Abstract
Purpose
To evaluate binding of P-selectin targeted microbubbles (MB) in tumor vasculature; a whole-body imaging and biodistribution study was performed in a tumor bearing mouse model.
Methods
Antibodies were radiolabeled with Tc-99m using the HYNIC method. Tc-99m labeled anti-P-selectin antibodies were avidin-bound to lipid-shelled, perfluorocarbon gas-filled MB and intravenously injected into mice bearing MDA-MB-231 breast tumors. Whole-body biodistribution was performed at 5 min (n=12) and 60 min (n=4) using a gamma counter. Tc-99m labeled IgG bound IgG-control-MB group (n=12 at 5 min; n=4 at 60 min), Tc-99 m-labeled IgG-control-Ab group (n=5 at 5 min; n=3 at 60 min) and Tc-99 m-labeled anti P-selectin-Ab group (n=5 at 5 min; n=3 at 60 min) were also evaluated. Planar gamma camera imaging was also performed at each time point.
Results
Targeted-MB retention in tumor (60 min: 1.8 ± 0.3% ID/g) was significantly greater (p=0.01) than targeted-MB levels in adjacent skeletal muscle at both time points (5 min: 0.7 ± .2% ID/g; 60 min: 0.2 ± 0.1% ID/g) while there was no significant difference (p=0.17) between muscle and tumor retention for the IgG-control-MB group at 5 min.
Conclusions
P-selectin targeted MBs were significantly higher in tumor tissue, as compared with adjacent skeletal tissue or tumor retention of IgG-control-MB.
Keywords: Biodistribution, cancer, microbubbles, P-selectin, targeted delivery
Introduction
Improving targeted delivery of anti-cancer drugs to a solid primary tumor can improve overall effectiveness of current systemic and targeted therapies, while reducing total dose and systemic toxicity. Ultrasound contrast agents are perfluorocarbon, gas-filled, lipid microbubbles (MBs) with a diameter of 1–3 μm. The stability of MBs within microvasculature, combined with their non-toxic and non-immunogenic properties has led to pre-clinical investigations of MBs to improve tumor delivery of therapeutic compounds [1], plasmids [2] and viral vectors [3]. Various drug delivery strategies have been investigated using MBs to improve cancer therapy. Some pre-clinical research utilizing MB-assisted delivery involves a physical association between the MB and therapeutic compound [2,4]. One such approach includes labeling hydrophilic pDNA to the exterior of protein-shelled MBs using non-covalent interactions [5]. Other studies have taken advantage of the unique lipid shell component in conjunction with lipophilic compounds, such as Paclitaxel, to physically join the compound to the MB core [1,6,7]. Additional approaches involve double-emulsified MBs that physically encapsulate hydrophilic macromolecules such as pDNA [8], Doxorubicin [9] and adenovirus [10]. In the latter studies, complete encapsulation of the agent was proven advantageous for systemic or localized delivery because the payload was shielded from immune response and sequestering mechanisms. In all of these strategies, the performance of the MB to transport and deliver a molecule to the targeted region is dependent upon the ability of the MB to specifically accumulate within that tissue.
Targeting MBs to commonly over-expressed receptors in a specified region-of-interest have been shown to improve overall MB accumulation at target sites [11,12]. The active targeting of MBs is achieved by conjugating receptor-specific ligands to the outer shell via biotin–avidin chemistry or covalent linkage [13]. Ligand-modified MBs bind specifically to molecular receptors within the vasculature of the targeted tissue, while unbound MBs are filtered from the circulation [14]. Improved MB accumulation using targeted strategies has been demonstrated in the molecular imaging of tumor angiogenesis [15–17], inflammation [13,18,19] and intravascular thrombi [6,7,20]. Radiolabeling MBs is not a novel concept, as many groups are exploring these techniques for dual-modality US/SPECT or US/PET imaging [21–23], as well as assessing MB distribution [24]. Using these established tools, it is hypothesized that we can better evaluate full body evaluation of P-selectin targeted MBs for imaging and drug delivery. One cellular target currently under investigation is the cell adhesion molecule, P-selectin (CD-62 P), which is commonly over-expressed in tumor endothelial cells [25]. P-selectin is expressed on stimulated endothelial cells and activated platelets; it contributes to the recruitment of leukocytes in areas of inflammation common in tumor vasculature [26,27]. In addition, the presence of P-selectin permits the adhesion of platelets and cancer cells to the tumor endothelium. Strategies for improving MB accumulation have utilized the expression of P-selectin in echocardiography, atherosclerotic plaque detection, and tumor detection [28–30]. The overexpression of P-selectin in the tumor vasculature by stimulated endothelial cells makes it a viable target for improving intravascular MB retention. In comparison to other targeting options for drug delivery, such as VEGFR2 and αVß3 integrin, our group has previously demonstrated that P-selectin showed the highest binding efficiency in SVR mouse endothelial cells, which is the basis for it being chosen in this study for further exploration [30].
The challenges associated with systemically delivered therapeutic agents include both non-specific sequestration and immunogenicity from toxic chemical compounds and viral therapy. The well characterized safety of MBs [31], combined with the ability to target specific molecules within the tumor makes this approach a viable tool for the safe and specific delivery of these agents to improve overall patient treatment and survival. The current study propels this drug delivery technique forward by elucidating the whole-body biodistribution of P-selectin targeted MBs.
Materials and methods
Culture methods and tumor model
MDA-MB-231 breast cancer cell lines were purchased from the American Tissue Type Collection (Manassas, VA) and maintained in DMEM, 10% FBS and 1% L-glutamine. The cell line was cultured at 37°C and 5% CO2 while maintained to 70–90% confluence before passaging. To generate the tumor model, 2 × 106 cells were subcutaneously implanted in the flank of 6-week-old athymic female nude mice (Frederick Cancer Research, Hartford, CT). Cell numbers were determined with hemocytometer and trypan blue dye exclusion. Tumors were allowed to grow to a mean diameter range of 8–10 mm. Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham approved all animal protocols.
Preparation of radiolabeled antibodies
Radiolabeling of biotinylated rat IgG anti mouse CD-62 P (PSGL-1; 553743, BD Pharmingen, San Diego, CA) and biotinylated rat IgG antibody (SouthernBiotech, Birmingham, AL) was performed using the HYNIC method as previously described [32]. Briefly, a fresh 1.8 mmol/L solution of succinimidyl 6-hydrazinonicotinate (HYNIC) in dimethylformamide was prepared. Forty picomoles were transferred to glass vials, followed by freezing at −90°C, and then solutions were vacuum dried using an Advantage Benchtop Freeze Dryer (Virtis Co., Inc., Gardiner, NY) with the shelf temperature at 75°C and trap at −90°C. The vials were sealed under vacuum and kept frozen at −80°C until use. Each vial was reconstituted with 1.0mL of sodium phosphate buffer (0.15 mol/L, pH 7.8) containing 0.3 mg of IgG antibody (HYNIC/antibody molar ratio of 18) [33]. After 3 h incubation at room temperature, the mixture was transferred to a Slide-ALyzer dialysis cassette having 10 000 molecular weight cutoff (Pierce, Rockford, IL) and immersed in 1.0 L phosphate buffered saline (PBS, pH 7.4) overnight at 4°C. The HYNIC-modified antibody was labeled with Tc-99m using SnCl2/tricine as the transfer ligand [34], and unbound Tc-99m was removed by G-25 Sephadex size-exclusion chromatography. Protein concentrations of the collected fractions were measured by Lowry assay [35]. The level of free Tc-99m was measured by thin layer chromatography (TLC) using separate strips eluted with saturated saline and methyl ethyl ketone. Experiments were separated into 2 days.
Targeted microbubbles
Streptavidin coated MBs (Targestar-SA) were obtained from Targeson (San Diego, CA). MBs were conjugated to the antibodies by means of biotin–streptavidin chemistry as previously described [30]. Briefly, streptavidin-bound MBs were incubated with the respective antibodies (100 μg per group) for 20 min followed by two times centrifuge washing (400 x for 3 min) to wash out unbound particles. The amount of antibody used during conjugation served to saturate the available streptavidin on the MB. MB concentration was determined via hemocytometer to ensure equal amounts of MBs were injected between groups.
The amount of antibody within the MB dose administered per injection was calculated by dividing activity injected by the decay-corrected specific activity (μCi/μg). To determine the μg/MB, the amount of antibody injected was divided by the number of MB administered per injection. This value was converted to micromoles, and then multiplied by 6.023 × 1017 (molecules per micromole) to yield number of molecules per MB.
Whole-body biodistribution
Prior to experiments, mice were sorted based on tumor sizes to achieve equal distribution of tumor size in all groups. Experiments were performed over a 2-day period with day one biodistribution performed 5 min post-intravenous (tail vein) injection of P-selectin-MB (n=12; 0.274μg, 2.88 × 105 MB), IgG-control-MB (n=12; 0.363μg, 2.88 ×105 MB), P-selectin-Ab (n=5; 0.274μg) and IgG-control-Ab (n=5; 0.363μg). Day two biodistribution was performed 60 min post-intravenous (tail vein) injection of P-selectin-MB (n=4; 0.239μg, 2.49 × 105 MB), IgG-control-MB (n=4; 0.378 μg, 2.88 × 105 MB), P-selectin-Ab (n=3; 0.239 μg) and IgG-control-Ab (n=3; 0.378 μg). All injections for MBs and antibody alone were diluted to a total volume of 60 μl with saline. Planar gamma camera imaging was performed on day 2. The biodistribution procedure was performed as previously described [36]. Briefly, syringes containing dose were counted before and after injection using an Atomlab 100-dose calibrator (Biodex Medical Systems, Shirley, NY) to determine the exact dose. At 5 min and 60 min post-dose, animals were sacrificed and all tissues collected in previously weighed scintillation vials. All tissue samples were then weighed and the Tc-99m activity was measured using a calibrated gamma ray counter (MINAXIg Auto-gamma 5000 series Gamma Counter; Packard Instrument Company), decay corrected to dosing time, and converted to absolute radioactivity. The percentage of injected dose per gram of tissue (%ID/g) was determined and used for comparison.
Gamma camera imaging
Imaging studies were conducted using X-SPECT, a SPECT/CT dual-modality imaging instrument manufactured by Gamma Medica, Inc. (Northridge, CA). Acquisitions (60 s) were performed in planar mode using high-resolution lowenergy parallel-hole collimators. Imaging was performed during day two at 4 min and 59 min post-injection. Injection of radioactivity and subsequent imaging was staggered between the groups to account to radionuclide and MB decay. Quantitative analysis was performed using exported images and ImageJ software. ROI masks were generated and applied to each tumor to uniformly measure mean pixel intensity. Data shown as mean pixel intensity ± SD.
Statistical analysis
Statistical analysis was completed using the SAS 9.2 software (Cary, NC). Analysis of variance (ANOVA) was used to analyze the differences between the group means and variation among and within the groups. Comparisons were performed using a Tukey–Kramer method (Tukey's HSD) multiple comparisons test. When comparing % ID/g per tissue within the same animal (e.g. muscle versus tumor), a paired t-test was utilized. All data is given as mean ± standard deviation (SD). A difference of p<0.05 was considered statistically significant.
Results
Radiolabeling
The antibodies and MBs were successfully labeled with Tc-99 m, with 4% or less free Tc-99m in the preparations. For day one radiolabeling, specific activity was determined to be 37.25 μCi/μg for the anti P-selectin radiolabeled antibody and 33.61 μCi/μg for the rat IgG radiolabeled antibody. During day 2 radiolabeling, specific activity was determined to be 22.04 μCi/μg for the anti P-selectin radiolabeled antibody and 29.54 μCi/μg for the rat IgG radiolabeled antibody. Values for μg/MB and antibody molecules/MB are presented in Table 1.
Table 1.
μg/MB and antibody molecules/MB for P-selectin-MB and IgG-control-MB.
| P-selectin-MB (day 1) | P-selectin-MB (day 2) | IgG-control-MB (day 1) | IgG-control-MB (day 2) | |
|---|---|---|---|---|
| μg/MB | 9.55 × 10–7 | 8.33 × 10–7 | 1.26 × 10–6 | 1.32 × 10–6 |
| Antibody molecules/MB | 3.83 × 106 | 3.34 × 106 | 5.07 × 106 | 5.28 × 106 |
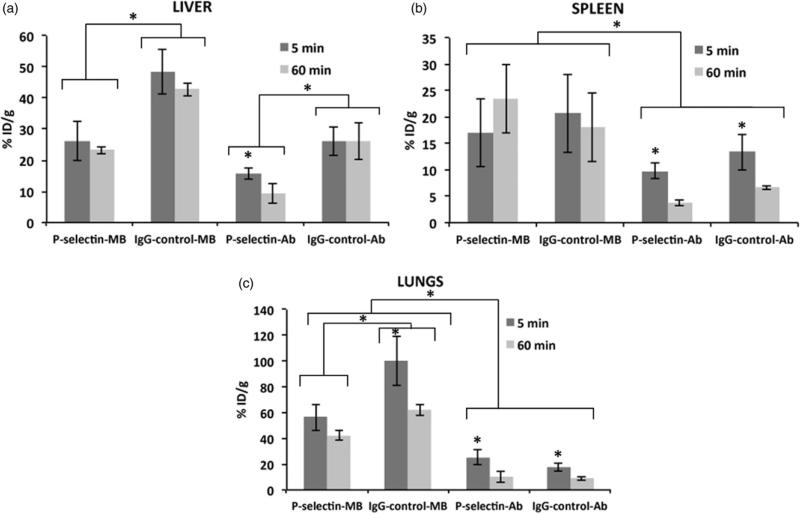
P-selectin-MB retention in liver, spleen and lungs
Liver retention was significantly higher (p<0.05) for the IgG-control-MB group (48.5±7.3% ID/g at 5 min, 42.7 ± 2.1% ID/g at 60 min) compared to all other groups (Figure 1a). Liver retention in all groups remained stable from 5 to 60 min with the exception of the P-selectin-Ab which decreased significantly (p<0.05) from 15.7 ± 1.8% ID/g at 5 min to 9.2 ± 3.1% ID/g at 60 min. Notably, liver retention was 82 and 84% higher at both 5 and 60 min in the IgGcontrol-MB group compared to the P-selectin-MB group. For the spleen, retention levels were greater for the MB groups compared with Ab groups, with no significant difference (p>0.05) between P-selectin-MB (16.9 ± 6.4% ID/g at 5 min, 23.4 ± 6.5% ID/g at 60 min) and IgG-control-MB (20.6 ± 7.4% ID/g at 5 min, 18.0 ± 6.6% ID/g at 60 min) at the 5 and 60 min time points (Figure 1b). Lung retention remained highest in the MB groups at both time points, however IgG-control-MB was significantly higher (p<0.05) than P-selectin-MB at both 5 min (100.2 ± 19.3% ID/g for IgG-control-MB, 56.3 ± 10.1% ID/g for P-selectin-MB) and 60 min (61.7 ± 4.1% ID/g for IgG-control-MB, 42.4 ± 4.1% ID/g for P-selectin-MB; Figure 1c).
Figure 1.
Comparison of percent injected dose per gram (%ID/g) values at 5 min and 60 min for P-selectin targeted microbubbles (MB), IgG targeted control MB, P-selectin antibody, and IgG control in (a) liver, (b) spleen, and (c) lungs. Data are means ±SD. Asterisk denotes statistically significant difference, p<0.05.
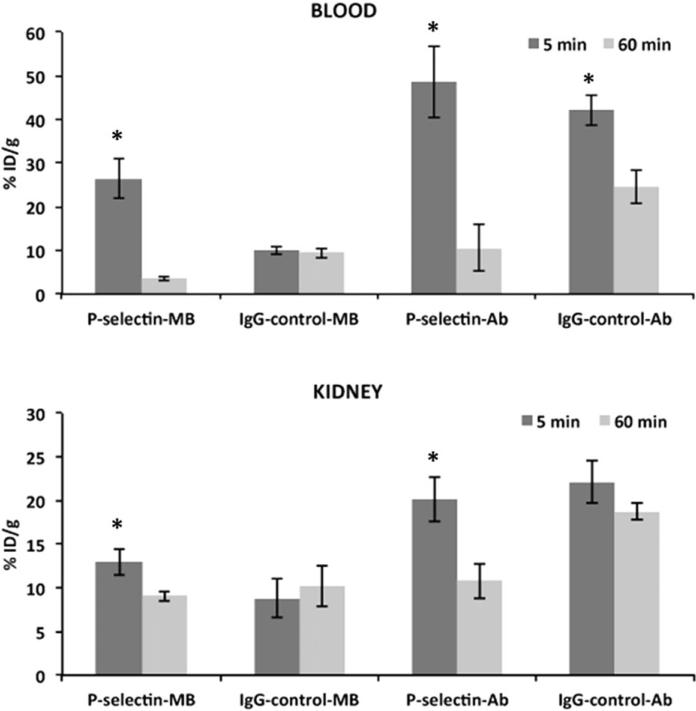
P-selectin-MB clearance in blood and kidney
Blood clearance from 5 min (26.4 ± 4.4% ID/g) to 60 min (3.6 ± 0.5% ID/g) was greatest (p<0.01) for the P-selectin-MB group (87%), while the IgG-control-MB group (9.9 ± 0.9% ID/g at 5 min, 9.4 ± 1.2% ID/g at 60 min) was not significantly different (p>0.05; Figure 2a). Likewise, the blood clearance was significantly greater (p<0.05) for the P-selectin-Ab (79%) over the control antibody (42%) from 5 min to 60 min. For the kidneys, there was significantly greater (p<0.05) retention of the P-selectin-MB (12.9 ± 1.4% ID/g) over IgG-control-MB (8.8 ± 2.3% ID/g) at the 5 min time point. However, P-selectin-MB were significantly cleared (p<0.05) at the 60 min time point (9.1 ± 0.6% ID/g), while the IgG-control-MB retention was not significantly different at the 60 min time point (10.2 ± 2.3% ID/g; Figure 2b).
Figure 2.
Comparison of percent injected dose per gram (%ID/g) values at 5 min and 60 min for P-selectin targeted microbubbles (MB), IgG targeted control MB, P-selectin antibody, and IgG control in (a) blood and (b) kidney. Data are means ±SD. Asterisk denotes statistically significant difference, p<0.05.
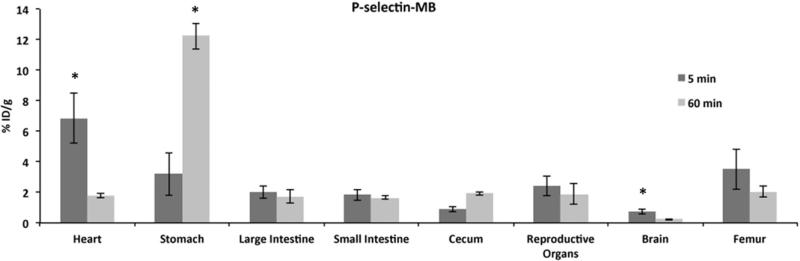
P-selectin-MB retention in other tissues
Figure 3 shows the retention of P-selectin-MB at the 5 and 60 min time points in heart, stomach, large intestine, small intestine, cecum, reproductive organs, brain and femur. The greatest P-selectin-MB retention was observed in the stomach (12.2 ± 0.9% ID/g) at 60 min. The stomach also demonstrated the greatest increase in retention from 5 min to 60 min (3.2 ± 1.4% ID/g at 5 min). The cecum also demonstrated an increase in P-selectin-MB retention from 5 min (12.2 ± 0.8% ID/g) to 60min (12.2 ± 0.8% ID/g). The heart (6.8 ± 1.6% ID/g at 5 min, 1.77 ± 0.1% ID/g at 60 min) and brain (0.7 ± 0.2% ID/g at 5 min, 0.2 ± 0.03% ID/g at 60 min) showed the only significant decrease (p<0.05) in retention over time. IgG-control-MB exhibited similar trends in these tissues compared to P-selectin-MB (p>0.05).
Figure 3.
Comparison of percent injected dose per gram (% ID/g) values at 5 min and 60 min for P-selectin targeted microbubbles in heart, stomach, large intestine, small intestine, cecum, reproductive organs, brain and femur. Data are means ±SD. Asterisk denotes statistically significant difference, p<0.05.
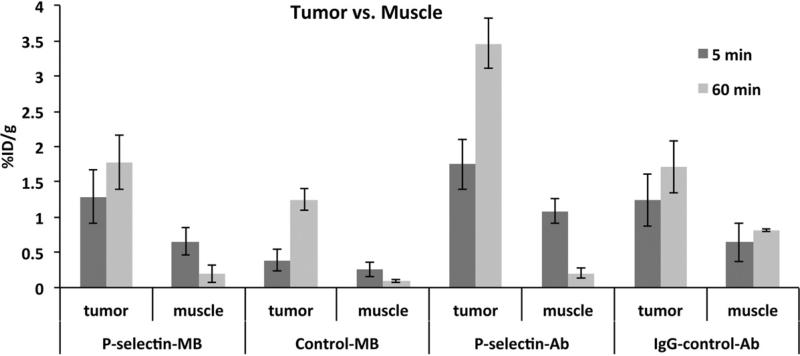
P-selectin-MB retention in tumor and muscle
For the tumor, there was significantly higher (p<0.05) retention of P-selectin-MB (1.3 ± 0.4% ID/g) over IgGcontrol-MB (0.4 ± 0.1% ID/g) at 5 min (Figure 4). Tumor retention for all groups increased from 5 to 60 min, which was unique to tumor. P-selectin-MB retention in tumor(1.3 ± 0.4% ID/g at 5 min, 1.8 ± 0.4% ID/g at 60 min) was significantly greater (p<0.01) than P-selectin-MB levels in adjacent skeletal muscle at both time points (0.6 ± 0.2% ID/g at 5 min, 0.2 ± 0.1% ID/g at 60 min). There was no significant difference (p=0.17) between muscle (0.3 ± 0.1% ID/g) and tumor (0.4 ± 0.1% ID/g) retention for the IgG-control-MB group at 5 min. The greatest tumor retention occurred at the 60 min time point with the P-selectin-Ab group (3.5 ± 0.3% ID/g).
Figure 4.
Comparison of percent injected dose per gram (%ID/g) values in tumor and muscle tissue at 5 min and 60 min P-selectin targeted microbubbles (MB), IgG targeted control MB, P selectin antibody and IgG control groups. Data are means ±SD.
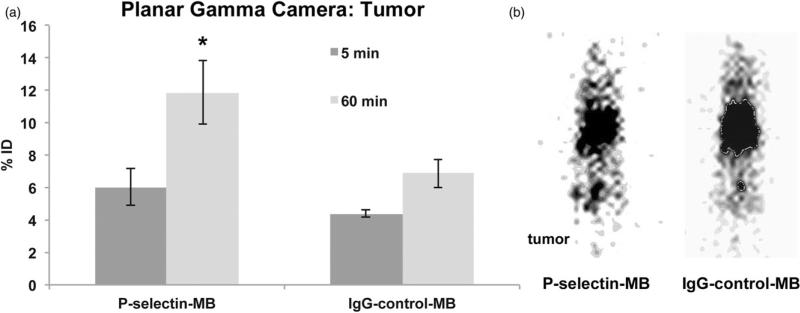
Planar gamma camera imaging
Planar gamma camera imaging was performed to compare with whole-body biodistribution. Figure 5(a) shows analysis of mean pixel intensity in tumor at 5 and 60 min time points for P-selectin-MB and IgG-control-MB groups. The trend of increased tumor retention for the MB groups was observed in the P-selectin-MB exhibiting a 98% increase (p=0.03) over time (6.0 ± 2.23% at 5 min, 11.9 ± 3.93 at 60 min). The IgG-control-MB group showed a 57% increase (p=0.07) from 5 to 60 min (4.4 ± 0.4% at 5 min, 6.9 ± 1.8% at 60 min). In Figure 5(b), a representative image of planar gamma camera imaging shows Tc-99m tumor retention for the P-selectin-MB group at 60 min.
Figure 5.
(a) Comparison of percent injected dose (%ID) at 5 min and 60 min during planar gamma camera imaging between P-selectin targeted microbubbles (MB) and IgG targeted control MB in tumor tissue. Data are means ±SD. (b) Representative image of P-selectin targeted MB during planar gamma camera imaging at 60 min. Asterisk denotes statistically significant difference, p<0.05.
Discussion
Accurate measurement of targeted MBs and appropriate controls, in all tissues will aid in the development of these novel-imaging agents. These steps establish specific targeting, as well as non-target tissues involved in elimination. Reported here is the whole body biodistribution of tumor bearing mice after intravenous injection of P-selectin-MBs at 5 and 60 min following systemic injections. Due to the relatively short half-life of MBs, these time points were chosen to best represent initial uptake in individual organs (5 min), as well as retention and clearance in those organs (60 min). This full body biodistribution comparing P-selectin-MBs and control MBs can be more generally applied for other mechanisms to target MBs.
Tumor retention of P-selectin-MBs was greater than IgG-control-MBs with a 3.2-fold enhancement of MB accumulation. In addition, there was greater retention of P-selectin-MBs in the tumor compared with adjacent skeletal muscle at both time points, 2-fold at 5 min and 9.3-fold at 60 min. This trend of enhanced tumor accumulation by P-selectin targeting was also observed in the antibody alone groups with greater tumor retention of P-selectin-Ab compared to the IgG-control-Ab. These results confirm the hypothesis that P-selectin targeting is beneficial for MB delivery to tumor. An observed trend that was unique to the tumor tissue was the increase in retention from 5 min to 60 min for all groups. This could be attributed to the enhanced permeability and retention (EPR) effect common to tumor tissue [37]. At 60 min post-injection, it is hypothesized that all the MBs have disintegrated and released their targeted antibodies or have been excreted due to their estimated 2-min half-life. Therefore, the EPR effect is generally suggesting entrapment of the targeted antibodies within the tumor, which can be especially beneficial for tumor drug delivery [38]. Differences in this EPR effect within the tumor as known to vary by tumor vascularity, tumor type, as well as size and properties of the molecule delivered. In a study by Willmann et al. [24], F-18 labeled anti-VEGFR2 MBs were evaluated in angiosarcoma tumor bearing mice using dynamic micro-PET imaging [24]. In that study, tumor retention was reported to be 1.14%ID/g at 4 min post-iv injection and 1.35%ID/g at 60 min post-iv injection while skeletal muscle retention was reported unchanged at 0.84% ID/g at 4 and 60 min. These results were relatively similar to the current study findings, however the deviation between the tumor and muscle tissue was greater for the P-selectin-MBs, which could also be attributed to the different modality, tumor type and receptor density used within the studies.
For the filtration organs, Tc-99m liver accumulation remained stable from 5 to 60 min for all groups except the P-selectin-Ab group, which was significantly reduced by 41%. This observation of static retention in the liver was also observed in the previously mentioned study performed by Willmann et al. These findings would imply that the capacity of the liver to sequester and process the injected doses requires more than 60 min. Delayed liver clearance was also shown to occur in a similar study of un-targeted MB biodistribution where MB clearance in the liver was first observed at the 6 h time point [39]. This retention by the liver is most likely due to MB uptake by Kupffer cells, whose normal function is to phagocytose foreign particles, and have been shown to play a crucial role in MB uptake in the liver [3]. At each time point, the lung and liver retention was significantly higher for the IgG-control-MB group compared to the P-selectin-MB group. However, P-selectin-MB retention was greater than IgG-control-MB group in the blood and tumor at each time point, which would account for the lack of retention in liver and lung. Unique to the lung, Tc-99m retention was significantly greater in the MB groups at both time points compared to antibody alone. Considering the minor amount of pulmonary macrophage contribution to lung clearance in mice [40], the increased MB retention could be explained by the non-specific pulmonary entrapment that often occurs using MBs with a diameter greater than 5 μm [41]. In the spleen, there was a slightly higher retention of MB groups compared with antibody alone; this was most likely caused by previously reported involvement of splenic macrophages and mononuclear phagocyte system in the clearance of MBs [2]. There was no significant difference in the spleen for the MB groups at 5 and 60 min however there was a significant decrease in targeted and control antibody from 5 to 60 min, a trend that further supported the role of splenic macrophages to engulf MBs in the spleen. In the kidneys, there was greater retention of the radiolabeled antibody groups compared to the MB groups at 5 min, a trend that was also observed in the Willmann et al. [24] study. However, there was a significant clearance of both P-selectin-MB and P-selectin-Ab in the kidneys that was not observed in the IgG control groups suggesting an additional benefit of targeting to improve P-selectin-MB circulation and bioavailability while limiting kidney accumulation over time. Expediting kidney clearance could prove useful in decreasing systemic circulation of unbound particles. As expected, organs filtered MBs and antibodies differently, illustrating the importance of the control groups used during the whole-body biodistribution. This analysis also demonstrates the differences between targeted and control MBs, with decreased filtration of targeted MBs compared to controls.
When analyzing P-selectin-MBs alone, retention was highest at the 5 min time point in the lung (56.3% ID/g) followed by the blood (26.4% ID/g) and then the liver (26.1%ID/g). The relatively high signal in the stomach and cecum is attributed to the normal gut localization of free Tc-99m pertechnetate that ultimately is an artifact of the radiolabeling strategy used in this study [42]. At the 60-min time point, P-selectin-MB retention remained relatively constant in the lung, liver, and spleen however there was an 86% clearance in the blood. This represented the greatest amount of blood clearance observed in all groups from 5 to 60 min. The constant levels in lung, liver and spleen suggest the Tc-99 m-labeled P-selectin was not released from the MBs in those tissues. Similarly, the blood clearance suggests the Tc-99 m-labeled P-selectin remained with the MBs and was also not released into the circulation where a longer half-life would be expected.
While P-selectin is overexpressed in tumor vasculature, it is also expressed in other regions of the body, including inflammation processes. This could be a limitation to the study; however, it was shown that targeting does enhance the retention of the targeted groups in the tumor when compared to controls. Other targeted agents explored in cancer therapies including VEGFR2, ICAM-1 and integrin αVß3 are also expressed in other tissues, and not specific to the tumor. Further studies would be beneficial to compare the performance of P-selectin targeted MBs to alternate targeted MBs. Also, there was significant amount of blood clearance after injection of the P-selectin-MB group in comparison to the other groups, exhibiting the necessary traits for filtration. Differences in the blood flow and shear stress can directly affect targeting and retention of molecules within a region of interest. There has recently been studies examining P-selectin targeting in ischemic tissues where blood flow is altered [43,44], however it has also been shown that targeting and shear stress is not linearly related [45]. The difference in blood flow is a limitation when comparing skeletal muscle to tumor. This study relies on targeting a receptor which is overexpressed within the tumor, and limitations included, still shows increased retention of targeted MBs over control MBs. Differences in blood flow also directly affect Abs and MBs differently, as MBs have a much shorter half-life than Ab. Ab have an estimated 11-day half-life [46], while MBs have an estimated 2-min half-life [2]. These differences can directly affect tumor uptake, as well as organ filtration and sequestration. MB half-life is dependent both on size of the MB and concentration in which they are injected, which can have a direct effect of the stability of the delivery system. The more stable a drug or molecule, the greater chance at heightened efficiency to for delivery to the intended site, leading to improved drug delivery and imaging.
One limitation of this study was the use of avidin chemistry to adhere the antibody to the MB. While this strategy ensures a stable bioconjugate, the avidin utility reduces the translational potential of the targeted MB construct due to the immunogenicity of the avidin molecule. Alternative strategies of MB targeting being developed include the use of targeted peptides that are covalently bound to MBs. Initial studies using this motif have been performed and results suggest the strategy to be safe and specific [16,47]. One study recently concluded a phase 0 clinical trial using VEGFR2 as the targeting moiety during prostate cancer evaluation [48]. This component would allow for a more feasible clinical translation of targeted MBs for both imaging and drug delivery. This study reveals interesting results for whole body interactions of targeted microbubble drug delivery, which has potential to lead to future investigations regarding individual organ uptake. A limitation to this study is organ information is only available at the 5- and 60-min time point. Utilizing ultrasound, SPECT or PET, future investigations with dynamic imaging of targeted microbubbles could also be evaluated in specific individual organs or tumor for addition information regarding uptake and clearance. Although there are limitations, this study provides initial evidence of a promising tool for targeted drug delivery using P-selectin and ultrasound contrast agents.
Conclusions
If MBs are continued to be investigated as drug delivery carriers, this study demonstrates that targeting improves tumor retention compared to non-targeting. The study also shows that targeting MBs to P-selectin reduces liver and lung retention compared to non-targeted MBs. While there is more MB retention in all tissues compared to tumor tissue, there is less retention of targeted MBs in tissues and greater tumor retention relative to non-targeted MBs. With the potential of targeted MBs to improve CEUS and drug delivery in patients, the current work is invaluable to elucidate the systemic biodistribution of targeted MBs in general. The innate role of P-selectin to sequester platelets and rolling monocytes is well suited to reproduce the desired mechanism for vascular MB adhesion. The P-selectin molecule is a promising candidate to improve MB accumulation in target tissues; however other cell adhesion molecules and receptors should be continually investigated and may prove to be more efficacious. This study provides validation of P-selectin targeted MBs as a more specific approach for systemic drug delivery in a solid tumor.
Acknowledgments
This work was supported the UAB Small Animal Imaging Shared Facility NIH Research Core Grant (P30CA013148) and the Susan G. Komen Breast Cancer Foundation (KG090969). The authors would like to thank Reshu Saini and Soojin Kim for their contributions to this project.
Footnotes
Declaration of interest
The authors declare that they have no conflict of interest.
References
- 1.Tartis MS, McCallan J, Lum AF, et al. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol. 2006;32:1771–80. doi: 10.1016/j.ultrasmedbio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–47. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SL, Rahim AA, Bush NL, et al. Targeted retroviral gene delivery using ultrasound. J Gene Med. 2007;9:77–87. doi: 10.1002/jgm.1003. [DOI] [PubMed] [Google Scholar]