Abstract
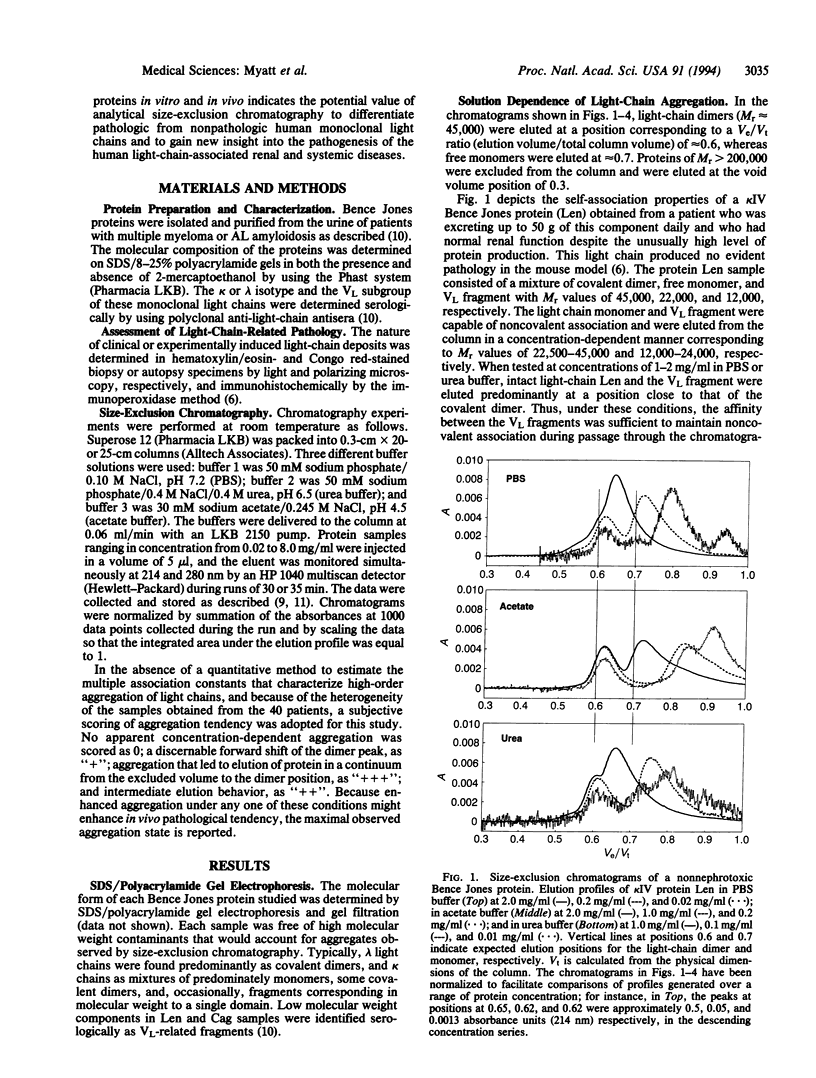
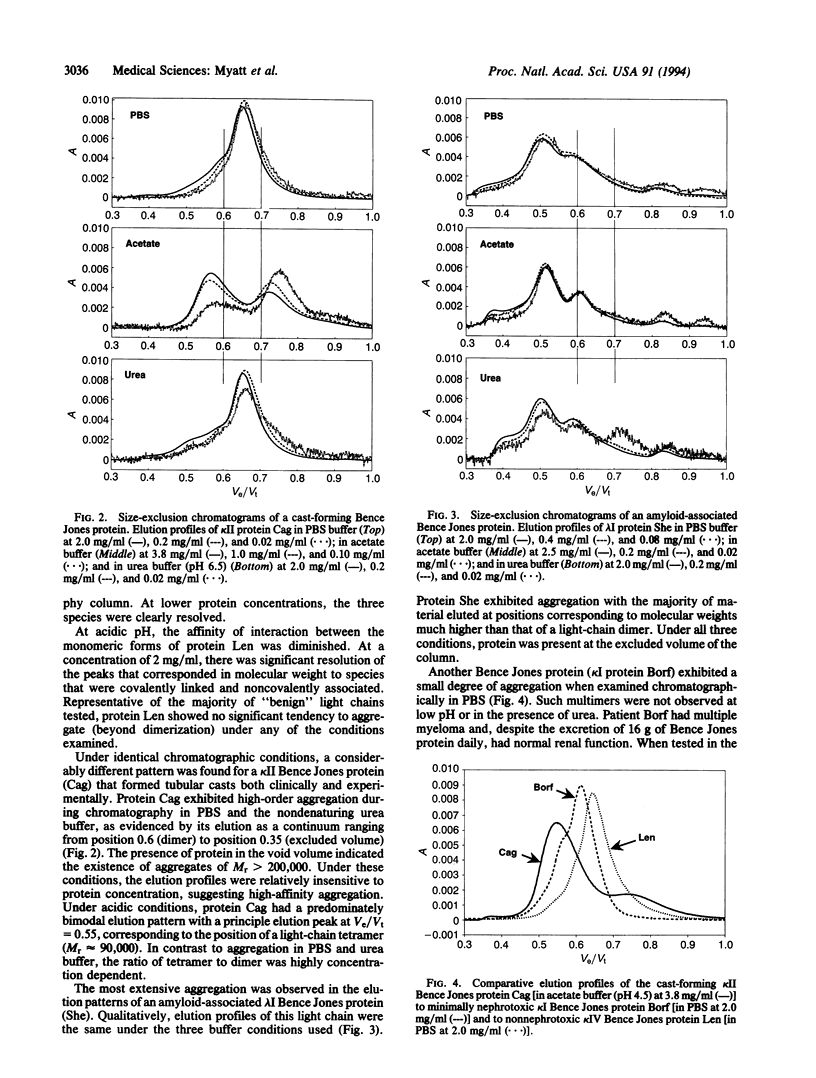
The deposition of certain Bence Jones proteins as tubular casts, basement membrane precipitates, or amyloid fibrils results in the human light-chain-associated renal and systemic diseases--myeloma (cast) nephropathy, light-chain deposition disease, and immunocyte-derived (primary or AL) amyloidosis. To determine if light-chain nephrotoxicity or amyloidogenicity is related to the propensity of these components to form high molecular weight aggregates under physiological conditions, we used a size-exclusion chromatographic system to study 40 different Bence Jones proteins. Each samples was tested over a wide range of protein concentration in three different buffers varying in pH, osmolality, and the presence or absence of low concentrations of urea. Thirty-three of the 35 proteins found clinically and/or experimentally to form in vivo pathologic light-chain deposits were shown to undergo high-order self-association and form high molecular weight aggregates. In contrast, of five nonpathologic proteins, one showed polymerization under the chromatographic conditions used. The correlation between the in vivo results achieved by size-exclusion chromatography and that found in vivo provides (i) a rapid diagnostic method to identify potential nephrotoxic or amyloidogenic Bence Jones proteins and (ii) an experimental means to gain new insight into the physicochemical basis of light-chain aggregation and the treatment of those invariably fatal disorders associated with pathologic light-chain deposition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Analysis and management of renal failure in fourth MRC myelomatosis trial. MRC working party on leukaemia in adults. Br Med J (Clin Res Ed) 1984 May 12;288(6428):1411–1416. doi: 10.1136/bmj.288.6428.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- BERNIER G. M., PUTNAM F. W. POLYMERISM, POLYMORPHISM, AND IMPURITIES IN BENCE-JONES PROTEINS. Biochim Biophys Acta. 1964 May 11;86:295–308. doi: 10.1016/0304-4165(64)90056-x. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. N., Chuba J. V., Hellman G. C., Solomon A., Gallo G. R. Monoclonal immunoglobulin deposition disease: light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology, and molecular analysis. Ann Intern Med. 1990 Mar 15;112(6):455–464. doi: 10.7326/0003-4819-76-3-112-6-455. [DOI] [PubMed] [Google Scholar]
- Clyne D. H., Pesce A. J., Thompson R. E. Nephrotoxicity of Bence Jones proteins in the rat: importance of protein isoelectric point. Kidney Int. 1979 Sep;16(3):345–352. doi: 10.1038/ki.1979.137. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kohler P. F. A case of tetramer Bence Jones proteinaemia. Clin Exp Immunol. 1968 Mar;3(3):277–285. [PMC free article] [PubMed] [Google Scholar]
- Holland M. D., Galla J. H., Sanders P. W., Luke R. G. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985 Jan;27(1):46–50. doi: 10.1038/ki.1985.8. [DOI] [PubMed] [Google Scholar]
- Huang Z. Q., Kirk K. A., Connelly K. G., Sanders P. W. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J Clin Invest. 1993 Dec;92(6):2975–2983. doi: 10.1172/JCI116920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky R., Snow A. The potential significance of sulphated glycosaminoglycans as a common constituent of all amyloids: or, perhaps amyloid is not a misnomer. Med Hypotheses. 1988 Aug;26(4):231–236. doi: 10.1016/0306-9877(88)90125-9. [DOI] [PubMed] [Google Scholar]
- Kosaka M., Iishi Y., Okagawa K., Saito S., Sugihara J., Muto Y. Tetramer Bence Jones protein in the immunoproliferative diseases. Angioimmunoblastic lymphadenopathy, primary amyloidosis, and multiple myeloma. Am J Clin Pathol. 1989 Jun;91(6):639–646. doi: 10.1093/ajcp/91.6.639. [DOI] [PubMed] [Google Scholar]
- Koss M. N., Pirani C. L., Osserman E. F. Experimental Bence Jones cast nephropathy. Lab Invest. 1976 Jun;34(6):579–591. [PubMed] [Google Scholar]
- Martinez-Maldonado M., Yium J., Suki W. N., Eknoyan G. Renal complications in multiple myeloma: pathophysiology and some aspects of clinical management. J Chronic Dis. 1971 Jul;24(4):221–227. doi: 10.1016/0021-9681(71)90076-2. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Putnam F. W. Characterization of the thermal denaturation of Bence-Jones proteins by ultracentrifugation at elevated temperatures. J Biol Chem. 1966 May 25;241(10):2320–2325. [PubMed] [Google Scholar]
- PUTNAM F. W., EASLEY C. W., LYNN L. T., RITCHIE A. E., PHELPS R. A. The heat precipitation of Bence-Jones proteins. I. Optimum conditions. Arch Biochem Biophys. 1959 Jul;83(1):115–130. doi: 10.1016/0003-9861(59)90016-5. [DOI] [PubMed] [Google Scholar]
- Sanders P. W., Booker B. B., Bishop J. B., Cheung H. C. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J Clin Invest. 1990 Feb;85(2):570–576. doi: 10.1172/JCI114474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Galla J. H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987 Dec;32(6):851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- Smolens P., Barnes J. L., Stein J. H. Effect of chronic administration of different Bence Jones proteins on rat kidney. Kidney Int. 1986 Dec;30(6):874–882. doi: 10.1038/ki.1986.267. [DOI] [PubMed] [Google Scholar]
- Solomon A. Clinical implications of monoclonal light chains. Semin Oncol. 1986 Sep;13(3):341–349. [PubMed] [Google Scholar]
- Solomon A., Frangione B., Franklin E. C. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982 Aug;70(2):453–460. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A. Light chains of human immunoglobulins. Methods Enzymol. 1985;116:101–121. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- Solomon A., McLaughlin C. L. Bence-Jones proteins and light chains of immunoglobulins. I. Formation and characterization of amino-terminal (variant) and carboxyl-terminal (constant) halves. J Biol Chem. 1969 Jun 25;244(12):3393–3404. [PubMed] [Google Scholar]
- Solomon A., Weiss D. T., Kattine A. A. Nephrotoxic potential of Bence Jones proteins. N Engl J Med. 1991 Jun 27;324(26):1845–1851. doi: 10.1056/NEJM199106273242603. [DOI] [PubMed] [Google Scholar]
- Solomon A., Weiss D. T. Ominous consequences of immunoglobulin deposition. N Engl J Med. 1993 Nov 4;329(19):1422–1423. doi: 10.1056/NEJM199311043291913. [DOI] [PubMed] [Google Scholar]
- Solomon A., Weiss D. T., Williams T. K. Experimental model of human light-chain-associated disease. Curr Top Microbiol Immunol. 1992;182:261–267. doi: 10.1007/978-3-642-77633-5_32. [DOI] [PubMed] [Google Scholar]
- Stevens F. J. Analysis of protein-protein interaction by simulation of small-zone size exclusion chromatography. Stochastic formulation of kinetic rate contributions to observed high-performance liquid chromatography elution characteristics. Biophys J. 1989 Jun;55(6):1155–1167. doi: 10.1016/S0006-3495(89)82912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens F. J. Analysis of protein-protein interaction by simulation of small-zone size-exclusion chromatography: application to an antibody-antigen association. Biochemistry. 1986 Mar 11;25(5):981–993. doi: 10.1021/bi00353a006. [DOI] [PubMed] [Google Scholar]
- Stevens F. J., Solomon A., Schiffer M. Bence Jones proteins: a powerful tool for the fundamental study of protein chemistry and pathophysiology. Biochemistry. 1991 Jul 16;30(28):6803–6805. doi: 10.1021/bi00242a001. [DOI] [PubMed] [Google Scholar]
- Sølling K., Sølling J., Lanng Nielsen J. Polymeric Bence Jones proteins in serum in myeloma patients with renal insufficiency. Acta Med Scand. 1984;216(5):495–502. doi: 10.1111/j.0954-6820.1984.tb05037.x. [DOI] [PubMed] [Google Scholar]
- Voltarelli J. C., Carvalho I. F. Experimental nephropathy induced by human polyclonal light chains. Braz J Med Biol Res. 1985;18(3):315–326. [PubMed] [Google Scholar]
- Wood S. P., Oliva G., O'Hara B. P., White H. E., Blundell T. L., Perkins S. J., Sardharwalla I., Pepys M. B. A pentameric form of human serum amyloid P component. Crystallization, X-ray diffraction and neutron scattering studies. J Mol Biol. 1988 Jul 5;202(1):169–173. doi: 10.1016/0022-2836(88)90529-3. [DOI] [PubMed] [Google Scholar]



