Abstract
Background
Reduced cerebrospinal fluid (CSF) α-synuclein has been described in synucleinopathies, including dementia with Lewy bodies (DLB). Common symptoms of DLB include visual hallucinations and visuospatial and executive deficits. Co-occurrence of Lewy body pathology is common in Alzheimer’s disease (AD) patients, but it is unknown if reduced CSF α-synuclein is associated with Lewy body-like symptomatology in AD.
Objective
Determine associations between CSF α-synuclein and Lewy body-like symptomatology.
Methods
We included 73 controls (NC), 121 mild cognitive impairment (MCI) patients, and 61 AD patients (median follow-up 3.5 years, range 0.6–7.8). We tested associations between baseline CSF α-synuclein and visual hallucinations and (longitudinal) cognition. Models were tested with and without co-varying for CSF total tau (T-tau), which is elevated in AD patients, and believed to reflect neurodegeneration.
Results
Hallucinations were reported in 20% of AD patients, 13% of MCI patients, and 8% of NC. In AD, low CSF α-synuclein was associated with hallucinations. When adjusting for CSF T-tau, low CSF α-synuclein was associated with accelerated decline of executive function (NC, MCI, and AD), memory (MCI and AD), and language (MCI).
Conclusion
The associations of low CSF α-synuclein with hallucinations and poor executive function, which are hallmarks of DLB, indirectly suggest that this biomarker may reflect underlying synuclein pathology. The associations with memory and language in MCI and AD suggests either that reduced CSF α-synuclein also partly reflects global impaired neuronal/synaptic function, or that non-specific overall cognitive deterioration is accelerated in the presence of synuclein related pathology. The findings will require autopsy verification.
Keywords: Alpha-synuclein, Alzheimer’s disease, cerebrospinal fluid, cognition, hallucinations, tau
INTRODUCTION
Alzheimer’s disease (AD) is associated with brain accumulation of amyloid-β (Aβ) and tau, and neurodegeneration [1], but co-occurrence of other pathologies is common [2–4]. About 10–40% of AD patients have concomitant Lewy bodies composed of α-synuclein [2, 3, 5–8], which may be partly responsible for the clinical heterogeneity of AD [9]. Analysis of cerebrospinal fluid (CSF) proteins may provide insight into mechanisms of neurodegenerative diseases, including disease heterogeneity [10]. CSF tau (total-tau, T-tau) and phosphorylated-tau (P-tau) are elevated in AD, where they may reflect the presence of neurodegeneration and neurofibrillary tangles (although it should be noted that several other diseases with neurodegeneration and tangles have normal levels of T-tau and P-tau), while CSF Aβ42 is reduced, likely due to peptide sequestration in senile plaques [11]. CSF α-synuclein has been explored as an in vivo marker of disorders associated with α-synuclein aggregates in pathological lesions. Patients with synucleinopathies, e.g., Parkinson’s disease, dementia with Lewy bodies (DLB), and multi-system atrophy often have reduced CSF α-synuclein compared to AD and controls [12, 13]. Although still controversial, this has been hypothesized to be due to protein entrapment in the brain parenchyma. In contrast, most [14–18] (but not all [19, 20]) studies of mild cognitive impairment (MCI) and AD have shown increased CSF α-synuclein in AD, correlating with increased CSF T-tau [18, 21]. In summary, α-synuclein pathology alone is often associated with reduced CSF α-synuclein, while AD pathology is most often associated with normal or increased CSF α-synuclein.
In AD, the relationship of CSF α-synuclein to DLB symptoms, including hallucinations, visuospatial impairment, and executive dysfunction [22–24] is unknown. Several autopsy studies (but not all [25]) have found higher rates of hallucinations, and worse visuospatial and executive performance in AD with Lewy bodies than in pure AD [26, 27]. Since reduced CSF α-synuclein may reflect brain synucleinopathy, it is possible that reduced CSF α-synuclein may also be associated with Lewy body-like symptoms. Few studies have tested associations between CSF α-synuclein and neurological-cognitive symptoms, but one study found that low CSF α-synuclein was related to poor performance on the Mini-Mental State Examination (MMSE) and category fluency in DLB [28], and a previous Alzheimer’s Disease Neuroimaging Initiative (ADNI) study found associations between low CSF α-synuclein and accelerated cognitive decline when testing a mixed group of healthy controls, MCI, and AD [18]. In this study (on the same subjects as [18]), we tested, within each diagnostic group, the hypotheses that lower CSF α-synuclein levels are associated with 1) hallucinations, a core clinical feature of DLB, 2) worse visuospatial performance, as determined by the constructional praxis test in the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-cog), and 3) overall executive function, as determined by a composite cognitive score. We also tested associations between CSF α-synuclein and a composite memory score and the Boston Naming Test, since deficits of memory and language are less characteristic for DLB. Associations were tested at baseline and longitudinally. Furthermore, since we aimed to estimate effects of CSF α-synuclein that were independent of AD neurodegenerative changes, we co-varied all models for CSF T-tau.
METHODS AND METHODS
Study design
Data were obtained from the ADNI database (http://adni.loni.usc.edu/). The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. For up-to-date information, see http://www.adni-info.org/
Participants
Our study population consisted of subjects from the ADNI-1 CSF sub-study with available CSF α-synuclein and hallucination data. Inclusion/exclusion criteria are described in detail at http://www.adni-info.org/. Briefly, all subjects included in ADNI-1 were between the ages of 55 and 90 years, had completed at least 6 years of education, were fluent in Spanish or English, and were free of any significant neurologic disease other than AD. NC subjects had MMSE score ≥24, and a Clinical Dementia Rating scale (CDR) score of 0. MCI subjects had MMSE score ≥24, objective memory loss as shown on delayed recall of the Wechsler Memory Scale Logical Memory II (>1.5 standard deviations below the normal mean), CDR of 0.5, preserved activities of daily living, and absence of dementia. AD dementia subjects fulfilled the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD, had MMSE scores between 20–26, and a CDR of 0.5 or 1.0. All patients underwent a structured neurological examination. A clinical diagnosis of dementia was made by the local (site) clinician, and included all available information, such as history, neurological exam and cognitive testing. Classification of MCI was done by the site principal investigator following the Petersen criteria [29] to define amnestic and non-amnestic subtypes, and results were adjudicated by a central committee. The site principal investigator and review committee used the detailed neuropsychological battery, the CDR, and the Functional Assessment Questionnaire to help to make this diagnosis. Biomarker data were not used at sites to assign clinical diagnoses.
CSF biomarkers
CSF was collected at baseline by lumbar puncture, and shipped frozen in polypropylene tubes to the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center for long term storage in −80°C. CSF Aβ42, T-tau, and P-tau was measured by xMAP with the INNOBIA AlzBio3 kit (Innogenetics, Ghent, Belgium) [30, 31]. CSF α-synuclein was measured using a xMAP bead-based assay by the Zhang laboratory at University of Washington [17, 18]. Only samples with CSF hemoglobin <200 ng/mL were used, to minimize the risk of contamination from blood-based α-synuclein [17]. Intra-assay CVs were <10%.
Clinical data
History of hallucinations was assessed using the informant-based Neuropsychiatric Inventory brief questionnaire form [32], with hallucinations confirmed or denied at every study visit. Constructional Praxis and Boston Naming Tests were administered and scored at every study visit according to the ADNI Procedures Manual (http://adni.loni.usc.edu). Composite scores aimed at measuring executive function and memory were defined previously [33, 34] as metrics with the mean 0 and the standard deviation 1, based on combinations of measures of executive function (Wechsler Adult Intelligence Scale-Revised (WAIS-R) digit symbol substitution, digit span backwards, trails A and B, category fluency, and clock drawing), and memory (Rey Auditory Verbal Learning (RAVL) Learning, RAVL Recall, two memory tasks from ADAS-Cog, WAIS R Logical Memory [immediate and delayed recall], and a recall task from MMSE). The executive score has been associated with progression to dementia, brain structure, CSF Aβ42, T-tau, and P-tau [33], and with Lewy body pathology in AD [8]. The memory score has been associated with progression to dementia and with brain structure [34].
Statistical analyses
Demographics were compared between groups using non-parametric tests (Mann-Whitney U or chi-square tests). α-synuclein data were log transformed and standardized. Associations between α-synuclein and demographic factors were evaluated using Mann-Whitney U tests and Spearman correlation tests.
For analysis of associations between α-synuclein and hallucinations, we used a binary variable (hallucinations reported in at least one study visit versus hallucinations denied at all study visits). This approach was used because hallucinations may be intermittent. Ordinary least squares linear regression models were tested with α-synuclein as the dependent variable and hallucinations as the independent variable, in each diagnostic group separately. Models where tested with and without adjusting for T-tau (and with and without adjusting for use of benzodiazepines and benzodiazepine-like hypnotic medications). For comparison, we also tested T-tau, P-tau, and Aβ42 as dependent variables.
Analyses of α-synuclein and cognitive data were done using mixed effects models, estimating effects at baseline and longitudinally. For constructional praxis (score 0–5), few data points had scores >1 (0 : 669; 1 : 739; 2 : 79; 3 : 18; 4 : 4; 5 : 1). Because of the sparsity of the data in the upper cells, we dichotomized the score (0 versus >0). Effects were tested assuming a binomial response, with constructional praxis as the dependent variable, and α-synuclein as the independent variable. For analysis of other cognitive data, we used linear mixed effects regression models. Each diagnostic group was tested separately. We evaluated regression models including the interactions α-synuclein × T-tau, α-synuclein × time, T-tau × time, and α-synuclein × T-tau × time (all main effects were also included). The three-way-interaction and the interaction between α-synuclein and T-tau were non-significant in all models (with one exception in MCI as explained below) and were removed. The final models included the interactions α-synuclein × time, T-tau × time, and the main effects of α-synuclein, T-tau, and time. Although the main objective was to assess α-synuclein, we also report model estimates for T-tau, when they were significant.
All regression models were adjusted for age, gender, and education, and all mixed effects models included a random slope and intercept. We assessed the applicability of models by evaluating the normality of residuals, associations between residuals and fitted values, and q-q plots. Statistical significance was determined at p < 0.05. All statistics were done using R (v. 3.0.1, The R Foundation for Statistical Computing). Mixed effects models were fit using the lme4 package (v. 1.0–5.) or the nlme package (v.3.1–111).
Ethics
Written informed consent was obtained from all participants and the study was conducted with prior institutional ethics approval.
RESULTS
The study included 73 NC, 121 MCI, and 61 AD patients (Table 1). There were no group differences in age, education, or gender, but there were more APOE ε4-positives in MCI and AD (p < 0.001). There were no associations between α-synuclein and demographic factors. Subjects were followed with multiple visits (up to 11) for a median of 3.5 years (range 0.6–7.8 years). Seventeen NC progressed to MCI, 2 NC to AD, and 62 MCI to AD, while 3 subjects reverted from MCI to NC, and 1 from AD to MCI. When used in combination with P-tau, T-tau, and Aβ42, reduced CSF α-synuclein was significantly associated with conversion from MCI to AD, as has been published previously [18].
Table 1.
Demographics
| Group | NC | MCI | AD | All |
|---|---|---|---|---|
| N | 73 | 121 | 61 | 255 |
| Gender, M:F (% F) | 38 : 35 (48%) p = 0.85 |
80 : 41 (34%) p = 0.81 |
34 : 27 (44%) p = 0.71 |
152 : 103 (40%) p = 0.94 |
| Age, y (mean, SD) | 75.6 (4.9) p = 0.12 |
74.0 (7.5) p = 0.46 |
74.6 (8.4) p = 0.92 |
74.6 (7.1) p = 0.37 |
| Education, y mean, SD) | 15.9 (2.5) p = 0.74 |
15.5 (3.0) p = 0.12 |
15.3 (3.1) p = 0.37 |
15.6 (2.9) p = 0.12 |
| APOE ε4, +:− (%+) | 16 : 57 (22%) p = 0.41 |
67 : 54 (55%) p = 0.54 |
38 : 23 (62%) p = 0.41 |
121 : 134 (48%) p = 0.052 |
| CSF α-synuclein, ng/mL (mean, SD) | 0.43 (0.14) | 0.50 (0.18) | 0.56 (0.20) | 0.49 (0.18) |
| Baseline constructional praxis | 0 : 45, 1 : 28 | 0 : 50, 1 : 71 | 0 : 13, 1 : 48 | 0 : 108; 1 : 147 |
| Baseline executive function (mean, SD) | 0.67 (0.63) | −0.017 (0.71) | −0.94 (0.83) | −0.041 (0.93) |
| Baseline memory function (mean, SD) | 0.93 (0.48) | −0.16 (0.60) | −0.74 (0.53) | 0.010 (0.83) |
| Baseline Boston Naming Test (mean, SD) | 27.4 (2.8) | 25.7 (4.2) | 23.9 (5.3) | 25.8 (4.3) |
p-values are for associations with each variable and CSF α-synuclein (log), tested by Spearman correlation (age, education, MMSE, clock-drawing) or Mann-Whitney U test (gender, APOE genotype). Constructional Praxis is dichotomized (0 versus >0). Executive function and memory function are composite scores, defined as explained in [33, 34].
Hallucinations
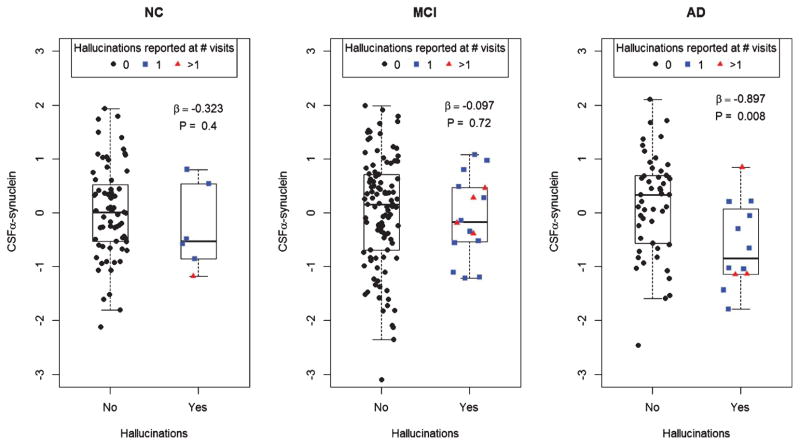
Hallucinations were reported by a total of 12 (20%) AD (9 at one visit, 3 at several visits), 16 (13%) MCI (12 at one visit, 4 at several visits), and 6 (8%) NC subjects (5 at one visit, 1 at several visits). Hallucinations were significantly associated with reduced α-synuclein in AD (Fig. 1). The results were similar when adjusting for T-tau, P-tau, and Aβ42, and when adjusting for use of benzodiazepines and benzodiazepine-like hypnotic medications (NC, n = 4; MCI, n = 6; AD, n = 4) (data not shown). There were no associations between hallucinations and T-tau, P-tau, or Aβ42 in any diagnostic group (data not shown).
Fig. 1.
CSF α-synuclein and hallucinations. CSF α-synuclein (logarithmized, centered, and scaled) in people with or without a medical history of hallucinations (grouped by baseline diagnosis). The data is adjusted for age, gender, and education. β-coefficients and p-values are for the statistical effects of hallucinations on CSF α-synuclein in respective diagnostic group (tested by ordinary least square linear regression).
Executive function
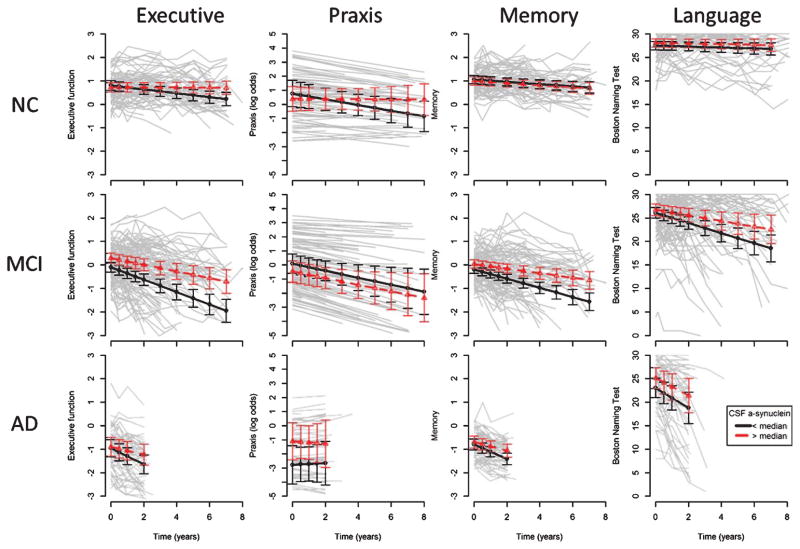
Baseline and longitudinal executive function scores are shown in Table 1 and Fig. 2, left column. The three-way-interaction between T-tau, α-synuclein and time was marginally significant in MCI (p = 0.039), but a detailed examination did not reveal any conclusive associations, wherefore the interaction was removed. Poor baseline executive function was associated with low α-synuclein in MCI (β = 0.20, p = 0.033). T-tau was not associated with baseline executive function. Accelerated decline of executive function was associated with low α-synuclein (NC, β = 0.044, p = 0.0039; MCI, β=0.089, p = 0.0008; AD, β = 0.15, p = 0.012) and high T-tau (NC, β = −0.054, p = 0.0005; MCI, β = −0.14, p < 0.0001; AD, β = −0.20, p = 0.0008).
Fig. 2.
CSF α-synuclein and cognition. First column: Composite score for executive function. Executive function worsened with time in NC (β = −0.045, p < 0.0001), MCI (β = −0.20, p < 0.0001), and AD (β = −0.26, p < 0.0001). Low α-synuclein was associated with accelerated decline in NC (p = 0.0039), MCI (p = 0.0006), and AD (p = 0.012). Second column: Constructional praxis (displayed as log odds, since scores were dichotomous and modeled using logistic regression). Higher scores indicate worse performance. Praxis performance worsened with time in NC (OR = 1.22, p = 0.013) and MCI (OR = 1.28, p = 0.018), but not in AD (OR = 0.94, p = 0.89). α-synuclein had no effects at baseline or longitudinally. Third column: Composite score for memory. Memory function worsened with time in NC (β = −0.047, p < 0.0001), MCI (β = −0.15, p < 0.0001), and AD (β = −0.25, p < 0.0001). Low α-synuclein was associated with poor baseline memory in MCI (β = 0.20, p = 0.0095), and accelerated decline in MCI (β = 0.049, p = 0.024) and AD (β = 0.12, p = 0.0057). Fourth column: Boston Naming Test. Language function worsened with time in MCI (β = −0.83, p < 0.0001) and AD (β = −1.98, p < 0.0001), but not significantly in NC (β = −0.084, p = 0.073). Low α-synuclein was associated with accelerated decline in MCI (β = 0.40, p = 0.017). For visualization purposes, the graphs include fits from mixed effects models, showing the intercepts and slopes for subjects with a-synuclein above (dashed, triangles) or below (solid, circles) the median (but the coefficients and significances in this section are from models using continuous biomarkers). All models were adjusted for time, age, gender, education, T-tau, and the interaction between time and T-tau.
Constructional praxis
Baseline and longitudinal constructional praxis scores are shown in Table 1 and Fig. 2, second column. There were no significant associations between α-synuclein and constructional praxis at baseline or longitudinally. High T-tau was associated with worse baseline constructional praxis in NC (OR = 2.4, p = 0.040) and AD (OR = 5.2, p = 0.029).
Memory
Baseline and longitudinal executive function scores are shown in Table 1 and Fig. 2, third column. Poor baseline memory was associated with low α-synuclein and high T-tau in MCI (β = 0.20, p = 0.0095; β = −0.31, p = 0.0001). Accelerated decline of memory was associated with low α-synuclein in MCI (β = 0.049, p = 0.024) and AD (β = 0.12, p = 0.0057), and high T-tau in all groups (NC, β = −0.033, p = 0.022; MCI, β = −0.097, p < 0.0001; AD, β = −0.16, p = 0.0006).
Language
Baseline and longitudinal language scores are shown in Table 1 and Fig. 2, fourth column. There were no associations between baseline language function and biomarkers. Accelerated decline of language was associated with low α-synuclein and high T-tau in MCI (β = 0.40, p = 0.017; β = −0.69, p = 0.0001).
DISCUSSION
The main findings were that 1) AD patients with hallucinations had reduced CSF α-synuclein, and that 2) low CSF α-synuclein was associated with accelerated decline of executive function in NC, MCI, and AD. Low α-synuclein was also associated with accelerated decline of language in MCI, and memory in MCI and AD. High T-tau was associated with longitudinal decline in several cognitive domains. These findings, especially the fact that α-synuclein levels were reduced in AD patients with hallucinations, indirectly suggest an association between reduced α-synuclein and DLB-like symptomatology in AD dementia patients. The finding that low α-synuclein was associated with accelerated decline of memory and language is difficult to explain since these domains are not considered characteristic of DLB. However, it is consistent with previous data on overall accelerated cognitive deterioration in patients with the Lewy body variant of AD [9, 35]. It is also possible that α-synuclein may be reduced partly due to overall reduced neuronal/synaptic function, as proposed in a study showing a correlation between low CSF α-synuclein and low MMSE in AD patients [20].
We are not aware of any previous study testing associations between hallucinations and CSF α-synuclein, but in a recent autopsy study, including some of the subjects in this paper, hallucinations were a strong predictor of co-incident Lewy body pathology in patients with AD pathology [8] (and subjects with Lewy body pathology had lower CSF α-synuclein than subjects without Lewy body pathology, but the difference was not significant, p = 0.13). Another autopsy study found that hallucinations were associated with DLB in early-stage dementia [22]. Studies of α-synuclein in AD have shown mixed results, both when comparing AD patients to controls, and when comparing AD with other dementias [14–16, 19, 20, 28]. Such discrepancies may be due to methodological differences between studies. Another possibility is that CSF α-synuclein is oppositely affected by different forces. According to this theory, significant neurodegeneration increases CSF α-synuclein levels (very high levels are seen in Creutzfeldt-Jakob’s disease [19]), while synucleinopathy reduces levels (low levels are seen in Parkinson’s disease and DLB [19]). Previous studies on the subjects analyzed in this paper found increased CSF α-synuclein in MCI and AD [17], and correlations between CSF α-synuclein and T-tau [18], but no major associations between CSF α-synuclein and regional brain atrophy rates [36]. It should be stressed, however, that mechanisms regulating CSF levels of these biomarkers likely reach beyond what is proposed above, given that not all diseases with significant neurodegeneration, including those with significant tauopathies, demonstrate increased CSF α-synuclein levels (or even tau for that matter) [16].
The finding that low α-synuclein was associated with longitudinal decline in performance on executive function, which is characteristic of DLB [23], supports the link between reduced α-synuclein and a possible presence of α-synuclein pathology (although we did not find any association between α-synuclein and constructional praxis performance). It should be noted that the MCI subjects enrolled in ADNI were selected to mainly include patients with dominating amnestic symptoms. Amnestic MCI patients are at high risk for AD dementia, while non-amnestic MCI patients are at high risk for DLB [37]. Further studies of α-synuclein and Lewy body-like symptoms are therefore needed in mixed MCI populations. The association between low α-synuclein and accelerated decline in healthy controls may suggest that this biomarker detects preclinical Lewy body pathology, similarly to how CSF Aβ42 may be reduced in preclinical AD [38]. Importantly, these associations were mainly seen when models were adjusted for T-tau, consistent with the theory that α-synuclein is oppositely affected by Lewy body pathology and typical AD pathology where T-tau is consistently elevated [11, 39].
The results that T-tau was associated with accelerated decline of executive function (NC, MCI, and AD), memory (NC, MCI, and AD), and language (MCI), confirms previous studies linking high T-tau to increased risk for cognitive deterioration [40–43]. To our knowledge, no study has reported that reduced α-synuclein and increased T-tau are independently associated with cognitive decline. This supports the notion that α-synuclein and T-tau partly reflects different pathological processes.
The associations between low α-synuclein and accelerated decline of executive function and memory are in line with a previous analysis of the same cohort [18], although there are several differences between these studies. For example, Toledo and colleagues [18] did not assess hallucinations, constructional praxis, or language, and used other metrics for executive function and memory. The studies also differed in statistical methods, including grouping (α-synuclein in all subjects was tested simultaneously in [18], while this study tested each diagnostic group separately), specifications of the statistical models (especially regarding how correction for tau was carried out), and levels of details in the reported results (this paper reports the independent effects of both α-synuclein and T-tau). However, both these studies support that notion that effects of α-synuclein on cognitive measures should be evaluated while co-varying for T-tau (or P-tau [18]), to avoid masking of α-synuclein’s effects.
The main limitation of this study is the lack of autopsy confirmation of Lewy body pathology. Also, the small number of people with hallucinations gives low power to detect associations with α-synuclein in NC and MCI, and we cannot rule out that hallucinations in AD were provoked by medications not accounted for or by or medical or mental illness unrelated to neurodegeneration. Parkinsonism and REM-sleep behavior disorder reflect brainstem pathology, whereas there is evidence that visuospatial deficits and hallucinations reflect cortical pathology in DLB. Reduced CSF α-synuclein levels may reflect cortical aspects of the DLB syndrome [44, 45]. The ADNI study did not systematically assess Parkinsonism or REM-sleep behavior disorder; therefore we have incompletely assessed DLB. Nevertheless, our findings link CSF α-synuclein to a prominent aspect of the DLB phenotype, which has been shown to distinguish DLB and AD in clinical-pathological studies. Another limitation is that we did not analyze levels of neurotransmitters, like acetylcholine, which may be linked to hallucinations. However, despite the fact that all AD patients have cholinergic deficits, not all develop hallucinations and measuring acetylcholine in CSF has not proven diagnostic in AD or DLB. A combination of a cholinergic deficit and specific cortical pathology may predict hallucinations in DLB or Parkinson’s disease dementia [44].
CONCLUSIONS
Low CSF α-synuclein is associated with hallucinations in AD, and (when adjusted for T-tau) with accelerated decline of executive function in NC, MCI, and AD, suggesting that reduced α-synuclein may reflect the presence of Lewy body pathology both in preclinical stages and in patients evaluated for cognitive decline. However, in AD and/or MCI, low α-synuclein is also related to decline in memory and language functions, which suggest either that Lewy bodies (as reflected by low CSF α-synuclein) are related to accelerated overall cognitive deterioration in these subjects, or that CSF α-synuclein is reduced in relation to overall neuronal or synaptic loss. These findings motivate further analyses, especially including autopsy confirmation, and perhaps of cohorts enriched for visual hallucinations, to validate and extend our findings.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimers Association; Alzheimers Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by the Swedish Research Council, Goteborgs Lakaresallskap, Svenska Lakaresallskapet, Sahlgrenska Universitetssjukhuset, Carl-Bertil Laurells fond, Klinisk Biokemi i Norden, the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs Medical Center of San Francisco, and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2445).
References
- 1.Zetterberg H, Mattsson N. Understanding the cause of sporadic Alzheimer’s disease. Expert Rev Neurother. 2014;14:621–630. doi: 10.1586/14737175.2014.915740. [DOI] [PubMed] [Google Scholar]
- 2.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 3.James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307:1798–1800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson JRT, Foa EB. Posttraumatic stress disorder: DSM-IV and beyond. American Psychiatric Press; Washington, DC: 1993. [Google Scholar]
- 5.Jellinger KA, Attems J. Prevalence and pathology of dementia with Lewy bodies in the oldest old: A comparison with other dementing disorders. Dement Geriatr Cogn Disord. 2011;31:309–316. doi: 10.1159/000327360. [DOI] [PubMed] [Google Scholar]
- 6.Hansen LA, Masliah E, Galasko D, Terry RD. Plaque-only Alzheimer disease is usually the lewy body variant, and vice versa. J Neuropathol Exp Neurol. 1993;52:648–654. doi: 10.1097/00005072-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, Householder E, Ayutyanont N, Roontiva A, Bauer RJ, Eisen P, Shaw LM, Davatzikos C, Weiner MW, Reiman EM, Morris JC, Trojanowski JQ Alzheimer’s Disease Neuroimaging, Initiative . Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013;1:65. doi: 10.1186/2051-5960-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson N. CSF biomarkers in neurodegenerative diseases. Clin Chem Lab Med. 2011;49:345–352. doi: 10.1515/CCLM.2011.082. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 12.Lim X, Yeo JM, Green A, Pal S. The diagnostic utility of cerebrospinal fluid alpha-synuclein analysis in dementia with Lewy bodies - a systematic review and meta-analysis. Parkinsonism Relat Disord. 2013;19:851–858. doi: 10.1016/j.parkreldis.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, Dardis A, Deganuto M, De Carlo C, Castrioto A, Balducci C, Paciotti S, Tambasco N, Bembi B, Bonanni L, Onofrj M, Rossi A, Beccari T, El-Agnaf O, Calabresi P. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov Disord. 2014;29:1019–1027. doi: 10.1002/mds.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tateno F, Sakakibara R, Kawai T, Kishi M, Murano T. Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson Disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:213–216. doi: 10.1097/WAD.0b013e31823899cc. [DOI] [PubMed] [Google Scholar]
- 15.Wennström M, Surova Y, Hall S, Nilsson C, Minthon L, Boström F, Hansson O, Nielsen HM. Low CSF levels of both α-synuclein and the α-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS One. 2013;8:e53250. doi: 10.1371/journal.pone.0053250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Håkan W, Decraemer H, Någga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 17.Korff A, Liu C, Ginghina C, Shi M, Zhang J Alzheimer’s Disease Neuroimaging, Initiative. α-Synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2013;36:679–688. doi: 10.3233/JAD-130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol. 2013;126:683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel JP, Locascio JJ, El-Agnaf OM, Schlossmacher MG. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett. 2009;450:332–335. doi: 10.1016/j.neulet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin J-J, De Deyn PP, Engelborghs S. Increased CSF α-synuclein levels in Alzheimer’s disease: Correlation with tau levels. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain. 2006;129:729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 23.Cagnin A, Gnoato F, Jelcic N, Favaretto S, Zarantonello G, Ermani M, Dam M. Clinical and cognitive correlates of visual hallucinations in dementia with Lewy bodies. J Neurol Neurosurg Psychiatr. 2013;84:505–510. doi: 10.1136/jnnp-2012-304095. [DOI] [PubMed] [Google Scholar]
- 24.Salmon DP, Galasko D, Hansen LA, Masliah E, Butters N, Thal LJ, Katzman R. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31:148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa H, Vonsattel JPG, Honig LS. Early neuropsychological discriminants for Lewy body disease: An autopsy series. J Neurol Neurosurg Psychiatry. 2013;84:1326–1330. doi: 10.1136/jnnp-2012-304381. [DOI] [PubMed] [Google Scholar]
- 26.Connor DJ, Salmon DP, Sandy TJ, Galasko D, Hansen LA, Thal LJ. Cognitive profiles of autopsy-confirmed Lewy body variant vs pure Alzheimer disease. Arch Neurol. 1998;55:994–1000. doi: 10.1001/archneur.55.7.994. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 28.Reesink FE, Lemstra AW, van Dijk KD, Berendse HW, van de Berg WDJ, Klein M, Blankenstein MA, Scheltens P, Verbeek MM, van der Flier WM. CSF α-synuclein does not discriminate dementia with Lewy bodies from Alzheimer’s disease. J Alzheimers Dis. 2010;22:87–95. doi: 10.3233/JAD-2010-100186. [DOI] [PubMed] [Google Scholar]
- 29.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 30.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 32.Kaufer DI. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imag Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D Alzheimer’s Disease Neuroimaging, Initiative. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, Kukull WA, Leverenz JB, Cherrier MM. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattsson N, Insel P, Tosun D, Zhang J, Jack CR, Jr, Galasko D, Weiner M for the Alzheimer’s Disease Neuroimaging, Initiative. Effects of baseline CSF α-synuclein on regional brain atrophy rates in healthy elders, mild cognitive impairment and Alzheimer’s Disease. PLoS One. 2013;8:e85443. doi: 10.1371/journal.pone.0085443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, Graff-Radford NR, Wszolek Z, Van Gerpen J, Uitti R, Pedraza O, Murray ME, Aakre J, Parisi J, Knopman DS, Petersen RC. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81:2032–2038. doi: 10.1212/01.wnl.0000436942.55281.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoonenboom NSM, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, Pijnenburg YAL, Blankenstein MA, Rozemuller AJ, Scheltens P, van der Flier WM. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 40.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, Sutphen CL, Benzinger TLS, Mintun MA, Holtzman DM, Morris JC. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR On behalf of the Alzheimer’s Disease Neuroimaging, Initiative. MRI and CSF biomarkers in normal, MCI, and AD subjects: Predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher DA, Parkkinen L, O’Sullivan SS, Spratt A, Shah A, Davey CC, Bremner FD, Revesz T, Williams DR, Lees AJ, Schrag A. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–3309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- 45.Fujishiro H, Iseki E, Kasanuki K, Chiba Y, Ota K, Murayama N, Sato K. A follow up study of non-demented patients with primary visual cortical hypometabolism: Prodromal dementia with Lewy bodies. J Neurol Sci. 2013;334:48–54. doi: 10.1016/j.jns.2013.07.013. [DOI] [PubMed] [Google Scholar]