Abstract
Background
Anesthesiologists face increasing pressure to demonstrate the value of the care they provide, whether locally or nationally through public reporting and payor requirements. In this article, we describe the current state of performance measurement in anesthesia care at the national level and highlight gaps and opportunities in performance measurement for anesthesiologists.
Approach
We evaluated all endorsed performance measures in the National Quality Forum (NQF), the clearing house for all federal performance measures, and classified all measures as follows: 1) anesthesia-specific; 2) surgery-specific; 3) jointly attributable; or 4) other. We used NQF-provided descriptors to characterize measures in terms of (1) structure, process, outcome or efficiency; (2) patients; disease and events targeted; (3) procedural specialty; (4) reporting eligibility; (5) measures stewards; and (6) timing in the care stream.
National Quality Forum Measures
Of the 637 endorsed performance measures, few (6, 1.0%) were anesthesia-specific. An additional 39 measures (6.1%) were surgery-specific, and 67 others (10.5%) were jointly attributable. “Anesthesia-specific” measures addressed preoperative antibiotic timing (n=4), normothermia (n=1), and protocol use for placement of central venous catheter (n=1). Jointly attributable measures included outcome measures (n=49/67, 73.1%) which were weighted towards mortality alone (n=24) and cardiac surgery (n=14). Other jointly attributable measures addressed orthopedic surgery (n=4), general surgical oncologic resections (n=12) or nonspecified surgeries (n=15), but none specifically addressed anesthesia care outside the operating room such as for endoscopy. Only 4 measures were eligible for value-based purchasing. No named anesthesiology professional groups were among measure stewards, but surgical professional groups (n=33/67, 47%) were frequent measure stewards.
Summary and Ways Forward
Few NQF performance measures are specific to anesthesia practice, and none of these appears to demonstrate the value of anesthesia care or differentiate high-quality providers. To demonstrate their role in patient-centered, outcomes-driven care, anesthesiologists may consider actively partnering in jointly attributable or team-based reporting. Future measures may incorporate surgical procedures not proportionally represented as well as procedural and sedation care provided in non-operating room settings.
Introduction
Performance measurement in anesthesia is the past, present, and future
While providing anesthesia as medical students, E. A. Codman and Harvey Cushing compared anesthesia records to determine the better anesthetist, thus beginning modern performance measurement.1,a Since then, performance measurement has become a core discipline in the science of health care delivery, and tracking performance with metrics has become a central activity of anesthesia practices.2,3 This shift has been accelerated as payors and administrators have developed and mandated performance measurement. While initially linking payment to reporting, payors are increasingly linking payment to performance.b
Current challenges: attribution, sample size and relevance
Defining anesthesia quality with discrete performance metrics has been uniquely challenging. Although surgical quality has been concerned with morbidity and mortality, attribution of outcomes and complications is complex both scientifically and politically. Many anesthesiologists are reluctant to share accountability for serious morbidity and mortality that has traditionally been attributed solely to surgeons or other health care providers. Anesthesia-specific outcomes, however, are problematic as metrics. Serious outcomes, such as deaths caused by anesthesia alone, are rare and thus unsuitable for benchmarking.4,5 More common anesthesia-specific complications, such as postoperative sore throat and nausea, are not broadly recognized as relevant because they do not easily align with the goals of the surgeon, referring physician, and hospital and may not be considered the highest priority by the patients themselves except in very low risk procedures.6 In addition to addressing these requirements of attribution, statistical utility and significance, performance measures for anesthesia would ideally reflect the spectrum of care provided for “perianesthetic” patients, including patients undergoing anesthesia with or without operations such as for imaging studies or sedation care such as for endoscopy. Potential gaps in performance measurement, whether in terms of patients, procedures or outcomes, also represent potential gaps in quality improvement and demonstration of value to other stakeholders in the care system, including patients.
The aim of this article
Given these imperatives and challenges to performance measurement, we wished to describe the state of performance measurement in anesthesia care as a starting point to identify gaps and opportunities for the future. Because links between performance and payment are currently strongest at the national level, we chose, as a starting point, to review all performance measures in the National Quality Forum (NQF) library of performance measures. Performance measures are more commonly addressed individually to re-evaluate scientific merit rather than be described as a group.7 In August 2009, the Surgery and Anesthesia Steering Committee of the NQF issued a document entitled “National Voluntary Consensus Standards for Surgery and Anesthesia—Additional Performance Measures 2008.” At that time, the NQF had endorsed more than 50 performance measures relevant to surgery and anesthesia, and the 2008 report highlighted 5 additional performance measures as follows: timely urinary catheter removal, perioperative temperature management, protocol for glycemic control, postoperative venous thromboembolism, and hair removal among ambulatory surgery patients. No effort to synthesize and update the current state of national anesthesia performance measures has been published since, by the NQF or another group. Since that time, the corpus of endorsed performance measures has been in flux, with some measures being endorsed and others retired from use.
The National Quality Forum
The NQF has become the designated clearinghouse for performance measures in the United States health care environment. The NQF was contracted by the United States Department of Health and Human Services in 2009 to establish “a portfolio of quality and efficiency measures that will allow the federal government to more clearly see how and whether healthcare spending is achieving the best results for patients and taxpayers.”c The NQF shepherds measures under consideration for use in federal public reporting and performance-based payment programs…”d Candidate performance measures are vetted through a transparent process within the NQF under the Measures Application Partnership. All measures are subject to public and member comment. The process of measure endorsement and details of all measures are described on the NQF website (qualityforum.org).
This library of measures is regularly changing as measures are proposed and endorsed, placed in reserve status or endorsement is withdrawn. The NQF uses review mechanisms to assure that only high-quality measures are endorsed at a given time. These include time-limited endorsement, transparent review, and periods for public comment to solicit diverse opinions. Although such changes can take months or years, there are exceptions. One example pertains to NQF measure #0500, Severe Sepsis and Septic Shock: Early Management Bundle. This measure was initially endorsed by the NQF on the condition that it be reviewed pending the results of highly relevant but ongoing clinical trials. Within 1 month of the release the ProCESS trial (Protocol-Based Care for Early Septic Shock), which demonstrated no difference between resuscitation strategies in sepsis with versus without a central venous catheter,8 NQF convened a panel that overturned its previous endorsement of guidelines calling for central venous catheterization.e The transparency and public commentary period make the NQF a critical audience for anesthesiologists to share new research and voice opinions relevant to performance measurement.
Describing national performance measures: National Quality Forum as substrate
We evaluated all measures endorsed by the NQF as of April 8, 2014. Two authors systematically reviewed and characterized measures in terms of specificity and relevance to anesthesia practice as well as characterized the processes, outcomes, diseases and populations targeted by these measures. One author has more than 10 years of experience directing a perioperative clinic that cares for more than 1600 patients per month. The preoperative clinic is under anesthesia physician supervision and evaluates patients from all surgical, procedural, and imaging services that require anesthesia services. The level of sedation that requires the presence of an anesthesiologist is defined by hospital policy. Another author is a board-certified anesthesiologist and intensivist. We first classified measures into mutually exclusive groups by specificity and relevance to surgery and anesthesia as follows (TABLE 1): A) measures “specific” to and attributable to anesthesia practice; B) measures for perioperative/perianesthetic patients that may be jointly attributable to anesthesia and surgical practice; C) measures for perioperative/perianesthetic patients that are specific to surgical practice and not attributable to anesthesia practice; D) other. This final group includes measures that may be intended for nonsurgical (medical) patients which may or may not be associated with anesthesia practice. Also included in this “other” group are measures intended for pain medicine, palliative care or intensive care practice or patient care such as prehospital transport that is commonly undertaken by anesthesiologists in other countries. These categories were formed though an inductive, recursive process with discussions over more than 6 months among the coauthors. After creation of categories, measures were assigned to categories by 2 authors and discussed individually. When faced with a measure for which classification was initially unclear, the authors queried measure stewards to clarify details of the measure and additionally consulted with experts, initiated a review of the literature, and consulted with co-authors. This process resulted in consensus of assignment for measures classifications. Throughout this process, it was recognized that local practice patterns may vary and that, in some instances, assignments may not be applicable. This review focuses on measures specific to anesthesia and surgical practice (A, B and C above).
Table 1.
Example classifications of performance measures, National Quality Forum (NQF), 2014.
| A. Measures specific to anesthesia practice |
| Example: |
| NQF # 0454 |
| Title: Anesthesiology and Critical Care: Perioperative Temperature Management |
| Description: Percentage of patients, regardless of age, undergoing surgical or therapeutic procedures under general or neuraxial anesthesia of 60 minutes duration or longer for whom either active warming was used intraoperatively for the purpose of maintaining normothermia, OR at least one body temperature equal to or greater than 36 degrees Centigrade (or 96.8 degrees Fahrenheit) was recorded within the 30 minutes immediately before or the 30 minutes immediately after anesthesia end time |
| B. Measures jointly attributable to anesthesia and surgical practice |
| Example: |
| NQF #0533 |
| Title: Postoperative Respiratory Failure Rate (PSI 11) |
| Description: Percentage of postoperative respiratory failure discharges among adult, elective surgical discharges in a one year time period |
| C. Measures specific to surgical practice |
| Example: |
| NQF # 0134 |
| Title: Use of Internal Mammary Artery (IMA) in Coronary Artery Bypass Graft (CABG) |
| Description: Percentage of patients aged 18 years and older undergoing isolated coronary artery bypass graft (CABG) who received an internal mammary artery (IMA) graft |
| D. Measures not focused on or intended for surgical patients |
| Example:includes non-surgical patients in measures |
| NQF# 0348 |
| Title: Transfusion Reaction (PSI 16) |
| Description: The count of medical and surgical discharges for patients age greater than or equal to 18 or in MDC 14 with ICD-9-CM code for transfusion reaction in any secondary diagnosis field. |
| Example: not intended for surgical patients |
| NQF # 0105 |
| Title: Antidepressant Medication Management |
| Description: The percentage of members 18 years of age and older who were diagnosed with a new episode of major depression and treated with antidepressant medication, and who remained on an antidepressant medication treatment (…) |
| Example:intended for palliative care or pain medicine practice |
| NQF # 1639 |
| Title: Hospice and Palliative Care -- Dyspnea Screening |
| Description: Percentage of hospice or palliative care patients who were screened for dyspnea during the hospice admission evaluation/palliative care initial encounter. |
Measures included in D are not further addressed in this analysis
The NQF-provided descriptions for each measure include targeted age group, measure type (i.e. structure, process, outcome, and efficiency), data source, measure steward (person or group who develops and oversees the measure), categorization within the national quality strategy, and eligibility for reporting purposes (including as part of value-based purchasing). We additionally reviewed measures to determine and classify other salient details including the type of outcome assessed (i.e., death, composite, length of stay), timing and location of action or event for a measure, or medical or surgical specialty of relevance, whether a presenting disease or disease category was addressed or if a specific complication was targeted by a measure.
What the National Quality Forum holds for anesthesia care
Six measures (6/637; 1.0%) were specific to, or entirely attributable, to anesthesia care (TABLE 2). All of these were process measures. Four concerned the timing of antibiotic prophylaxis, with specifications for surgical setting as ambulatory surgery, adult surgery, cesarean delivery, or not specified. One measure concerned normothermia as either use of active warming or achievement of normothermia. The remaining anesthesia-specific measure addressed use of a care bundle during insertion of a central venous catheter. None of these measures included an anesthesiology society as a named steward. The measure steward for #0264 was noted as the Ambulatory Surgery Collaborative, of which the American Society of Anesthesiologists (ASA) is a participating member. All 6 measures were eligible for public reporting and benchmarking, and 1 measure (#0527 for preincision antibiotic timing) was applicable to value-based purchasing.
Table 2.
Characteristics of anesthesia-specific performance, National Quality Forum, 2014
| NQF # | Primary Issue | Type | Timing | Population | Steward |
|---|---|---|---|---|---|
| 0264 | Antibiotic timing | Process | Pre-incision | Ambulatory | ASC Quality Collaboration |
| 0269 | Antibiotic timing | Process | Pre-incision | Adult | AMA-PCPI |
| 0454 | Normothermia | Process | OR or PACU | Any | AMA-PCPI |
| 0464 | CRBSI care bundle | Process | CVC insertion | Any | AMA-PCPI |
| 0472 | Antibiotic timing | Process | Pre-incision | Cesarean section | MGH/Partners Health Care System |
| 0527 | Antibiotic timing | Process | Pre-incision | Any | CMS |
ASC= Ambulatory Surgery Center - Quality Collaboration
CRBSI= catheter related blood stream infection
AMA-PCPI = American Medical Association - Physician Consortium for Performance Improvement
MGH=Massachusetts General Hospital
CMS= Centers for Medicare and Medicaid Services
Measures exclusive to perioperative patients but not relevant to anesthesia
In all, 39 measures (39/637; 6.1%) were identified as specific to surgical care but not relevant to anesthesia practice (TABLE 3). Reasons that specific measures were determined to be not relevant to anesthesia practice were diverse and included assessment of care that is surgeon-driven (12; 30.8%), specification of surgical technique (7; 18.0%), requirement for preoperative orders (4; 10.3%); requirement for orders after discharge from the postanesthesia care unit (4; 10.3%) or hospital discharge orders (4; 10.3%). Only a small number of measures in this group, primarily for cataract surgeries, focused on true surgical outcomes (4; 10.3%).
Table 3.
Summary of measures specific to surgical care not relevant to anesthesia practice, National Quality Forum, 2014
| Reason Not Relevant To Anesthesia | TOTAL | Outcome | Process | Example(s) | |
|---|---|---|---|---|---|
|
| |||||
| N | (%) | N | N | ||
| Surgeon-driven perioperative care | 12 | (30.8) | 1 | 11 | #0219 Post breast conservation surgery irradiation # 0567 Appropriate work up prior to endometrial ablation procedure |
| Surgical technique | 7 | (18.0) | 6 | 1 | #0134 Use of Internal Mammary Artery in Coronary Artery Bypass Graft #0363 Foreign Body Left During Procedure |
| Require preoperative orders | 4 | (10.3) | 0 | 4 | #0458 Pulmonary Function Tests Before Major Anatomic Lung #0268 Selection of Prophylactic Antibiotic |
| Post-PACU in-hospital orders | 4 | (10.3) | 0 | 4 | #0300 Cardiac Surgery Patients With Controlled Postoperative Blood Glucose #0128 Duration of Antibiotic Prophylaxis for Cardiac Surgery Patients |
| True surgical outcome | 4 | (10.3) | 4 | 0 | #0565 20/40 or Better Visual Acuity within 90 Days [after] Cataract Surgery #1536 Improvement in Visual Function within 90 Days [after] Cataract Surgery |
| Discharge Orders | 4 | (10.3) | 0 | 4 | #0117 Beta Blockade at Discharge #1519 Statin Therapy at Discharge after Lower Extremity Bypass |
| Hair removal | 2 | ( 5.1) | 0 | 2 | #0515 Ambulatory surgery patients with appropriate method of hair removal |
| Variable anesthesia penetration | 2 | ( 5.1) | 1 | 1 | #0716 Healthy Term Newborn |
|
| |||||
| Total, N (%) | 39 | (100) | 27 (69.2) | 12 (30.8) | |
No measures in this group were structure measures or efficiency measures.
Measures jointly attributable to anesthesia and surgical care
Sixty-seven measures (10.5%) were categorized as jointly attributable (TABLE 4). Of these, most (n=49, 73.1%) were outcome measures (TABLE 5). The most common outcomes were death (n=24), death or complication (n=9) or complication(s) alone (n=12). Outcomes were assessed as in-hospital and up to 120 days postoperatively. Other outcomes represented included length of stay, transfer (admission after ambulatory surgery), and patient experience. This outcome measure for patient experience was an adaptation of the Surgical Consumer Assessment of Healthcare Providers and Systems that assesses patient experience with surgical care and is the only jointly attributable measure categorized by the NQF as addressing person- and family-centered care.
Table 4.
Features of anesthesia-relevant performance measures related to administration and quality improvement, National Quality Forum, 2014.
| Jointly attributable measures | ||
|---|---|---|
|
| ||
| N | (%) | |
| Total | 67 | (100.0) |
| Measure Type | ||
| Structure | 8 | (11.9) |
| Process | 7 | (10.5) |
| Outcome | 49 | (73.1) |
| Efficiency | 3 | ( 4.8) |
| National Quality Strategy | ||
| Patient Safety | 33 | (49.3) |
| Cardiovascular Diseases | 23 | (34.3) |
| Affordable Care | 8 | (11.9) |
| Communication and Coordination | 1 | ( 1.5) |
| None specified | 1 | ( 1.5) |
| Person- and Family-Centered Care | 1 | ( 1.5) |
| Measure Stewards | ||
| Society of Thoracic Surgeons | 24 | (35.8) |
| Agency for Healthcare Research and Quality | 13 | (19.4) |
| Centers for Medicare and Medicaid Services | 8 | (11.9) |
| Society for Vascular Surgery | 5 | ( 7.5) |
| American College of Surgeons | 4 | ( 6.0) |
| Ambulatory Surgical Centers Quality Collaborative | 4 | ( 5.8) |
| Leapfrog Group | 3 | ( 4.5) |
| Boston Children’s Hospital | 1 | ( 1.5) |
| The Children’s Hospital of Philadelphia | 2 | ( 3.0) |
| American College of Cardiology Foundation | 1 | ( 1.5) |
| Centers for Disease Control and Prevention | 1 | ( 1.5) |
| Optum | 1 | ( 1.5) |
| American Society of Anesthesiologists | 0 | ( 0.0) |
| Data source (non-exclusive) | ||
| Claims | 28 | (41.8) |
| Registry | 30 | (44.8) |
| Level of Analysis (non-exclusive) | ||
| Integrated Delivery System | 4 | ( 6.0) |
| Facility | 63 | (94.0) |
| Group/Practice | 29 | (43.3) |
| Individual | 10 | (14.9) |
| Eligible Use (non-exclusive) | ||
| Quality Improvement (internal) | 67 | (100) |
| Quality Improvement (external benchmarking) | 67 | (100) |
| Public Reporting | 67 | (100) |
| PQRS | 7 | (10.5) |
| Eligible for Value-Based Purchasing | 3 | ( 4.5) |
Table 5.
Performance measures by patients and diseases, National Quality Forum, 2014
| Jointly attributable measures | ||
|---|---|---|
|
| ||
| N | (%) | |
| Type of Measure and Complication Focus | ||
| Structure measures | n=8 | |
| Volume reporting or database participation | 8 | |
| Process measures | n=7 | |
| Infection-wound | 2 | |
| Infection-urinary tract | 1 | |
| Cardiovascular diseases | 4 | |
| Beta-blocker | 3 | |
| Aspirin | 1 | |
| Outcome measures | n=49 | |
| Death alone | 24 | (50.0) |
| Complications composite (including death) | 9 | (18.4) |
| Complication solitary (not including death) | 12 | (24.5) |
| Length of stay | 1 | ( 2.0) |
| Patient Experience | 1 | ( 2.0) |
| Transfer (admission after ambulatory surgery) | 1 | ( 2.0) |
| Readmission | 1 | ( 2.0) |
| Efficiency measures, types and foci | n=3 | |
| Cost of care (hip/knee replacement) | 1 | |
| Appropriate preoperative cardiac imaging | 2 | |
No measures for depression, imaging, breast cancer, prostate cancer, renal cancer or vision disturbance
Four outcome measures include venous thromboembolism and seven include wound infection.
Other jointly attributable measures addressed common perioperative issues. Among process measures (n=7), 3 addressed perioperative use of beta-blockade and 1 addressed use of aspirin. Among the 3 efficiency measures, 2 tracked cost-effective use of perioperative cardiac imaging. Only 3 measures had been designated as applicable to value-based purchasing. These included #0453 for perioperative beta-blockade usage, #0284 for selection of appropriate perioperative antibiotics, and #0528 for removal of urinary catheter on postoperative day 1.
Measure stewards and stakeholders
Surgical societies and federal agencies were common among named measure stewards while anesthesiology societies were absent (TABLE 4). The Society of Thoracic Surgeons (n=24), the Society for Vascular Surgery (n=5), and the American College of Surgeons (n=4) accounted for nearly half of all jointly attributable measures and were more numerous than government agencies (Agency for Healthcare Research and Quality, n=13; Centers for Medicare and Medicaid Services, n=8).
Measure stewardship and physician stakeholders were correspondingly weighted towards cardiothoracic surgeons, cardiac surgery, esophagectomy and lung resection. Patients from these populations were similarly emphasized. No specialty was designated for 15 of the measures. No measures specifically addressed a number of surgical and procedural specialties whose patients are well-represented in anesthesia practice (TABLE 6). These included urology, head and neck surgery, neurosurgery, endoscopy, radiology and psychiatry/neurology.
Table 6.
Descriptions of jointly attributable performance measures by surgeon or proceduralist, National Quality Forum, 2014
| Jointly attributable measures | ||
|---|---|---|
|
| ||
| N | (%) | |
| Patient Age Group Targeted | ||
| Not specified | 59 | (88.1) |
| Pediatric only | 4 | ( 6.0) |
| Aged >65 only | 4 | ( 6.0) |
| Presenting Disease Specified* | ||
| None specified | 15 | (22.4) |
| Examples: | ||
| #0697, Elderly surgery outcomes | ||
| #0533, Postoperative respiratory failure | ||
| #0450, Postoperative venous thromboembolism | ||
| Cardiovascular disease | 27 | (40.3) |
| Musculoskeletal/Orthopedic | 4 | ( 6.0) |
| Common cancer surgeries | 12 | (17.5) |
| Colorectal | 2 | |
| Hysterectomy | 1 | |
| Pancreatic | 3 | |
| Esophageal or Lung | 6 | |
| Pediatric cardiac | 5 | ( 7.5) |
| Pediatric non-cardiac | 1 | ( 1.5) |
| Proceduralist | ||
| None specified | 17 | (26.9) |
| Surgeon | ||
| Cardiac (adult) | 18 | (26.9) |
| Thoracic (adult) | 7 | (10.5) |
| Vascular only | 9 | (13.4) |
| Cardiac (pediatrics) | 5 | ( 7.5) |
| General Surgery (nos) | 4 | ( 5.9) |
| Orthopedics only | 4 | ( 5.8) |
| Multiple specialties designated | 3 | ( 4.5) |
| Pediatric non-cardiac surgery | 1 | ( 1.5) |
| Non-surgeon | ||
| Cardiology (pediatrics) | 1 | ( 1.5) |
No anesthesia-relevant measures were identified for trauma, obstetrics/gynecology, urology only, Ear, nose, throat only, neurosurgery, oral and maxillofacial only.
No anesthesia-relevant measures were identified including the following non-surgeon physicians: gastroenterology/endoscopy, radiology or psychiatry/neurology.
Performance in the Care Stream
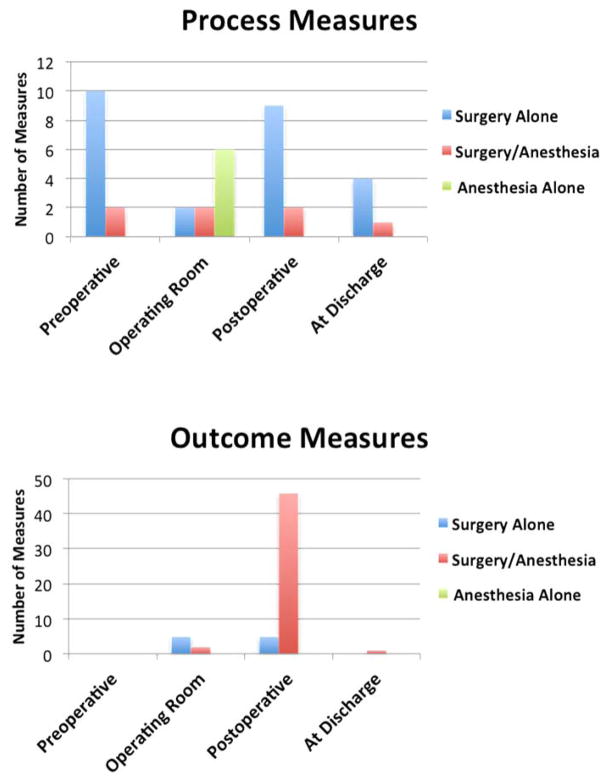
We present the array of perioperative performance measures in the stream of patient care (FIGURE 1). These measures are differentiated by specificity and relevance to anesthesia and surgery practice. Structural measures, such as participation in a national registry, were excluded as were 6 obstetric measures which were unclassifiable in terms of timing of the event(s) in the care stream. The bulk of measures of any kind are postoperative outcome measures.
Figure 1.
Presentation of performance measures by measure type and occurrence during the care stream, National Quality Forum, 2014
What can we learn from the measures in the National Quality Forum?
Current anesthesia-specific measures do not meet the needs of our specialty or our patients
Only 6 measures were anesthesia-specific, or uniquely attributable to anesthesia care, and all of these were process measures; not a single outcome measure was identified. Process measures are problematic for a variety of reasons. An emphasis on care processes may increase vulnerability to performance-payment mismatch. This can occur when specific care processes continue to be rewarded even after competing or even contrary evidence comes to light. Despite the review mechanisms in the NQF, evidence may change faster than either expert guidelines or panel votes.9, 10 An additional limitation of process measures comes from the perspective of the anesthesia provider. Compliance with the 6 anesthesia-specific process measures does not measure the excellence that the best anesthesia groups can deliver. At one time, maintenance of normothermia and timely antibiotic prophylaxis were not standard approaches to care, but performance has become standardized.11,12 Thus, these process measures are unlikely to differentiate between high- and low-quality anesthesia care or demonstrate the value of good anesthesia care in an integrated perioperative system such as the proposed Perioperative Surgical Home. Finally, from the perspective of the patient, current anesthesia-specific measures miss the mark for quality and patient-centered care by focusing on what the physician does rather than how the patient does. Given the opportunity, patients are unlikely to ask their perioperative teams about rates of normothermia and Surgical Care Improvement Project compliance when they could ask about important outcomes such as postoperative pain control or major complications. In this sense, these measures fail to capture what really matters to patients.13
Additional anesthesia-specific measures may not be the solution
The review of the NQF identified no measures that addressed historically “core” aspects of anesthesia care such as airway management, intraoperative hemodynamic stability, postoperative pain, nausea or vomiting, operating room efficiency, case delay and cancellation, or effective resuscitation after arrest. These components of anesthesia care may not be ideal for use in performance measures. There are no data describing important hospital-level variation in airway management, nor does a national measure for case cancellation seem practical since delays or cancellations may be local phenomena requiring local solutions. Future work into the suitability of anesthesia-specific measures is critical with the understanding that these measures may be more important at the local level rather than national level. Regardless, national payers will continue to seek suitable outcome measures. Anesthesia-specific outcomes, as discussed, are typically too rare to be used for benchmarking or not serious enough to capture the attention of surgeons, hospital administrators, or payors.
Risks, rewards, and inevitability of embracing team-based assessment of performance
Anesthesiologists have, over time, struggled to distinguish the uniqueness of “anesthesia quality” from those outcomes, good or bad, attributable to surgeons or the surgical care episode. Transformations in health care may severely alter the terms of this problem. At the regional and national levels, health care and it measurement are transforming to become both patient-centered (rather than physician-centered) and outcomes-based (called “no outcome, no income”). Individual, fee-for-service models within smaller anesthesia groups are to be replaced by comprehensive payment models applied across large practices including multispecialty practices and accountable care organizations. In these settings, physicians are frequently salaried, and incentives may be contingent on some aspect of measured performance. Such models could serve to explicitly align the incentives of anesthesiologists and surgeons.
When attempting to measure anesthesia quality, the numerous jointly attributable measures described here may include alternatives to anesthesia-specific measures. The American College of Surgeons has formally proposed the formation of “clinical affinity groups” which use patient-oriented, outcomes-based, risk-adjusted quality measures as metrics for evaluation of teams of clinical providers who care for a specific condition, disease, or patient population.f Under such a proposal, the performance of anesthesiologists would be evaluated as part of the clinical affinity groups and share performance reporting with other members, including other physicians, within these groups. In such a model, an oncologist, thoracic surgeon, anesthesiologist, and an intensivist could share reporting on a single outcome or composite measure. An alternative bipartisan legislative proposal addressing the Sustainable Growth Rate formula introduced a “Merit-Based Incentive Payment System.” Professionals enumerated under this proposal would include anesthesiologists and nurse anesthetists. In this context, performance measurement is an important part of practice expertise for anesthesiologists and may change how attribution of complications is perceived. Perioperative patient care is team-based care, and the causal contribution, or attribution, of anesthesia care to diverse postoperative outcomes is determined, in part, by the extent to which anesthesiologists guide perioperative care.14,15
Integrated surgical care delivery, Surgical Home and the Anesthesia Quality Institute
The concept of the “Surgical Home” as proposed by the ASA is 1 of many approaches to formally integrate anesthesiologists across the spectrum of perioperative care, both in terms of timing of care episodes and the decision-making that is required during those episodes. New metrics, local and national, may be needed to evaluate the success of integrated surgical care models such as the Perioperative Surgical Home.16 This review illustrates that current perioperative surgical performance measures span the scope of perioperative care from preoperative decision-making process to postoperative outcomes including discharge and beyond to 90 and even 120 days. A Surgical Home model would also allow anesthesiologists to formally participate in the process of implementing perioperative performance measures that are not typically part of anesthesia practice but may impact subsequent outcomes that are relevant to anesthesia. For example, in a Surgical Home model, a multispecialty team including anesthesiologists may design and implement a protocol for prevention of surgical site infection (SSI) that includes multiple performance measures, including surgeon-specific measures. Such as protocol may include the selection of antibiotics (NQF #0268), appropriate hair removal (NQF #0301), timing of antibiotics (NQF #0527), maintenance of normothermia (NQF # 0452), and appropriate cessation of antibiotics (NQF #0529). This chain of events would aim to decrease SSI (NQF #0753).
No current performance measures are designed to assess the combination of safety, quality, and resource efficiency that reflects the successful performance of a Surgical Home model; there is early work suggesting methodologies for how these might be developed.17 Team-based care is necessary to achieve good outcomes; risk of wound infection, for example, is linked to comprehensive perioperative management rather than a single process.18 Formal participation by anesthesiologists across the care spectrum may be valuable to achieving a variety of postoperative outcomes. In addition to the above series of measures, new approaches by anesthesiologists may contribute to performance improvement. For instance, the application of regional anesthesia may reduce risk of wound infection and pneumonia in joint arthroplasty.19,20,21,22
Anesthesiologists are being asked increasingly to select performance measures for public reporting and consider some form of performance-based payment.23 The ASA has addressed this issue by creating an internal Quality Division staffed with performance improvement methodologists. ASA is applying to take over stewardship of anesthesia-specific measures within NQF, and will be working to develop and steward new measures for perioperative care. One resource in this process is the Anesthesia Quality Institute (AQI), an important, emerging anesthesia quality organization. The AQI has published a list of “Anesthesia Outcomes of Interest” that includes candidate outcomes.g The AQI is acquiring anesthesia-related performance data that should facilitate the process of creating and vetting meaningful performance metrics, locally and nationally, for anesthesia care including perioperative outcomes. In May 2014, the AQI received approval of the National Anesthesia Clinical Outcomes Registry as a Qualified Clinical Data Registry for anesthesiologists to use in regulatory reporting. This mechanism includes the opportunity to include specialty-specific measures that are not already used in the Physician Quality Reporting System.
Future Challenges and Opportunities
Anesthesiology is not well represented among measure stewards. Although the ASA participates in sponsoring performance measures and has applied to NQF to become the measure steward for several measures originally managed by the American Medical Association Physician Consortium for Performance Improvement,h we found no named professional representation by any anesthesiology societies among measure stakeholders. This is in contrast to the 33 measures stewarded by named surgical societies. The importance of these disparities in the setting of linked performance and payment is unclear. The relative lack of anesthesia-specific measures may lead to the under-valuing of services provided by anesthesiologists.
Demonstrating the value of anesthesiologists outside the operating room
A noted gap among national measures is the absence of measures, whether anesthesia-specific or jointly attributable, that specifically address the quality of general anesthesia and sedation care provided outside the operating room. None of the more than 664 performance measures addressed procedural performance for electroconvulsive therapy or endoscopy (upper or lower). These care settings, which also include imaging and interventional radiology procedures, are not uniformly “low risk.”24 Anesthesia for imaging may be associated with a greater risk of mortality than anesthesia for many general surgical procedures.25 Electroconvulsive therapy occurs in settings where26,27 anesthesiologists provide antecedent consultation in 60 to 70% of settings.28 Although performance measures are not needed for every combination of procedure and setting, these care episodes, and the role of anesthesia during them, may be considered for future performance metrics.
Endoscopy (upper and lower) is a procedure for which anesthesiologists are important stakeholders. Anesthesiologists are regularly involved in hospital sedation practices or direct care of patients undergoing endoscopy, and anesthesia involvement has been associated with increased risk of postendoscopy complications in the literature.29 The GI Quality Improvement Consortium, co-sponsored by the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, began registry tracking upper endoscopy in 2013.i This registry includes its own performance measures, including documentation of ASA Physical Status, but no other sedation or anesthesia-related information. This registry, as well as the registries operated by the Society of Thoracic Surgeons and American College of Surgeons, may present partnership opportunities for anesthesiologists to take a public role in patient safety and performance measurement for endoscopy.
Over- and under-represented procedures
In contrast to current anesthesia-specific measures, jointly attributable performance measures were heavily weighted towards outcome measures. Only 1 outcome measure addressed overall patient experience or patient satisfaction, and this measure, as constructed, did not include any questions specific to the anesthetic experience. Jointly attributable outcome measures disproportionately emphasized patients undergoing cardiothoracic surgeries and primarily focused on 30-day mortality alone. While these surgeries are high-cost surgeries with high mortality compared to noncardiac surgery, their representation is more likely a reflection of the historical strengths of specialty-specific data registries administered by the Society for Thoracic Surgery, the Society for Vascular Surgery and the American College of Surgeons National Surgical Quality Improvement Program. Cardiovascular surgery accounts for only a small percentage of all anesthetics administered nationally, and many anesthesia groups do not practice in settings where cardiac surgery is performed.30 Future targets for performance measurement may emphasize those surgeries, such as knee arthroplasty, hip replacement, spinal fusion, and cesarean delivery, which account for a large fraction of national spending on surgical care.j Other common perioperative patients were not specifically addressed by current performance measures. Examples highlighted in this review include trauma, urology, neurosurgery, and head and neck surgery. Additionally, no measures specifically addressed anesthesia involvement in obstetric care beyond administration of antibiotics for cesarean delivery.
Limitations of this article
This article has focused on NQF-endorsed metrics as a starting point to evaluate performance measurement in anesthesiology. Other metrics outside the NQF are used by providers, groups, and health systems to measure performance. The exclusive focus on NQF was intentional because this organization is the federally designated clearinghouse for measures intended for value-based purchasing, and the NQF has publicized periods of review where the public is encouraged to provide feedback on proposed measures. This mechanism allows patient and provider groups, including anesthesiologists, to voice opinions about any NQF measure. This article does not address the evidence base for any measures or assess their utility, and it is clear that the evidence base for performance measures can be questioned as new data and methods emerge. Examples include both process and outcome measures such as postoperative glucose control after cardiac surgery,31 perioperative beta-blockade in cardiac surgery,32 normothermia,33 colorectal SSI,34 and venous thromboembolism.35 In a broad sense, this demonstrates that all performance measures require periodic reassessment and that outcome measures are not uniformly superior to process measures.
Finally, the extent to which any measures are jointly attributable to both anesthesia care and surgical care may vary across settings or institutions. In this review, the classification of measures as jointly attributable to anesthesia care is intentionally broad, such as for case volume for complex cases. These classifications are not intended to be an expert opinion about attribution of performance to both anesthesia and surgical care for all settings. The role of anesthesia care in outcomes occurring more than a few days postoperatively may not be apparent to all anesthesiologists. For instance, anesthesiologists may not appreciate SSI as a jointly attributable outcome despite their taking ownership of antibiotic administration and maintenance of normothermia. These issues of attribution will require local-level discussions but may become irrelevant as bundled payments become more common and surgical care is delivered in integrated care environments such as the perioperative Surgical Home. What will remain important is that national level performance measures are intended to encourage high-value care for patients across diverse settings irrespective of local patterns of attribution.
Conclusion
Few current NQF metrics address performance entirely attributable to anesthesia. By contrast, numerous measures address performance with shared attribution by anesthesia providers, surgeons and others in the care stream, but these disproportionately represent cardiothoracic surgery. Developing and implementing meaningful perianesthetic metrics is important for quality improvement and demonstrating value, particularly as payment systems evolve. Adapting existing team-based performance measures with shared attribution by multiple specialties as part of team-based care is 1 alternative to creating new anesthesia-specific measures. In addition, efforts to develop national databases or engage with established surgical registries to contribute anesthesia-related information should receive strong support. Such efforts would permit available data to inform practice, improve patient care and pioneer candidate patient-centered outcomes.
Acknowledgments
Funding: N/A
Footnotes
“Health Policy Brief: Pay-for-Performance,” Health Affairs, October 11, 2012.
https://www.qualityforum.org/Setting_Priorities/Partnership/Measure_Applications_Partnership.aspx (Accessed February 13, 2014)
http://www.modernhealthcare.com/article/20140422/NEWS/304229955 (Accessed June 3, 2014)
Personal communication with Richard P Dutton, MD, MBA, July 8, 2014
http://gi.org/media/09062013-3. Accessed March 14, 2014.
http://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.pdf (Accessed June 3, 2014)
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Joseph A Hyder, MD, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Joseph A Hyder approved the final manuscript
Name: Jonathan Niconchuk, MD
Contribution: This author helped write the manuscript
Attestation: Jonathan Niconchuk approved the final manuscript
Name: Laurent G Glance, MD
Contribution: This author helped analyze the data and write the manuscript
Attestation: Laurent G Glance approved the final manuscript
Name: Mark D Neuman, MD
Contribution: This author helped write the manuscript
Attestation: Mark D Neuman approved the final manuscript
Name: Robert R Cima, MD, MA
Contribution: This author helped analyze the data and write the manuscript.
Attestation: Richard R Cima approved the final manuscript
Name: Richard P Dutton, MD
Contribution: This author helped write the manuscript
Attestation: Richard P Dutton approved the final manuscript
Name: Louis L Nguyen, MD, MBA, MPH
Contribution: This author helped design the study and write the manuscript
Attestation: Louis L Nguyen approved the final manuscript
Name: Lee A Fleisher, MD
Contribution: This author helped write the manuscript
Attestation: Lee A Fleisher approved the final manuscript
Name: Angela M Bader, MD, MPH
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Angela M Bader approved the final manuscrip
This manuscript was handled by: Steven L. Shafer, MD
Contributor Information
Joseph A. Hyder, Department of Anesthesiology, Mayo Clinic, Rochester, Minnesota.
Jonathan Niconchuk, Department of Anesthesiology, Vanderbilt University School of Medicine, Nashville, Tennessee.
Laurent G. Glance, Department of Anesthesiology, University of Rochester School of Medicine, Rochester, New York.
Mark D. Neuman, Department of Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, Pennsylvania.
Robert R. Cima, Department of Surgery, Mayo Clinic, Rochester, Minnesota.
Richard P. Dutton, Anesthesia Quality Institute, Park Ridge, Illinois; Department of Anesthesia and Critical Care, University of Chicago, Chicago, Illinois.
Louis L. Nguyen, Department of Surgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts.
Lee A. Fleisher, Department of Anesthesiology and Critical Care, University of Pennsylvania Health System, Philadelphia, Pennsylvania.
Angela M. Bader, Department of Anesthesiology, Perioperative and Pain Medicine, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts.
References
- 1.The joint commission recipients of 2007 Ernest Amory Codman awards. National health care award for performance measurement. The Joint Commission Perspectives. 2008 Jan;28(1):3, 10. [PubMed] [Google Scholar]
- 2.Pronovost PJ, Demski R, Callender T, Winner L, Miller MR, Austin JM, Berenholtz SM National Leadership Core Measures Work Groups. Demonstrating high reliability on accountability measures at the Johns Hopkins hospital. Jt Comm J Qual Patient Saf. 2013;39(12):531–44. doi: 10.1016/s1553-7250(13)39069-2. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenfeld JM, McEvoy MD, Furman WR, Snyder D, Sandberg WS. Automated near-real-time clinical performance feedback for anesthesiology residents: One piece of the milestones puzzle. Anesthesiology. 2014;120(1):172–84. doi: 10.1097/ALN.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimick JB, Ghaferi AA, Osborne NH, Ko CY, Hall BL. Reliability adjustment for reporting hospital outcomes with surgery. Ann Surg. 2012;255(4):703–7. doi: 10.1097/SLA.0b013e31824b46ff. [DOI] [PubMed] [Google Scholar]
- 5.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: The importance of reliability adjustment. Health Serv Res. 2010;45(6 Pt 1):1614–29. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glance LG, Fleisher LA. Anesthesiologists and the transformation of the healthcare system: A call to action. Anesthesiology. 2014;120(2):257–9. doi: 10.1097/ALN.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 7.Haller G, Stoelwinder J, Myles PS, McNeil J. Quality and safety indicators in anesthesia: A systematic review. Anesthesiology. 2009;110(5):1158–75. doi: 10.1097/ALN.0b013e3181a1093b. [DOI] [PubMed] [Google Scholar]
- 8.ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion, International Consortium for Evidence Based Perfusion. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 10.Wijeysundera DN, Mamdani M, Laupacis A, Fleisher LA, Beattie WS, Johnson SR, Kolstad J, Neuman MD. Clinical evidence, practice guidelines, and beta-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes. 2012;5(4):558–65. doi: 10.1161/CIRCOUTCOMES.112.965632. [DOI] [PubMed] [Google Scholar]
- 11.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–34. [PubMed] [Google Scholar]
- 12.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303(24):2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 13.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 14.Silber JH, Kennedy SK, Even-Shoshan O, Chen W, Mosher RE, Showan AM, Longnecker DE. Anesthesiologist board certification and patient outcomes. Anesthesiology. 2002;96(5):1044–52. doi: 10.1097/00000542-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Vetter TR, Goeddel LA, Boudreaux AM, Hunt TR, Jones KA, Pittet J. The Perioperative Surgical Home: how can it make the case so everyone wins? BMC Anesthesiology. 2013;13:6. doi: 10.1186/1471-2253-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vetter TR, Ivankova NV, Goeddel LA, McGwin G, Jr, Pittet JF UAB Perioperative Surgical Home Group. An analysis of methodologies that can be used to validate if a perioperative surgical home improves the patient-centeredness, evidence-based practice, quality, safety, and value of patient care. Anesthesiology. 2013;119(6):1261–74. doi: 10.1097/ALN.0b013e3182a8e9e6. [DOI] [PubMed] [Google Scholar]
- 18.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303(24):2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: A population-based study. Anesthesiology. 2010;113(2):279–84. doi: 10.1097/ALN.0b013e3181e2c1c3. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Ma C, Elkassabany N, Fleisher LA, Neuman MD. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg. 2013;117(4):1010–6. doi: 10.1213/ANE.0b013e3182a1bf1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memtsoudis SG, Sun X, Chiu YL, Stundner O, Liu SS, Banerjee S, Mazumdar M, Sharrock NE. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118(5):1046–58. doi: 10.1097/ALN.0b013e318286061d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193–9. doi: 10.2106/JBJS.K.01682. [DOI] [PubMed] [Google Scholar]
- 23.Glance LG, Fleisher LA. Anesthesiologists and the transformation of the healthcare system: A call to action. Anesthesiology. 2014;120(2):257–9. doi: 10.1097/ALN.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 24.Malviya S, Voepel-Lewis T, Prochaska G, Tait AR. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics. 2000;105(3):E42. doi: 10.1542/peds.105.3.e42. [DOI] [PubMed] [Google Scholar]
- 25.Girshin M, Shapiro V, Rhee A, Ginsberg S, Inchiosa MA., Jr Increased risk of general anesthesia for high-risk patients undergoing magnetic resonance imaging. J Comput Assist Tomogr. 2009;33(2):312–5. doi: 10.1097/RCT.0b013e31818474b8. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer PN, Valenstein M, Hoggatt KJ, Ganoczy D, Maixner D, Miller EM, Zivin K. Electroconvulsive therapy for major depression within the veterans health administration. J Affect Disord. 2011;130(1–2):21–5. doi: 10.1016/j.jad.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner RD, Prudic J. Electroconvulsive therapy in the United States: How often is it used? Biol Psychiatry. 2013;73(2):105–6. doi: 10.1016/j.biopsych.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Gilron I, Delva N, Graf P, Chan P, Enns M, Gosselin C, Jewell M, Lawson JS, Martin B, Milev R, Paltry S. Canadian survey of perianesthetic care for patients receiving electroconvulsive therapy. J Ect. 2012;28(4):219–24. doi: 10.1097/YCT.0b013e31825927a2. [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: A population-based analysis. JAMA Intern Med. 2013;173(7):551–6. doi: 10.1001/jamainternmed.2013.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semel ME, Lipsitz SR, Funk LM, Bader AM, Weiser TG, Gawande AA. Rates and patterns of death after surgery in the united states, 1996 and 2006. Surgery. 2012;151(2):171–82. doi: 10.1016/j.surg.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 31.LaPar DJ, Isbell JM, Kern JA, Ailawadi G, Kron IL. Surgical care improvement project measure for postoperative glucose control should not be used as a measure of quality after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147(3):1041–8. doi: 10.1016/j.jtcvs.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 32.LaPar DJ, Crosby IK, Kron IL, Kern JA, Fonner E, Jr, Rich JB, Speir AM, Ailawadi G. Preoperative beta-blocker use should not be a quality metric for coronary artery bypass grafting. Ann Thorac Surg. 2013;96(5):1539, 44. doi: 10.1016/j.athoracsur.2013.05.059. discussion 1544–5. [DOI] [PubMed] [Google Scholar]
- 33.Melton GB, Vogel JD, Swenson BR, Remzi FH, Rothenberger DA, Wick EC. Continuous intraoperative temperature measurement and surgical site infection risk: Analysis of anesthesia information system data in 1008 colorectal procedures. Ann Surg. 2013;258(4):606, 12. doi: 10.1097/SLA.0b013e3182a4ec0f. discussion 612–3. [DOI] [PubMed] [Google Scholar]
- 34.Lawson EH, Ko CY, Adams JL, Chow WB, Hall BL. Reliability of evaluating hospital quality by colorectal surgical site infection type. Ann Surg. 2013;258(6):994–1000. doi: 10.1097/SLA.0b013e3182929178. [DOI] [PubMed] [Google Scholar]
- 35.Bilimoria KY, Chung J, Ju MH, Haut ER, Bentrem DJ, Ko CY, Baker DW. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482–9. doi: 10.1001/jama.2013.280048. [DOI] [PubMed] [Google Scholar]