. doi: 10.1097/SHK.0b013e318033e5da. [DOI] [PubMed] [Google Scholar]
- 23.Ho J, Al-Musalhi H, Chapman M, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–14. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
- 24.Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–45. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 25.Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009;15:308–18. doi: 10.1111/j.1469-0691.2009.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh GCKW, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31:379–88. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care. 2006;12:315–21. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 28.Krinsley JS, Fisher M. The diabetes paradox: diabetes is not independently associated with mortality in critically ill patients. Hosp Pract (1995) 2012;40:31–5. doi: 10.3810/hp.2012.04.967. [DOI] [PubMed] [Google Scholar]
- 29.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 30.Brunkhorst F, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 31.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 32.Devos P, Preiser JC, Melot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycemia: final results of the Glucontrol study. Intensive Care Med. 2007;33:S189. [Google Scholar]
- 33.Paz-Filho G, Mastronardi C, Franco BC, Wang KB, Wong ML, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metab. 2012;56:597–607. doi: 10.1590/s0004-27302012000900001. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro NI, Khankin EV, Van Meurs M, et al. Leptin exacerbates sepsis-mediated morbidity and mortality. J Imunol. 2010;185:517–24. doi: 10.4049/jimmunol.0903975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behnes M, Brueckmann M, Lang S, et al. Alterations of leptin in the course of inflammation and severe sepsis. BMC Infect Dis. 2012;12:217. doi: 10.1186/1471-2334-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch A, Weiskirchen R, Zimmermann HW, Sanson E, Trautwein C, Tacke F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediators Inflamm. 2010;2010:473540. doi: 10.1155/2010/473540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 38.Bracho-Riquelme RL, Reyes-Romero MA. Leptin in sepsis: a well-suited biomarker in critically ill patients? Crit Care. 2010;14:138. doi: 10.1186/cc8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumour necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care. 2010;14:R33. doi: 10.1186/cc8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S, Dabla PK, Kumar S, Dublis S. Thyroid hormone dysfunction and CRP levels in neonates with sepsis. J Endocrinol Metab. 2013;3:62–6. [Google Scholar]
- 41.Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. Association between thyroid function test at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. 2011;164:147–55. doi: 10.1530/EJE-10-0695. [DOI] [PubMed] [Google Scholar]
- 42.Zuppa AF, Nadkarni V, Davis L, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004;32:2318–22. doi: 10.1097/01.ccm.0000146133.52982.17. [DOI] [PubMed] [Google Scholar]


