Abstract
Objective
This study was conducted to evaluate clinically and radiographically the use of a cellular dermal matrix allograft (Alloderm) in combination with PLA/PGA (Fisiograft) around immediate implants.
Materials and Methods
Fourteen patients were included in this study, three patients received two implants, total of seventeen implants were placed. Periapical radiographs and orthopantomographs were taken. The selected teeth were extracted atraumatically after the reflection of full thickness flaps. One-piece Zimmer implants were placed immediately into the sockets. Weeks from implantation, radiographic evaluation was made at 6 Fisiograft in powder form was placed in the osseous defects around the implants. The implants were immediately restored with provisional crowns free from occlusion. Patients were clinically evaluated at 3, 6, and 14 months after loading which was done after 6 weeks from implantation. Radiographic evaluation was made at 6 and 14 months from implant placement.
Results
showed that immediate implantation was successful in sixteen out of seventeen implants, clinical parameters regarding plaque index, gingival index, there was a slight decrease through the follow-up periods from 3 to 14 months but it was non-significant, while there was a significant decrease in the probing depth. Radiographically there was a significant increase in the bone density from 6 to 14 months post loading, while the vertical bone defect was significantly decreased. The fisiograft functioned well as space maker and scaffolding material. The Alloderm performed well as a membrane to be used in association with immediate implants and it has a good potentiality for increasing the width of the keratinized gingiva, which is an important feature for implant esthetics.
Conclusion
the combination technique between the bone graft and the membrane proved to be successful to overcome dehiscence and osseous defects around immediate implants.
Introduction
Implant dentistry is defined as the art and science concerned with the restoration of function, esthetics, comfort and health of partially or completely edentulous patients, since the therapeutic goal of implant dentistry it is not only replacement of missing tooth, but also complete rehabilitation. (1, 2)
Over the last decade numerous studies have documented successful placement of endosseous dental implants in fresh extraction sockets, and methods have been developed to provisionalize both full arch and single tooth implant cases at the time of surgery. (3) Implantation immediately after tooth extraction offers several advantages for both patients and clinicians including shorter treatment time, less bone resorption, fewer surgical sessions, and easier definition of the implant position. It makes the use of longer implants possible due to the preservation of the ridge height and width. Moreover it provides better opportunities for osseointegration because of the healing potential of then fresh extraction socket. (4–6)
Successful long term use of dental implants depends on integration within and support from osseous tissues. (7) Guided bone regeneration (GBR) involves the creation of an environment that is potentially favorable for new bone formation. This involves the generation of bone beneath a barrier membrane that prevents the ingrowth of connective tissue and epithelium. (8–10) This was first described in orthopedic research in 1959 by Hurley et al. (11)
GBR has been used to augment the quantity and quality of host bone in areas of localized ridge defects either prior to or in conjunction with implant placement. (12, 13) Lazzara, (14) used immediate implants associated with a membrane to allow osseointegration and bone regeneration within the extraction site after a root fracture extraction procedure. However if there are problems such as defects with buccal plate destruction, it is necessary to use grafting materials either autogenous, xenogenic, synthetic bone substitutes or membranes, or a combination of them to create sufficient space for cell migration and bone regeneration. (15, 16)
Absorbable synthetic biopolymers have been used as bone fillers, proving effective stimulants to bone regeneration in some cases. (17–19) A new product has been developed (Fisiograft) that is made from a copolymer of PLA-PGA and which in its different forms (sponge, powder, and gel), has a density that permits its complete absorption within a short period of time (4 to 8 months). (20, 21, 22) This synthetic bone replacement biomaterial demonstrated osteoconductivity, considerable practical flexibility and excellent biocompatibility. (18, 20, 21, 22) Problems associated with the GBR procedures such as premature exposure of the membranes to the oral cavity and consequently contamination, have been reported. Acellular dermal matrix (ADM) graft material (Alloderm) is presently used to treat soft tissue problems. (23) This allograft is a skin preparation from which the cellular component is removed. (24, 25, 26) The ultra- structural integrity of the extra-cellular matrix is maintained and the collagen and elastin matrices remain undamaged. (27, 28, 29) In several case reports in periodontal surgeries, it has been observed that ADM material consistently integrates into the host tissue. It maintains the structural integrity of the tissue and re-vascularizes via preserved vascular channels. (29, 30, 31) This material is presently used with success as a free graft to increase the width of attached gingiva along teeth and implants, (30, 31) for root coverage, (32) and in the management of soft tissue ridge deformities. (33) Given what have been observed in these studies, the ADM is probably not significantly colonized by periodontopathic bacteria, thus healing is uneventful and the material is incorporated into the tissues. (34)
According to our knowledge few studies, has been regarding the evaluation of the Fisiograft in combination with Alloderm as a GBR procedure. Thus this study was conducted, using clinical assessment and digital radiographic analysis for evaluation of these materials in management of osseous defects around one piece immediate implant.
Material and Methods
This study was conducted on fourteen adult patients selected from the outpatient dental clinics in Oral Surgery and Periodontology Departments, Faculty of Dentistry, Alexandria University. From the patients selected, four are females and ten are males, their ages ranged from 28–45 years of average 36.5. They were all free from any systemic diseases contraindicating the use of implants. Smokers were excluded, the patients were seeking treatment with implants for their traumatized maxillary anterior teeth and those indicated for extraction when other conservative treatment failed to save them. Total of seventeen implants were placed in fourteen patients where three patients received two implants each. An informed consent form was signed by all the patients participating in this study according to Helsinki’s Declaration Statement. (35)
Patients who met the following criteria were recruited for the study;
Tooth extraction was indicated due to, root fracture, severe periodontitis without purulent discharge, untreatable endodontic failure or other factors that caused the tooth to have a hopeless prognosis.
Following extraction the residual socket was of inadequate dimensions, so that it will result in osseous defect after placement of a dental implant.
The bone remaining was adequate to stabilize a dental implant.
No para-functional habits and the patient occlusion were adjusted when needed.
Materials
A) Polylactic Polyglycolic Acid (PLA-PGA) (Fisiograft)*
According to the manufacturer, it is a copolymer of polylactic/polyglycolic acid. It has a spongy open-cell structure enabling it to be colonized by osteoblasts. It is available in sponge, powder, and gel forms, and has a density that permits its complete absorption within a short period of time, (4–8 months). The absorption time varies according to the quality of the material implanted, and where it is implanted, also on the reactivity of the patient.
(B) Acellular Dermal Matrix Membrane (Alloderm) **
This membrane was prepared and rehydrated using saline (100) ml. per piece, at least 10 minutes before being used but not more than 4 hours according to manufacturer. The membrane has distinct upper and lower surfaces, to enable correct orientation, each piece of it contains an orientation slit that must not be trimmed away before application. The material was placed in a sterile dish with 50 ml. After flotation of the protecting piece of the back side paper, the dermal material was transferred to another dish with 50ml. of sterile saline for another 10 minutes. Using sterile gloves or forceps, the rehydrated dermal graft was transferred to the osseous defects, which were filled with the alloplastic bone graft, with the basement membrane side up and connective tissue side down, using the orientation slit as a reference, which must be horizontal in either the upper left or the lower right corners. The correct orientation was further determined by the physical characteristics of the dermal membrane. The basement membrane side presents a smooth surface. Firm pressure was applied to membrane with a sterile moist gauze pad for 3–5 minutes, to adapt and to adhere the graft to the socket. The flap was then sutured with 000 black silk sutures without tension.
(C) Implant
The implant system used in this study is the Zimmer system.*** It is physically biocompatible grade IV pure titanium: UTS -550MPa.
It is formed of one piece threaded type fixture, different thread depths for gradual fitting, initial stability and high anchorage. It has three self-tapping specially profiled, longitudinal grooves for gradual self-tapping, higher initial stability and anti-rotational mechanical lock. The abutment is with internal hex for implant tightening. A shoulder is present for anti-rotational mechanical lock between abutment and prosthetic part.
The implant surface is sandblasted with calculated particles size and regime –except of the upper 2-mm portion which is electromechanically polished. The implant is etched and electromechanically treated to optimize the bone-implant interface conditions.
Each implant is sealed in a gamma sterilized, doubled packing with special design to guarantee safe implant handling and making its placement easier, avoiding any finger contact. The color indicator on the outer vial cap indicates the diameter of the implant.
Methods
(A) Pre-surgical Phase
Clinical assessment of the patients (oral hygiene, and general health) was performed. Primary impression for diagnostic upper and lower stone casts was taken and registration of bite blocks for standardization of the serial radiographs was performed. Initial periodontal therapy (phase I) was performed for each subject including plaque control instructions, scaling and root planning.
The procedures of dental implantation were explained to all subjects who in turn agreed to participate. Written consents were obtained from each patient. A pre-operative prophylactic antibiotic (Amoxicillin trihydrate-potassium Clavulanate 1 g every twelve hours) was administered 24 hours before surgery and for one week postoperatively. All patients were instructed to rinse with 0.1chlorhexidine gluconate for 30 seconds, 3 times a day, one day before surgery, and for six weeks postoperatively.
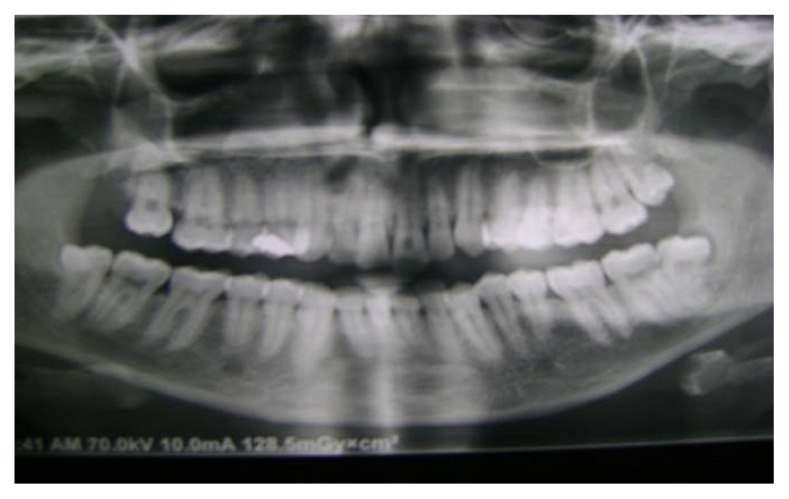
Orthopantomogram (OPG) (fig. 1), and periapical films were taken to study the bone condition and to exclude any pathologic lesions, such as cysts, tumors or bony abnormalities and to determine the length of the implant required.
Fig. (1).
Pre-operative panoramic view for the maxillary left central incisor with horizontally broken root due to trauma.
(B) Surgical Procedure
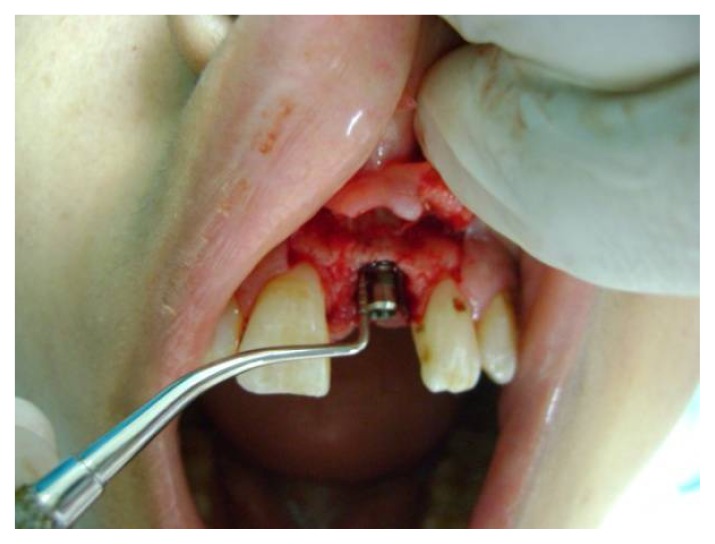
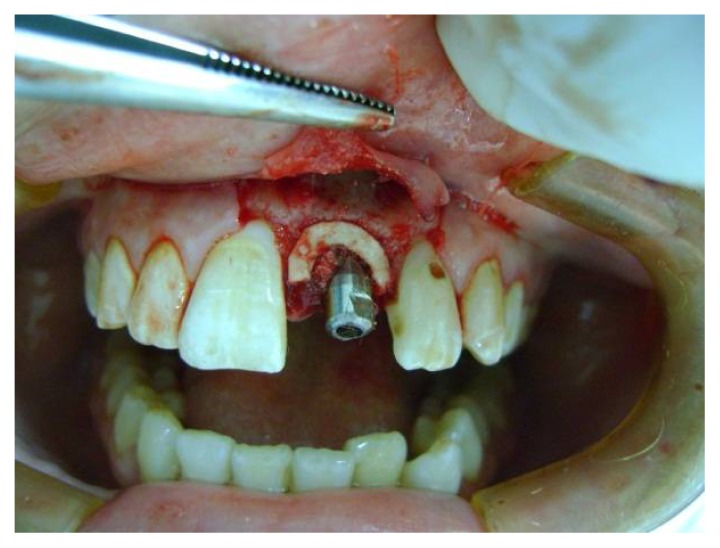
All patients were operated upon under local anesthesia using infra-orbital nerve block supplemented with infiltration of the interlacing fibers. A full thickness mucoperiosteal flap was reflected. The tooth was extracted with minimal trauma to preserve the cortical bone. Then the socket was curetted and filed to remove any infected or inflammatory tissues as well as remnants of periodontal ligament. Socket shaping and deepening was accomplished with appropriate sizing drills, so that maximum lateral contact could be achieved with the placed Implant body. Drilling was done 3 to 4 mm beyond the apex so that the implant would be anchored in healthy bone beyond the bottom of the socket. The appropriate length and width of the one piece Zimmer implant was selected and placed in the extraction socket (Fig. 2). The alloplastic material (bone graft) was placed in osseous defects after implant placement around the implant. Then the membrane was trimmed and placed over the bone graft (Fig. 3), and secured under the flap then, it was sutured using 000 silk sutures. The implant was then immediately restored with a provisional crown that was freed from occlusion. Final impression was taken using rubber base impression material and a permanent crown was placed six weeks later (loading was done after six weeks from implantation).
Fig. (2).
the one-piece implant placed in the extraction socket.
Fig. (3).
Alloderm membrane around implant.
(C) Post-surgical phase
Postoperative instructions were given to the patients that included applying cold packs on the first day and rinsing with warm chlorhexidine mouthwash twice daily in the second postoperative day and for six weeks later. The antibiotic was continued for 7 days and non- steroidal anti-inflammatory drugs and analgesics were prescribed when needed. The sutures were removed after one week.
(D) Follow-up Phase
Patients were evaluated clinically at three, six, and fourteen months after implant loading. Clinically the patients were examined for presence of pain, or discomfort, plaque index, (36) gingival inflammation, (37) probing depth according to Glavind and Loe, (38) and mobility according to Miknney and Koth. (39)
(E) Radiographic evaluation
Indirect digital radiography was done by using digital scanner and special software (40, 41) that has the ability to convert the X-ray image into digital data. Image J *1.31 Public domain software was used to capture the X-ray views and transfer them into TI-FF computer image and the change in bone density and bone height (vertical bone defect) around the implant were measured at 6, and 14 months after implant placement (Figs.4&5).
Fig. (4).
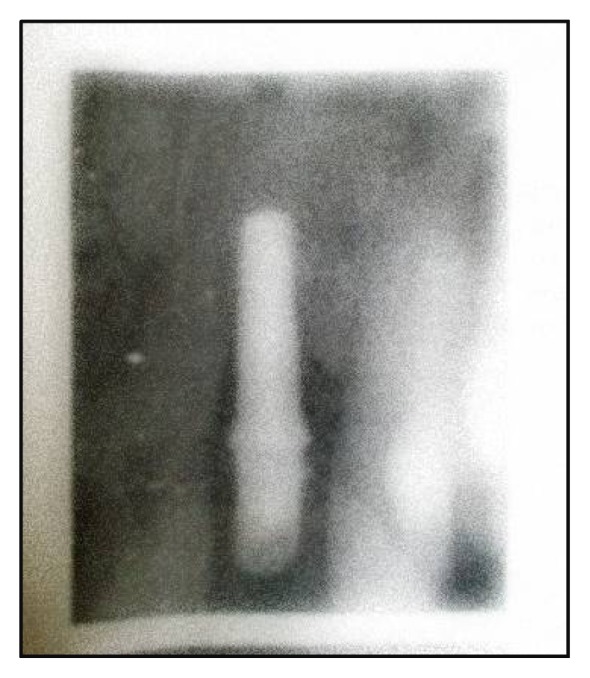
Periapical radiograph at six months after implant placement.
Fig. (5).
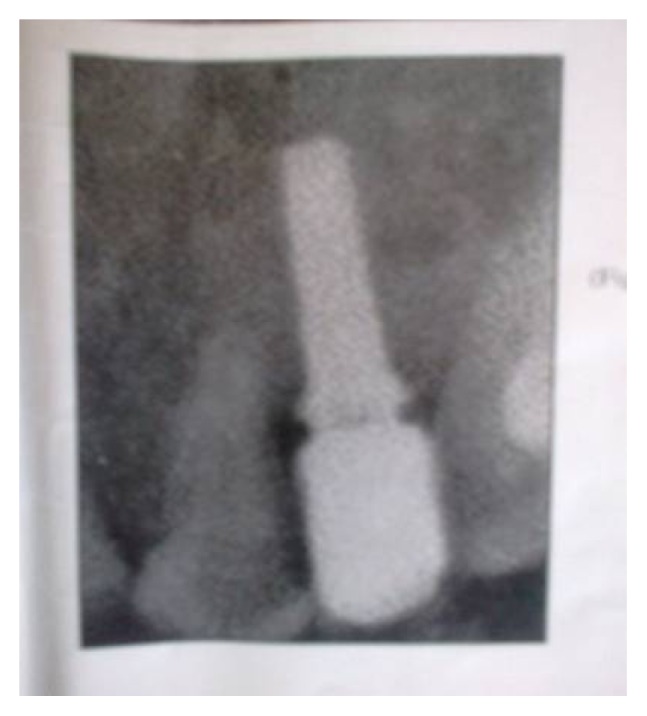
Periapical radiograph after fourteen months from implant placement.
Peri-implant bone height measurement
The computer program (image j) was used for evaluation of the distance from the shoulder of the implant to the first visible bone to implant contact that was determined by linear measurement. The measurements in mm were noted both mesially and distally and the mean was calculated. In addition the length of the implant was measured in order to determine the magnification factor in the radiograph. The measurements of the bone levels were then adjusted according to magnification.
Densitometry Analysis
The region of interest (ROI) was selected which was in close level, to bone implant interface in the mesial and distal aspects. The degree of blackening and whitening (radiolucency and radio-opacity) was expressed in numbers from 0 to 225.
Results
None of the studied patients complained of pain or discomfort. Sixteen out of seventeen implants healed and functioned well. Only one implant was lost three weeks after placement.
Clinical results
Sixteen implants showed no signs of mobility all-over the evaluation period, i.e. mobility score was 0. However one implant exhibited mobility at three weeks after placement.
Only some minor changes in plaque accumulation occurred throughout the study. The mean plaque index scores at three, six, and fourteen months after loading are shown in Table I the decrease in PI scores from the third to the fourteenth months was found to be statistically non-significant.
Table I.
Statistical comparison of mean differences of different clinical parameters at 3, 6, and, 14 months after implant loading
| Variables | Follow-up period | t-Test (P) | ||
|---|---|---|---|---|
|
| ||||
| 3 months | 6 months | 14 months | ||
|
| ||||
| Plaque index Range | 0.5–0.75 | 0.25–0.75 | 0.00–0.75 | T1=0.696 NS |
| Mean ± SD | 0.62±0.35 | 0.50±0.22 | 0.42±0.26 | T2=1.387 NS |
|
| ||||
| Gingival index Range | 0.25–0.75 | 0.25–0.50 | 0.00–0.50 | T1=1.000 NS |
| Mean ±SD | 0.46± 0.19 | 0.37± 0.14 | 0.25±0.22 | T2=1.746 NS |
|
| ||||
| Probing Depth Range | 2.25–4.25 | 1.75–3.25 | 1.75–2.75 | T1=4.781* |
| Mean ± SD | 3.37 ± 0.77 | 2.71±0.53 | 2.33±0.41 | T2=6.934* |
SD= Standard Deviation. T1 = paired (t-test) between 3 and 6 months post-surgery. T2= paired (t-test) between 3 and 14 months post -surgery. NS =non-significant
significant at P ≤0.05.
Very mild inflammatory reactions were detected around the implants throughout the period of observation. Mean gingival index scores at three, six, and fourteen months after loading are shown in Table I. No significant difference was found in gingival index scores from three to fourteen months from loading.
The mean probing depth scores at three, six, and fourteen months after loading are shown in Table I. A statistical difference in mean probing depth values was observed between the third, sixth, and fourteenth months after loading.
Radiographic evaluation
The mean vertical defect depth scores are shown in Table II. The decrease in vertical defect depth from six to fourteen months after implant placement was found to be statistically significant.
Table II.
Statistical comparison of mean differences in bone density and vertical defect depth at 6 and 14 months after implant placement.
| Variables | Follow-up | Period | t-test (P) |
|---|---|---|---|
|
| |||
| 6MONTHS | 14MONTHS | ||
|
| |||
| Bone Density Range | 86–94 | 112–124 | 23.175* |
| Mean ±SD | 90.00-± 3.41 | 120.17±4.62 | (0.000) |
|
| |||
| Vertical Depth Defect Range | 3.67–4.08 | 1.94–2.50 | 17.173* |
| Mean ±SD | 3.91±0.17 | 2.20±0.20 | (0.000) |
SD= standard deviation, t= paired t-test between 6 and 14 months post-surgery.
= significant at P≤0.05.
Bone densities at six and fourteen months after implant placement is shown in Table II The increase in bone density from six to fourteen months was found to be statistically significant.
Discussion
Immediate implant placed into fresh extraction socket sites is considered a predictable and acceptable procedure. The main biological advantage is the preservation of alveolar bone height and width. (42) The selected patients in the present study were all nonsmokers, as smoking is one of the factors often discussed in relation to implant failure. (43, 44) There is no doubt about the negative effects of active cigarette smoking in humans. It is well recognized that cigarette smoking is associated with impaired wound healing after surgical treatment in the oral cavity, (44) reduced bone height, (45) increased bone loss, (46) increased resorption of the alveolar ridge, (47) and higher incidence of periodontitis. (48) In addition, smoking has been found to be an important factor in peri-implant soft tissue changes. (49)
In the present study, a screw type one piece non submerged implant, followed by loading with a provisional acrylic crown. The screw type implant allows precise placement and provides the stability necessary for bone regeneration in the tooth socket as recommended by Lazzara. (14) As a result non-submerged implants have evolved into widespread human use. (50, 51) Schroeder et al (52) confirmed the predictability of non-submerged fixtures. The clinical results of one part non-submerged implant coated with a titanium sprayed plasma surface placed in the edentulous mandible demonstrated success rates greater than 90%. (53) In addition to expending treatment and eliminating a second surgical procedure, another advantage of this immediate one piece implant technique might be the preservation of soft and hard tissues. (54) It has been reported that immediate implant placement associated with immediate loading by provisional crowns, without occlusal contact, provided the patient with immediate esthetics and comfort without any complications during the post loading follow up period. (55, 56) One major disadvantage of the standardized dental implant placement protocol with a two stage approach is the necessary healing period between implant placement and restoration. This healing period is often psychologically and socially unacceptable for many patients. (57)
The results of the present work demonstrated slight decrease in PI scores from three to fourteen months which was found to be statistically insignificant. These low values of mean plaque accumulation could be attributed to the strong patient’s motivation for oral hygiene measures and to the highly polished titanium surface of the gingival collar part of the implant that is resistant to plaque accumulation as stated by Apse et al. (58) The results of the current study agree with the findings of other investigators who reported that the percentage of examined abutments of titanium fixtures without plaque was 70 to 75% and that it remained always constant throughout the study period. (59) It is worth mentioning that there is some controversy regarding the role of bacterial plaque in implant fixture. Some authors stated that since there is no periodontal ligament around an implant, plaque has no path of invasion beyond the gingivae and that it plays an insignificant role in implant failure. (60) Other authors have shown that periodontal pathogens can colonize implants (61) and that a relationship exists. (62, 63)
In the current study, very mild inflammatory reaction was detected around few implants as reflected by the low gingival index scores throughout the periods of observation. No significant difference was found in gingival index scores from three to fourteen months after loading. These results would be due to the oral hygiene instructions and measures, that the patients were instructed to follow during the follow-up periods. Also, the reduction of inflammation might be due to the rapid and favorable healing process that took place. Furthermore the good contouring of the crowns with the gingiva, allows for self-cleansing action mechanism maintenance. The results of the present study are consistent with the findings of other investigators, who reported that marginal tissue around titanium fixtures, in most examined patients had no gingivitis throughout the study. (59, 60)
Clinical probing is regarded as an important and reliable diagnostic parameter in the continuous monitoring of both periodontal and peri-implant tissues. (61, 62) The results of the present study demonstrated that the probing depths constantly decreased throughout the study periods. This decrease was regarded as statistically significant. This finding might be due to successive adaptation of the sulcular lining epithelium as suggested by Hansson et al. (63) The results of the present investigation are also in accordance with the studies of Adel et al. (59) and Lekholm et al. (64) In the present study there was no detected clinical mobility in sixteen implants throughout the evaluation period. Thus, osseointegration was achieved and maintained by the sixteen implants. Osseointegration and absence of implant mobility are considered as important criteria for implant success. (65, 66) However one of the implant exhibited mobility at three weeks after placement. It has been reported that the cause of peri-implant crestal bone loss could be multifactorial: bacterial infection and biomechanical factors can both be contributing factors. Other etiologic factors such as traumatic surgical techniques, inadequate amount of host bone resulting in an exposed implant surface at the time of placement and a compromised host response, could act as cofactors in the development of peri-implant disease. (67) Radiographic interpretation of alveolar bone loss has proven to be one of the most valuable means to clarify implant success. (68) Digitalizing intraoral radiographs using a flatbed scanner was a mean of choice since it was not expensive when compared to direct digital X-ray system and it is believed to be an essential feature of dental practice. (69) In the present study changes in alveolar bone seen in indirect digital radiographs, revealed a decrease in the mean vertical defect. This decrease was statistically significant at fourteen months after placement. Also, bone density was significantly increased from one to fourteen months after placement. The use of bone grafts and guided bone regeneration (GBR) separately or in combination, considerably improves the treatment outcome of bone defects. The encountered fenestrations, dehiscence, or other bone volume deficiencies during implant placement could be resolved by GBR. (8, 9, 70, 71)
In the present study, the efficacy of Alloderm as a bio-absorbable barrier for GBR was performed to increase the width of keratinized gingivae. Concerning the alloplastic bone graft, the synthetic polymer that was used in the current work, studies in several post-extraction and periodontal cases have demonstrated that this material is extremely easy to handle with good healing properties. In agreement with our findings, this new biomaterial proved to be biocompatible, non-allergic and did not produce any inflammatory response. (18, 19, 22) Several investigators utilized this product in its various form as a space maintainer either by itself, or as a support for absorbable and non-absorbable membranes. They reported that the degradation and the absorption of the copolymer permit a progressive and orderly reconstruction of the bone tissue. They concluded that evaluated ADM is known as a biocompatible material that does not exhibit any signs of foreign body reactions or rejection. (29, 25) This property proved to be highly important for GBR as there were no signs of soft tissue complications or any need for early membrane removal. ADM, in the present study, functioned as a resorbable barrier membrane and succeeded in protecting the underlying bone graft used Fisiograft during the early healing phase. Formation of adequate new bone fill in vertical osseous defects around implants was noticed as evidenced by the radiographic and densitometry findings at fourteen months post implant placement.
These results are in line with the findings of several investigators (22, 23, 71) who agreed that ADM graft performed well as membrane for GBR in association with immediate implants and that it has also the potential to increase the width of keratinized tissues (an important feature for implant esthetics). It is worth mentioning that an increased width of attached gingiva was noticed in most cases. In the present study, although no special technique the process of GBR appears particularly important for repair of large defects in the alveolar bone and when it is necessary to obtain an increase of the bone tissue at a peri-implant site, thus gaining stability and consequently success of immediate post extraction implantology. (25, 22, 72) Fisiograft has been recently used in several trials including ridge preservation following tooth extraction and in a new simplified technique for major augmentation of the maxillary sinus. It has been proven to be completely absorbed within the 4–8 months and replaced by well mineralized lamellar bone as confirmed by histological examination. (24, 22, 73) These findings in accordance with the results of the present study as evidenced by the radiographic study that demonstrated a well mineralized lamellar bone at the end of the follow-up period. It is apparent from the previous discussion that the combination of ADM and Fisiograft could be safely and effectively used for guided bone regeneration of osseous defects around immediate implants.
Conclusions
The materials used were safe and effective and fulfilled the aims that they were used for. The Fisiograft functioned well as a space maker and scaffolding material, to allow mineralized tissue formation. The ADM graft performed well as a membrane to be used in association with immediate implants because it, not only functioned well as a barrier membrane for GBR, but it also has the potential to increase the width of keratinized tissues, which is an important feature for implant esthetics.
Footnotes
Ghimas : Via Fucini, Gasalecchio di Rins (BO) Italy
Life Cell in-corporation-Texas, USA
Zimmer|dental (www.zimmerdental.com) 1900Aston Avenue Carlsbad, CA 92008-7308USA
Image j*: 1.31 Public Domain Software, downloaded through the internet from National Institute of Health, USA.
References
- 1.La Mar F. Implant dentistry today. J Oral Implant. 1997;23:49. [Google Scholar]
- 2.Korinski TE, Showronski RI. Immediate implant loading: A case report. J Oral Implan. 2002;28(2):87. doi: 10.1563/1548-1336(2002)028<0087:IILACR>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Beagle JR. Immediate placement/immediate load. A single–tooth case study utilizing the ITI dental implant system. J Indiana Dent Assoc. 2002;81(3):1. [PubMed] [Google Scholar]
- 4.Simsek B, Simsek S. Evaluation of success rates of immediate and delayed implants after tooth extraction. J CliMed. 2003;116(8):1216. [PubMed] [Google Scholar]
- 5.Lekovic V, Kenney EB, Weinlaender M. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68(6):563–570. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 6.Granin AN, Klein M, Simons A. Cited in Atlas of Oral implantology. Thieme Medical Publ; New York: 1993. Immediate placement of root-form implant into extraction sites. [Google Scholar]
- 7.Meraw SJ, Reeve CM. Qualitative analysis of peripheral peri-implant bone and influence of Alendronate sodium on early bone regeneration. J Periodontol. 1999;70:1228. doi: 10.1902/jop.1999.70.10.1228. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi HM, Parhiz A, Ghafari S. Vestibuloplasty: allograft versus mucosal graft. International Journal of Oral and Maxillofacial Surgery. doi: 10.1016/j.ijom.2011.09.014. Online publication date: 1-Nov-2011. [DOI] [PubMed] [Google Scholar]
- 9.Becker W, Becker BE. Guide tissue regeneration for implants placed into extraction sockets and for implant dehiscences: Surgical techniques and case report. Inter J Periodont Rest Dent. 1990;10:376. [PubMed] [Google Scholar]
- 10.Meraw SJ, Reeve CM, Wollan PC. Use of alendronate in peri-implant defect regeneration. J Periodontol. 1999;70:151. doi: 10.1902/jop.1999.70.2.151. [DOI] [PubMed] [Google Scholar]
- 11.Hurley LA, Stinchfield FE, Bassett AL, Lyon WH. The role of soft tissues in osteogenesis: An experimental study of canine spine fusions. J Bone Joint Siurg. 1959;41A:1243. [PubMed] [Google Scholar]
- 12.Belser U, Bernard JP, Buser D. Implant placement in the esthetic zone. In: Lindhe J, Karring T, Lang ND, editors. Clinical Periodontology and Implant Dentistry. 4th ed. Vol. 915. Blackwell Munksgaard Publ Co; UK, Tokyo, Berlin, Paris: 2003. Cited in. [Google Scholar]
- 13.Buser D, Dula K, Belser U, Hirts HP, Berthold H. Localized ridge augmentation using guided tissue regeneration. I. Surgical procedure in the maxilla. Inter J Periodont Rest Dent. 1993;13:137. [PubMed] [Google Scholar]
- 14.Lazzara RJ. Immediate implant placement into extraction sites. Surgical and restorative advantages. Inter J Period Res Dent. 1989;9:332. [PubMed] [Google Scholar]
- 15.Yunke RA, Castellon P, Saez – Nasr AM. Evaluation of hard tissue replacement composite soft material. A ridge preservation/augmentation material in conjunction with immediate hydroxyl-apatiten coated dental implant. J Periodontol. 2003;74:679. doi: 10.1902/jop.2003.74.5.679. [DOI] [PubMed] [Google Scholar]
- 16.Matin K, Senpuku H, Hanada N. Bone regeneration by recombinant human bone morphogenetic protein -2 around immediate implants. A pilot study in rats. J Oral MaxillofacImplants. 2003;18:211. [PubMed] [Google Scholar]
- 17.Lundgren D, Nyman S, Mathisen T, Isakson S, Kinge B. Guided tissue regeneration of cranial defects using biodegradable barriers. An experimental pilot study in rabbits. J Craniomaxillofac Surg. 1992;20:257. doi: 10.1016/s1010-5182(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 18.Niyamoto S, Takaoka K, Okadas T, Yoshkawa H, Hashimoto J, Suzuki S, Ono K. Evaluation of polylactic acid homopolymers as carriers for bone morphogenetic protein. J Clin Orthoped Related Res. 1992;278:274. [PubMed] [Google Scholar]
- 19.Winet H, Hollinger JO. Incorporation of polylactide/polyglycolide in a cortical defect; Neo-osteogenisis in a bone chamber. J Biomed Mater Res. 1993;27:667. doi: 10.1002/jbm.820270514. [DOI] [PubMed] [Google Scholar]
- 20.Sabattini VB, Salvatorelli G. New simplified technique for major augmentation of the maxillary sinus in preparation for implant. 35th Annual meeting of the Continental European Division of the International Association for Dental Research; Montpellier: IADR/CED; 1999. [Google Scholar]
- 21.Stancari F, Zanni B, Bernardi F, Calandriello M, Salvatorelli G. Use of PCA-PGA (copolymerized polylactic/polyglycolic acid) as a bone filler: Clinical experience and histologic study of a case. Quintessenz (Germany) 2000;51(1):47. [Google Scholar]
- 22.Hassan KS. Autogenous bone graft combined with polylactic polyglycolic acid polymer for treatment of dehiscence around immediate dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):e19–25. doi: 10.1016/j.tripleo.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Park JB. Healing of Extraction Socket Grafted with De-proteinized Bovine Bone and Acellular Dermal Matrix: Histomorphometric Evaluation. Implant Dentistry. 2010;19(4):307–313. doi: 10.1097/ID.0b013e3181e5abbc. [DOI] [PubMed] [Google Scholar]
- 24.Borges JG, Novaes AB, Grisi MFM, Palioto DB, Taba M, De Souza SLS. Acellular dermal matrix as a barrier in guided bone regeneration: A clinical, radiographic and histomorphometric study in dogs. Clinical Oral Implants Research. 2009;20(10):1105–1115. doi: 10.1111/j.1600-0501.2009.01731.x. [DOI] [PubMed] [Google Scholar]
- 25.De Andrade PF, De Souza SLS, Macedo GDO, Novaes AB, Grisi FMS, et al. Acellular Dermal Matrix as a Membrane for Guided Tissue Regeneration in the Treatment of Class II Furcation Lesions: A Histometric and Clinical Study in Dogs. Journal of Periodontology. 2007;78(7):1288–1299. doi: 10.1902/jop.2007.060325. [DOI] [PubMed] [Google Scholar]
- 26.Santos A, Goumenos G, Pascual A. Management of Gingival Recession by the Use of an Acellular Dermal Graft Material: A 12-Case Series. Journal of Periodontology. 2005;76(11):1982–1990. doi: 10.1902/jop.2005.76.11.1982. [DOI] [PubMed] [Google Scholar]
- 27.Becker W, Goldstein M. Immediate implant placement: treatment planning and surgical steps for successful outcome. Periodontology. 2008;47(1):79–89. doi: 10.1111/j.1600-0757.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 28.WainWright DJ. Use of an acellular allograft dermal matrix (Alloderm) in the management of a full thickness burns. J Dermatol. 1995;21:47. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 29.Tal H. Sub gingival acellular dermal matrix allograft for the treatment of gingival recession. A case report. J Periodontol. 1999;20:1118. doi: 10.1902/jop.1999.70.9.1118. [DOI] [PubMed] [Google Scholar]
- 30.Schulman J. Clinical evaluation of an acellular dermal allograft for increasing the zone of attached gingiva. Pract Periodontics Aesthet Dent. 1996;8:20. [PubMed] [Google Scholar]
- 31.Silverstein LH, Callan DP. An acellular dermal matrix allograft substitute for palatal donor tissue. Postgrad Dent. 1996;2:14. [Google Scholar]
- 32.Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft. A clinical and histological evaluation of a case report. J Periodontol. 1998;69:1305. doi: 10.1902/jop.1998.69.11.1305. [DOI] [PubMed] [Google Scholar]
- 33.Batista EL, Batista FC, Novaes AB. Management of soft tissue ridge deformities with acellular dermal matrix. Clinical approach and outcome after six months of treatment. J Periodontol. 2001;72 doi: 10.1902/jop.2001.72.2.265. [DOI] [PubMed] [Google Scholar]
- 34.Novaes AB, Papalexiou V, Luczyszyn SM, Muglia VA, Souza SLS, Taba M. Immedite implant in extraction socket with acellular dermal matrix graft and bioactive glass: A case report. Implant Dent. 2002;11(4):24. doi: 10.1097/00008505-200211040-00013. [DOI] [PubMed] [Google Scholar]
- 35.The Declaration of Helsinki (2002) (Edinburgh, Scotland). Note of clarification on Paragraph 29 added by the WMA General Assembly, Washington.
- 36.Silness J, Loe H. Periodontal disease in pregnancy: II. Correlating between oral hygiene and periodontal condition. Acta Odont Scand. 1964;22:121. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 37.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odont Scand. 1963;21:533. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 38.Glavind L, Loe H. Errors in the clinical assessment of periodontal destruction. J Periodont Res. 1967;2:180. doi: 10.1111/j.1600-0765.1967.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 39.Steflik DE, Koth DL, Robinson FG, McKinney RV, Davis BC, Morris CF, Davis QB. Crystal sapphire endosteal dental impant in humans. Ten –year results. J Oral Implantol. 1995;21(1):8. [PubMed] [Google Scholar]
- 40.Bourgeois M, Skorski A, Wood RE. Educational use of indirect digital radiographic imaging. J Canad Dent Assoc. 1995;61(11):968. [PubMed] [Google Scholar]
- 41.Gordel P, Hildeolt CT, Akdenize PJ. Technical report. The effect of different image file format and image analysis software program on dental radiometric digital evaluations. Dentomaxillofac Radiol. 2001;30:50. doi: 10.1038/sj/dmfr/4600570. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz Arad D, Gulayev N, Chaushu G. Immediate versus non-immediate implantation for full arch fixed reconstruction following extraction of all residual teeth: A retrospective comparative study. J Periodontal. 2000;71(6):923. doi: 10.1902/jop.2000.71.6.923. [DOI] [PubMed] [Google Scholar]
- 43.Bain CA, Moy PK. The association between the failure of dental implants and cigarette smoking. Inter J Oral Maxillofac Implants. 1993;8:609. [PubMed] [Google Scholar]
- 44.Hass R, Haimbock W, Mailath G, Watzek G. The relationship of smoking on peri-implant tissue. A retrospective study. J Proathetic Dent. 1996;76:592. doi: 10.1016/s0022-3913(96)90435-7. [DOI] [PubMed] [Google Scholar]
- 45.Meecham JG, MacGregor ID, Rogers SN, Hobson RS, Bate GP, Dennison M. The effect of smoking on immediate post-extraction socket filling with blood and the incidence of painful socket. Br J Oral Maxillofac Surg. 1988;26:402. doi: 10.1016/0266-4356(88)90093-9. [DOI] [PubMed] [Google Scholar]
- 46.Bolin A, Eklund G, Lavestdt S. The effect of changed smoking habits on marginal alveolar bone loss. A longitudinal study. Swed Dent J. 1993;64:16. [PubMed] [Google Scholar]
- 47.Holm G. Smoking as an additional risk for tooth loss. J Periodontol. 1994;65:996. doi: 10.1902/jop.1994.65.11.996. [DOI] [PubMed] [Google Scholar]
- 48.Haber J, Wattles J, Crowely M, Mandel R, Josphepora K, Kent RL. Evidence for cigarette smoking as a major risk for periodontotos. J Periodontol. 1993;64:16. doi: 10.1902/jop.1993.64.1.16. [DOI] [PubMed] [Google Scholar]
- 49.Weyant RJ. Characteristics associated with the loss of peri-implant tissue health of endosseuos dental implants. Inter J Oral Maxillofac Implants. 1994;9:95. [PubMed] [Google Scholar]
- 50.Buser D, Weber HP, Lang NP. Tissue integration of non-submreged implants. One year results of prospective study with with 100 (T) hollow –cylinder and hollow screw implants. Clin Oral Implant Res. 1990;1:33. doi: 10.1034/j.1600-0501.1990.010105.x. [DOI] [PubMed] [Google Scholar]
- 51.Buser D, Mericske-Stern R, Bernard J. Long term evaluation of non-submerged (T) implants. Clin Oral Implant Res. 1997;8:161. doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 52.Schroeder A, Van Der Zypen E, Slich H, Shutter H. The reaction of bone, connective tissue and epithelium to endosteal implants with sprayed titanium surfaces. J Oral Maxillofac Surg. 1981;3:161. doi: 10.1016/s0301-0503(81)80007-0. [DOI] [PubMed] [Google Scholar]
- 53.Babbush CA, Kent JN, Misiek DJ. Titanium plasma sprayed (TPS) screw implants for the reconstruction of the edentulous mandible. J Oral Maxillofac Surg. 1986;44:274. doi: 10.1016/0278-2391(86)90078-9. [DOI] [PubMed] [Google Scholar]
- 54.Gomez A, Lozada JL, Caplanis N, Klienman A. Immediate loading of a single hydroxyl-apatite coated threaded root formed implant: A Clinical report. J Oral Implantol. 1999;24(3):159. doi: 10.1563/1548-1336(1998)024<0159:ILOASH>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Schiroli G. Immediate tooth extraction, placement of a tapered screw–vent implant and provisionalization in the esthetic zone. A case report. Implant Dent. 2003;12(2):123. doi: 10.1097/01.id.0000055822.98106.94. [DOI] [PubMed] [Google Scholar]
- 56.Gray W, Coatoam DDS. Induced sinus augmentation procedure using one stage anatomically shaped root form implant. J Oral Implantol. 1999;23:25. [PubMed] [Google Scholar]
- 57.Salama H, Rose L, Salama M, Betts N. Immediate loading of bilaterally splinted titanium root form implants in fixed prosthodontics. A Technique re-examined, two case reports. Inter J Period Res Dent. 1995;15:345. [PubMed] [Google Scholar]
- 58.Apse P, Zarb GA, Schmitt A, Lewis DW. The longitudinal effectiveness of osseointegration of dental implants. The Toronto Study: Peri implant mucosal response. Inter J Period Res Dent. 1991;11:95. [PubMed] [Google Scholar]
- 59.Adell R, Lekholm U, Rockier B, Branemark PJ, Lindhe J, Ericksson B, Shordon L. Marginal tissue reactions at osseointegrated titanium fixture (1) A three year longitudinal prospective study. Inter J Oral Maxillofac Surg. 1986;15:39. doi: 10.1016/s0300-9785(86)80010-2. [DOI] [PubMed] [Google Scholar]
- 60.Nakous M, Mikx THM, Osterwal PJM, Kruijen JCWM. Early microbial colonization of peri-mucosal implants in edentulous patients. J Dent Res. 1987;66:1654. doi: 10.1177/00220345870660111001. [DOI] [PubMed] [Google Scholar]
- 61.Sanz M, Newman MG, Nachmani S, Holt R, Stewart R, Fleming T. Characterization of the subgingival microbial flora around endosseal sapphire dental implants in partially edentulous patients. Inter J Oral Maxillofac Implants. 1990;5:31. [PubMed] [Google Scholar]
- 62.Niklaus P, Lindhe L, Lindhe J. Maintenance of the implant patient. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. 4th ed. Vol. 1024. Blackwell Munksgaard Publ. Co; UK, Tokyo, Berlin, Paris: 2003. Cited in. [Google Scholar]
- 63.Hansson HA, Alberktason T, Branemark PJ. Structural aspects of the interface between tissue and titanium implants. J Prosthet Dent. 1983;50:108. doi: 10.1016/0022-3913(83)90175-0. [DOI] [PubMed] [Google Scholar]
- 64.Lekholm U, Adell R, Lindhe J, Branemark PI, Ericksson B, Rockler B, Lindvall AM, Yoneyama T. Marginal tissue reactions at osseointegrated titanium fixtures, A cross sectional retrospective study. Inter J Oral Maxillofac Surg. 1986;15:53. doi: 10.1016/s0300-9785(86)80011-4. [DOI] [PubMed] [Google Scholar]
- 65.Smith DE, Zarb GA. Criteria for success of osseointegrated endosseous implants. J Prosthet Dent. 1989;62:567. doi: 10.1016/0022-3913(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 66.Jovanovic SA. The management of peri-implant breakdown around functioning osseointegrated dental implants. J Periodontol. 1993;64:1176. doi: 10.1902/jop.1993.64.11s.1176. [DOI] [PubMed] [Google Scholar]
- 67.Janfrom A, Vanginkel FC, Vanamerongen JP, Vanderstelt PF. Scanning resolution and detection of proximal caries. Dentomaxillo Fac Radiol. 2001;30:166. [Google Scholar]
- 68.Dehlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconst Surg. 1988;5:672. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Brunell G, Brocard D, Duffort JF, Jacquet E, Justumus P, Simonet T, Benque E. Bioabsorbable materials for guided tissue regeneration prior to implant placement and 7-year follow –up. Report of 14 cases. J Periodontl. 2001;72:257. doi: 10.1902/jop.2001.72.2.257. [DOI] [PubMed] [Google Scholar]
- 70.Fowler EB, Breault LG, Rebitski G. Ridge preservation utilizing an acellular dermal allograft and demineralized freeze-dried bone allograft. Part II Immediate endosseous implant placement. J Periodontal. 2000;71:1670. doi: 10.1902/jop.2000.71.8.1360. [DOI] [PubMed] [Google Scholar]
- 71.Novaes AB, Souza SL. A cellular dermal matrix graft as a membrane for guide bone regeneration. A case report. Implant Dent. 2001;10:192. doi: 10.1097/00008505-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Leghissa GC, Salvatorelli G, Gulinati AM, Ansanel D, Marchetti MG. A new material for guided tissue regeneration. Modern Dentistry. 1997;6:77. [Google Scholar]
- 73.Serino G, Bianew S, Lezzi G, Piatelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolic sponge as space filler. A Clinical and Histologic study in humans. Clin Oral Implant Res. 2003;14:651. doi: 10.1034/j.1600-0501.2003.00970.x. [DOI] [PubMed] [Google Scholar]