Abstract
Background
During pregnancy and labor, the immune response is physiologically impaired and women are more susceptible to infections. Since many drugs may have potentially adverse effects on the fetus and newborn, less aggressive treatment regimens should be considered in pregnant and lactating patients. The aim of our study was to present the management of toxoplasmic retinochoroiditis during pregnancy, postpartum period, and lactation.
Material/Methods
A retrospective study was undertaken of the clinical records of 24 women during pregnancy, postpartum period, and lactation who were referred in the years 1994–2014 to the Department of Zoonoses and Tropical Diseases or the Department of Ophthalmology, Medical University of Warsaw for toxoplasmic retinochoroiditis. The diagnosis was based on the typical ophthalmoscopic picture, confirmed by serological testing using an ELISA method.
Results
A total of 28 attacks of toxoplasmic retinochoroiditis were observed in 24 patients during pregnancy, postpartum period, and lactation. The choice of treatment was guided by the character and location of the inflammatory lesion and the gestational age. Topical (steroidal/nonsteroidal eye drops) and systemic treatments with spiramycin or azithromycin, Fansidar (pyrimethamine 25 mg/sulfadoxine 500 mg), and prednisone were used.
Conclusions
Management of toxoplasmic retinochoroiditis during pregnancy, postpartum period, or lactation must be individualized and guided by the gestational age and location of the active lesion. Women of childbearing age with toxoplasma ocular lesions should be informed by their doctors about possible active recurrences during pregnancy and followed carefully by an ophthalmologist when pregnant.
MeSH Keywords: Chorioretinitis, Pregnancy, Therapeutics, Toxoplasmosis
Background
Toxoplasmosis is the most common parasitic infection in humans and probably the most common zoonosis in Poland [1,2]. It is estimated that approximately 30% to 50% of the Polish population are infected with T. gondii, although over 80% of these are asymptomatic [3,4].
Inflammation in the posterior eye segment occurs much later than the primary infection and is caused by a local reactivation resulting from rupture of tissue cysts that release dormant bradyzoites. This is due to weakened host immunity with the resulting inability to clear tachyzoites effectively, and is not due to an increased activity of the parasite [3,5,6].
Świtaj et al., using a polymerase chain reaction (PCR) method, demonstrated genetic material of T. gondii in peripheral blood samples from nearly all patients with reactivation of ocular toxoplasmosis [7]. Similar findings were reported by Silveira et al. [8] and Park et al. [9]. There is a potential risk to the fetus as there have been reports of mother-to-child transmission during reactivation of maternal ocular toxoplasmosis in pregnancy [10,11].
During pregnancy and postpartum period, the immune response is physiologically impaired and women are more susceptible to infections. Theoretically, this may predispose patients to reactivation of toxoplasmic retinochoroiditis [12–16]. Focal retinochoroiditis usually occurs many years after primary infection. Retinochoroiditis developing at the time of primary infection is very rare [17,18].
The aim of this study was to present the management of toxoplasmic retinochoroiditis during pregnancy, postpartum period, or lactation.
Material and Methods
A retrospective study was undertaken of the clinical records of 24 women, aged 15 to 39 years, during pregnancy, postpartum period, or lactation, who were treated at the Department of Zoonoses and Tropical Diseases and the Department of Ophthalmology, Medical University of Warsaw in the years 1994–2014. The diagnosis was based on the typical ophthalmoscopic picture, confirmed by serological testing for anti-T. gondii IgG and IgM, based on the enzyme-linked immunosorbent assay (ELISA) method. The choice of treatment was guided by the character and location of focal inflammation and the gestational age. Inflammation was considered to have been successfully treated when a scar was formed and the effusion resolved.
The study was approved by the Ethics Review Committee at the Medical University of Warsaw.
Results
A total of 28 attacks of acute toxoplasmic retinochoroiditis were observed in 24 women. In 20 cases, these were recurrences and the other 8 patients reported their problem as the first attack, although an ophthalmologic examination in 7 patients revealed postinflammatory retinal scars, confirming previous reactivations. The 7 patients were probably not aware of their condition because the foci were clinically “silent”, had peripheral location, or possibly were the result of childhood or even congenital infection. One patient (KSM, No. 16) actually presented with a first episode of toxoplasmic retinochoroiditis as she had no previous scars and a serologic test in the third trimester was negative. Judging from her serial serologic testing (i.e., the IgM and IgG results, and IgG avidity test) we estimate that seroconversion occurred approximately 7 months after delivery and 2 months before retinochoroiditis became evident.
Fifteen (15) of the patients were pregnant (4–32 weeks of pregnancy), 2 were in the postpartum period and 11 were lactating. In 2 pregnant patients, subsequent recurrences were observed in the postpartum period during lactation. One patient (RA, No. 1) had a history of acute toxoplasmic retinochoroiditis during her first pregnancy in 1991, followed by 3 reactivations: during her second pregnancy in 1994, when not pregnant in 1997, and again in 2002 during lactation after her third pregnancy. This is the most interesting case in our series and its rare nature prompted us to write this report. The retinal lesions in this patient are shown in Figures 1 and 2.
Figure 1.
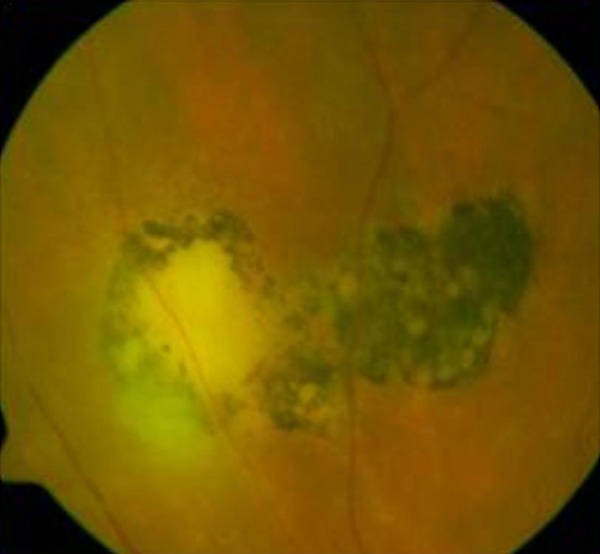
Fourth reported reactivation of toxoplasmic retinochoroiditis in patients RA, No. 9.
Figure 2.

This same eye 1 month later after the treatment – formed retinal scar.
To date, 17 of the patients have had no reactivation toxoplasmic retinochoroiditis after completing their treatment, 9 had 1 reactivation, and another 4 had more than 1 relapse.
Clinical details of the patients are given in Table 1.
Table 1.
Clinical details of the patients.
| Patient No. | Patient initials | Age | Date of first visit | Pregnancy (months) | Number of pregnancy | Lactation (months) | Treatment | Number of the retinal scars | Reactivation before pregnancy | Reactivation after pregnancy | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RA 2x | 27 | 06-1994 | 18 | 2 | 0 | 0 | 2 | 1 | 2 | Previous reactivation in first pregnancy in 1991 |
| 2 | GM | 15 | 08-1995 | 19 | 1 | 0 | T | 2 | 0 | 2 | 0 |
| 3 | AMA | 20 | 03-1997 | 11 | 1 | 0 | T | 1 | 0 | 3 | 0 |
| 4 | NB | 25 | 11-1997 | 0 | 1 | 6 | S+F+A +P+E, T | 3 | 1 | 2 | 0 |
| 5 | GA | 28 | 05-1999 | 21 | 2 | 0 | S+F+FA | 4 | 1 | 2 | Risk of damage to the macula |
| 6 | FJE | 24 | 10-2000 | 15 | 1 | 0 | S+F+FA | 3 | 2 | 0 | Risk of damage to the papillo-macular area |
| 7 | SA | 22 | 10-2001 | 0 | 1 | 3 | S+F+A T | 4 | 0 | 0 | |
| 8 | KM | 23 | 12-2001 | 24 | 1 | 0 | S+F+FA | 1 | 1 | 0 | Risk of damage to the macula |
| 9 | RA 3x | 35 | 01-2002 | 0 | 3 | 6 | S+F+A, T | 4 | 3 | 0 | Another reactivation in Patient No 1 |
| 10 | WJI | 22 | 10-2002 | 4 | 1 | 0 | S+F+A+P, | 3 | 2 | 1 | Not aware of her pregnancy. Stopped treatment at 17 Hbd |
| 11 | JZ1x | 29 | 01-2004 | 32 | 1 | 0 | S | 1 | 0 | 1 | 0 |
| 12 | MSM | 24 | 07-2004 | 4 | 1 | 0 | S | 2 | 0 | 0 | 0 |
| 13 | KJ | 22 | 10-2004 | 15 | 1 | 0 | S | 3 | 1 | 0 | 0 |
| 14 | JZ2x | 30 | 06-2005 | 0 | 1 | 12 | S+F+A+P, T | 1 | 1 | 0 | Another reactivation in Patient No 1 |
| 15 | ZK | 30 | 03-2006 | 0 | 1 | 0,5 | S+R+A+P, T | 1 | 0 | 0 | 0 |
| 16 | KSM | 39 | 08-2006 | 0 | 1 | 9 | S+F+A+P, T | 0 | 0 | 0 | Seroconversion 8 months earlier |
| 17 | RE | 25 | 05-2008 | 0 | 2 | 9 | S+F+A+P, T | 4 | 5 | 0 | 0 |
| 18 | ChSK | 28 | 05-2008 | 25 | 2 | 0 | S+P | 6 | 2 | 1 | 0 |
| 19 | BCM | 33 | 08-2008 | 11 | 1 | 0 | S+P | 4 | 1 | 0 | 0 |
| 20 | DW | 24 | 12-2009 | 0 | 1 | 6 | S+F+A+P, T | 2 | 0 | 0 | 0 |
| 21 | AP 1x | 19 | 12-2009 | 12 | 2 | 0 | S | 2 | 1 | 1 | 0 |
| 22 | BD | 29 | 04-2009 | 0 | 2 | 6 | S+F+A+P, T | 3 | 1 | 0 | 0 |
| 23 | TD | 38 | 07-2010 | 0 | 1 | 6 | S+F+A+P, T | 3 | 1 | 0 | 0 |
| 24 | AP 2x | 20 | 07-2010 | 0 | 2 | 1 | S+F+A+P, T | 3 | 2 | 0 | Another reactivation in Patient No 21 |
| 25 | GP | 22 | 12-2011 | 0 | 1 | 2 | S+P, T | 6 | 2 | 1 | 0 |
| 26 | PBK | 34 | 01-2012 | 0 | 1 | 2 | S+F+A+P, T | 1 | 1 | 1 | 0 |
| 27 | GBM | 25 | 07-2013 | 12 | 1 | 0 | S+P | 4 | 1 | 0 | 0 |
| 28 | MK | 30 | 02-2014 | 28 | 1 | 0 | S+P | 3 | 2 | 0 | 0 |
S – spiramycin; F – fansidar (pyrimethamine+sulfadoxine); A – azitromycin; P – prednisone; FA – folinic acid; T – topically.
Pregnant patients
All pregnant patients, except 1 who first presented when the active lesion had already settled, received topical treatment. Of those patients, 11 patients received spiramycin at doses of 3 million IU TID usually for 10 days and in 9 patients the inflammation was successfully treated. The 2 patients who did not respond to spiramycin were additionally prescribed Fansidar (pyrimethamine 25 mg/sulfadoxine 500 mg) 2 tablets/day for 2 days as a loading dose followed by 1 tablet/day for 19 days. Fansidar was also used in another patient who was at a risk of permanent damage of the macula. All women treated with Fansidar were >12 weeks of pregnancy and were supplemented with 15 mg of folinic acid per a day.
Patient WJI (No. 10) was treated with spiramycin, Fansidar, azithromycin, and prednisone without folinic acid supplementation, because at the time of treatment she was not aware that she was pregnant. In 2 pregnant patients, topical treatment (steroidal/nonsteroidal anti-inflammatory eye drops) only was used because the patients were referred at the time when the peripherally situated active lesion was already settling. Prednisone was used depending on the effusion level in the vitreous body at a starting dose of 40 mg, which was then gradually tapered.
Non-pregnant patients
Two episodes of retinochoroiditis reactivation were observed in the postpartum period and 11 episodes occurred during lactation. In all cases topical treatment (steroidal/nonsteroidal anti-inflammatory eye drops) was given and in 12 cases the patients were treated with a multidrug regimen including Fansidar, spiramycin (at a doses of 3 million IU TID for 10 days), and azithromycin (at a dose of 0.5g/day for 6 days). Prednisone was used depending on the effusion level in the vitreous body at a starting dose of 40 mg, which was then gradually tapered.
Fansidar was used after suppressing lactation. The patients took 1 tablet BID for 2 days, followed by 1 tablet QD for 19 days and 1 tablet twice a week for 6 months. The aim was to achieve permanent remission of recurrent retinochoroiditis. Platelet depletion as an adverse effect of anti-folinic drugs (Fansidar) was not observed.
The offspring of both pregnant and non-pregnant patients were not included in the present study.
Discussion
In pregnancy, the immune responses are physiologically impaired to prevent the rejection of the fetus and this weakening of the immune system may continue in the postpartum period and during lactation. Reactivation of inflammation associated with infectious disease is not infrequent and higher rates of ocular toxoplasmosis recurrence may be expected [5,12]. Our literature search yielded only a few reports of toxoplasmic retinochoroiditis in pregnant patients [12–14,18–20].
It is generally acknowledged that primary T. gondii infection in pregnancy carries a risk of transplacental infection with potentially serious neurological and ocular complications in the newborn. The risk increases with the gestational age, with the highest rate in the third trimester and the lowest rate in the first trimester, the average transmission rate being 30% [14,17,18]. A review of the literature covering the last 30 years revealed no evidence of a reduction in transplacental transmission rates of T. gondii resulting from the treatment of active toxoplasmosis in pregnancy, although decreased severity of congenital toxoplasmosis symptoms has been observed [21,22]. Thus, treatment does not have any effect on the potential disease in the offspring, but it may be effective in the mother. However, potential adverse effects in the fetus are a considerable concern [21]. Generally, spiramycin is a first-choice drug in toxoplasmosis in pregnancy and it can prevent transplacental transmission of T. gondii.
Importantly, toxoplasmic retinochoroiditis is a self-limiting condition and may resolve without treatment within 6 to 8 weeks [23,24]. In his report, Holland found spontaneous resolution of active inflammation with a resulting scar in patients with peripheral, not sight-threatening, locations of the active lesion [17]. According to Rothova et al., in cases of peripheral lesions or when there are contraindications for treatment (e.g., pregnancy), ocular toxoplasmosis does not require treatment [24].
In the reported series of female patients we did not find any massive inflammation in the vitreous and retina or related complications as described by Kump et al. [12]. The course of retinochoroiditis did not differ from that in non-pregnant women or in men.
The question of whether to treat ocular toxoplasmosis has been disputed for many years because the therapy is expensive and may be associated with adverse events, and when the lesions are peripheral and not sight-threatening it may offer no benefits to the patients.
Transplacental transmission of T. gondii is potentially possible in mothers with focal retinitis that occurred after remote primary infection. Treatment that is safe for the fetus should be instituted in each case to decrease the likelihood of maternofetal transmission [7–11,18].
In pregnancy the management of toxoplasmic retinochoroiditis should be individualized. It is necessary to consider a possible threat to sight in the mother, protection of the fetus against potential risk of transplacental transmission, and potential adverse effects of the treatment to the fetus, which is very susceptible to the effects of medication during organogenesis and then less so in the second and third trimesters.
Nowadays, specific treatments for T. gondii infection include products belonging to the classes of macrolide antibiotics, sulfonamides, and pyrimethamine [1,25–27].
Spiramycin was prescribed in pregnant women treated at our institutions as a first-choice drug because it is effective against T. gondii, and although it crosses the placental barrier in small amounts, it is not teratogenic. Spiramycin was given until focal retinochoroiditis resolved.
Treatment with the anti-folinic product (pyrimethamine 25 mg/sulfadoxine 500 mg), which is considered to be the most effective, was prescribed to pregnant women in the case of vision-threatening lesions or when there was no response to spiramycin, and only in late pregnancy. During lactation, the patients were advised to discontinue breast-feeding and then they were treated with anti-folinic products.
Fansidar, a very potent drug, is a combination of pyrimethamine and sulfadoxine, which suppress the synthesis of folic acid in protozoa, thus blocking cell division. Fansidar crosses the placental barrier and in animal studies (rat) it was embryotoxic, but no teratogenic effects have been reported in humans [28–30]. However, it produces folic acid deficiency in patients, with subsequent bone marrow suppression. Pregnant women treated with Fansidar were advised to take folinic acid, which is converted in the body to active folate, to prevent folate deficiency [1,27].
When there is no response to spiramycin, it is usually substituted with azithromycin, which has a much greater capacity to cross biological barriers, with effective intraocular penetration when the inflammation is settling. Azithromycin crosses the placental barrier easily, which is why its use is avoided it during pregnancy.
WJI (No. 10) was not aware of her pregnancy when treatment was initiated with Fansidar and spiramycin (swiched after 10 days for azitromycin for 6 days), followed by Fansidar alone, which she discontinued at 17 Hbd. She did not recive any folinic acid supplementation. However, she gave birth to a baby who had no signs of folate deficiency and whose further growth and development were normal.
Although the patients’ offspring were not included in this study, obstetricians and perinatologists were informed about maternal toxoplasma reactivation and the potential risk for placental transmission. To the best of our knowledge, none of newborns had any clinical signs of congenital toxoplasmosis, which was additionally confirmed by serologic testing.
Conclusions
The importance of secondary prophylaxis should be borne in mind, and Fansidar (pyrimethamine/sulfadoxine) may be administered at low doses for 6 months after recurrent toxoplasmic retinochoroiditis has resolved. Ocular toxoplasmosis may be in remission for many months or even years and then become reactivated, which must be always considered, especially in women of childbearing age [31].
Footnotes
Source of support: Departmental sources
References
- 1.Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis – a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9(3):e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sroka J, Wojcik-Fatla A, Szymanska J, et al. The occurrence of Toxoplasma gondii infection in people and animals from rural environment of Lublin region – estimate of potential role of water as a source of infection. Ann Agric Environ Med. 2010;17(1):125–32. [PubMed] [Google Scholar]
- 3.Foster CS, Vitale AT. Diagnosis and Treatment of Uveitis. In: Oréfice F, Vasconelos-Santos DV, Azerdo-Cordeiro C, Lambert Oréfice J, Costa Alves R, editors. Toxoplasmosis. Second edition. Chapter 35. Jaypee-Highlights Medical Publishers, INC; 2013. [Google Scholar]
- 4.Paul M, Petersen E, Szczapa J. Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznań region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. J Clin Microbiol. 2001;39(5):1912–16. doi: 10.1128/JCM.39.5.1912-1916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Mc Hugh TD, Bathgate T, Mangan J, et al. Recognition of tissue cyst-specific antigens in reactivating toxoplasmosis. J Med Microbiol. 1997;46(7):587–95. doi: 10.1099/00222615-46-7-587. [DOI] [PubMed] [Google Scholar]
- 7.Świtaj K, Master A, Borkowski PK, et al. Association of ocular toxoplasmosis with type I Toxoplasma gondii strains: direct genotyping from peripheral blood samples. J Clin Microbiol. 2006;44(11):4262–64. doi: 10.1128/JCM.01786-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95:396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 9.Park YH. Toxoplasma gondii in the peripheral blood of patients with ocular toxoplasmosis. Br J Ophthalmol. 2012;96:766. doi: 10.1136/bjophthalmol-2011-301068. author reply 766. [DOI] [PubMed] [Google Scholar]
- 10.Silveira C, Ferreira R, Muccioli CR, Belfort R., Jr Toxoplasmosis transmitted to a newborn from the mother infected 20 years earlier. Am J Ophthalmol. 2003;136(2):370–71. doi: 10.1016/s0002-9394(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 11.Andrade GM, Vasconcelos-Santos DV, Carellos EV, et al. Congenital toxoplasmosis from a chronically infected woman with reactivation of retinochoroiditis during pregnancy. J Pediatr (Rio J) 2010;86(1):85–88. doi: 10.2223/JPED.1948. [DOI] [PubMed] [Google Scholar]
- 12.Kump LI, Androudi SN, Foster CS. Ocular toxoplasmosis in pregnancy. Clin Experiment Ophthalmol. 2005;33(5):455–60. doi: 10.1111/j.1442-9071.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- 13.Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002;109:869–78. doi: 10.1016/s0161-6420(02)00990-9. [DOI] [PubMed] [Google Scholar]
- 14.Garweg JG, Scherrer J, Wallon M, Kodjikian L, Peyron F. Reactivation of ocular toxoplasmosis during pregnancy. BJOG. 2005;112:241–42. doi: 10.1111/j.1471-0528.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 15.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47(4):554–66. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 16.Braakenburg AM, Crespi CM, Holland GN, et al. Recurrence Rates of Ocular Toxoplasmosis During Pregnancy. Am J Ophthalmol. 2014;157(4):767–73. doi: 10.1016/j.ajo.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol. 1999;128(4):502–5. doi: 10.1016/s0002-9394(99)00263-9. [DOI] [PubMed] [Google Scholar]
- 18.Ramchandani M, Weaver JB, Joynson DH, Murray PI. Acquired ocular toxoplasmosis in pregnancy. Br J Ophthalmol. 2002;86(8):938–39. doi: 10.1136/bjo.86.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman CT, Knox DL. Variations in recurrent active retinochoroiditis. Arch Ophthalmol. 1969;81:481–93. doi: 10.1001/archopht.1969.00990010483005. [DOI] [PubMed] [Google Scholar]
- 20.Braakenburg AM, Rothova A. Clinical features of ocular toxoplasmosis during pregnancy. Retina. 2009;29(5):627–30. doi: 10.1097/IAE.0b013e31819a5ff0. [DOI] [PubMed] [Google Scholar]
- 21.Wallon M, Liou C, Garner P, Peyron F. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. BMJ. 1999;318(7197):1511–14. doi: 10.1136/bmj.318.7197.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyron F, Wallon M, Liou C, Garner P. Treatments for toxoplasmosis in pregnancy. Cochrane Database Syst Rev. 2000;(2):CD001684. doi: 10.1002/14651858.CD001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guex-Crosier Y, Auer C, Bernasconi O, Herbort CP. Toxoplasmic retinochoroiditis: resolution without treatment of the perilesional satellite dark dots seen by indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol. 1998;236(6):476–78. doi: 10.1007/s004170050108. [DOI] [PubMed] [Google Scholar]
- 24.Rothova A, Meenken C, Buitenhuis HJ, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115:517–23. doi: 10.1016/s0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- 25.Balaskas KJ, Vaudaux J, Boillat-Blanco N, Guex Y. Azithromycin versus Sulfadiazine and Pyrimethamine for non-vision-threatening toxoplasmic retinochoroiditis: a pilot study. Med Sci Monit. 2012;18(5):CR296–302. doi: 10.12659/MSM.882735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodjikian L. Toxoplasmose et grossesse. J Fr Ophthal. 2010;33:362–67. doi: 10.1016/j.jfo.2010.03.002. [in French] [DOI] [PubMed] [Google Scholar]
- 27.McLeod R, Kieffer F, Sautter M, et al. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104(2):320–44. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland GN, Lewis KG. An update on current practices in the managment of ocular toxoplasmosis. Am J Ophtalmol. 2002;134(1):102–14. doi: 10.1016/s0002-9394(02)01526-x. [DOI] [PubMed] [Google Scholar]
- 29.Luntamo M, Kulmala T, Mbewe B, et al. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am J Trop Med Hyg. 2010;83(6):1212–20. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deloron P, Bertin G, Briand V, et al. Sulfadoxine/pyrimethamine intermittent preventive treatment for malaria during pregnancy. Emerg Infect Dis. 2010;16(11):1666–70. doi: 10.3201/eid1611.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borkowski PK. Współczesne poglądy na toksoplazmozę narządu wzroku. Przegląd Epidemiologiczny. 2001;55:483–93. [in Polish] [PubMed] [Google Scholar]


