Abstract
Background/Aims
Advances in endoscopic submucosal dissection (ESD) techniques have led to the development of expanded criteria for endoscopic resection of early gastric cancer (EGC). The aim of this study was to evaluate the short- and long-term outcomes for ESD using indication criteria.
Methods
A total of 1,105 patients underwent ESD for EGC at six medical centers. The patients were classified into the following two groups based on the lesion size, presence of ulceration and pathological review: an absolute criteria group (n=517) and an expanded criteria group (n=588).
Results
The curative resection rates (91.1% vs 91.3%, p=0.896) were similar in the absolute criteria group and the expanded criteria group. The en bloc resection rates (93.4% and 92.3%, respectively; p=0.488) and complete resection rates (98.3% and 97.4%, respectively; p=0.357) did not differ between the groups. The cumulative disease-free survival rates and the overall survival rates were similar between the groups (p=0.778 and p=0.654, respectively). Independent factors for the curative resection of EGC included tumor location (upper vs middle and lower, 2.632 [1.128–6.144] vs 3.497 [1.560–7.842], respectively) and en bloc resection rate 12.576 [7.442–21.250].
Conclusions
The expanded criteria for ESD in cases of EGC is comparable with the widely accepted pre-existing criteria.
Keywords: Stomach neoplasms, Endoscopy, gastrointestinal, Criteria
INTRODUCTION
Gastric cancer is the most prevalent malignant neoplasm in Korea, and the second leading cause of cancer-related death in the world.1,2 Early gastric cancer (EGC) is defined as gastric cancer that is confined to the mucosa or submucosa (T1 cancer), irrespective of the presence of regional lymph node metastasis.3 As current growing number of health examinations and developments in endoscopic technology, more cases of EGC are being detected, corresponding to 47.4% of all gastric cancers in Korea as of 2004.4 Endoscopic mucosal resection (EMR) is widely accepted as an alternative treatment of EGC with a low risk of lymph node metastasis, as it is minimally invasive and has a good safety profile.5,6 At present, the standard guideline criteria for endoscopic resection, which were established by the Japanese Gastric Cancer Association, have been generally accepted, and as follows: a differentiated-type adenocarcinoma without ulcerative findings, of which the depth of invasion is clinically diagnosed as T1a and the diameter is ≤2 cm.7 However conventional EMR nearly always results in piecemeal resection when lesions are larger than 20 mm in diameter, and is not reliable for lesions with ulcer findings.8,9 Conventional EMR is associated with a high risk of local recurrence (2% to 35%), especially when resections are not accomplished en bloc or the margins are not clear.10
Endoscopic submucosal dissection (ESD) has been developed to dissect directly along the submucosal layer using specialized devices, and has advantage over conventional EMR for removing larger or ulcerated EGC lesions in an en bloc manner.8,11 ESD technique enabled to expand the range of criterias of endoscopic treatment in cases of EGC and Gotoda et al.12 and the Japanese Gastric Cancer Association7 recently defined expanded criteria, based on an analysis of the risk of lymph node involvement in more than 5,000 EGCs: (1) mucosal cancer without ulcer findings irrespective of tumor size, (2) mucosal cancer with ulcer findings ≤3 cm in diameter, (3) minute (<500 μm from the muscularis mucosae) submucosal invasive cancer ≤3 cm in size, and (4) undifferentiated type mucosal cancer ≤2 cm in size without ulceration. However, the outcomes of EGC patients treated by ESD that fulfilled the new expanded inclusion criteria remain uncertain. The purpose of this study was to evaluate the feasibility and efficacy of expanded criterias for ESD as a treatment of EGC and to compare it with other criteria.
MATERIALS AND METHODS
1. Patients
A total of 1,105 EGCs in 1,105 consecutive EGC patients were treated by ESD at the six hospitals in the Daegu Kyungpook area in Korea from February 2003 to May 2010. The patients were enrolled based on the criteria proposed by Gotoda et al.12 and the Japanese Gastric Cancer Association.7 EGCs which did not meet these criteria were recommended to receive a gastrectomy with removal of lymph nodes.
The study was approved by the Institutional Review Board of each medical center. Before ESD, all patients provided oral and written informed consent for the procedure. We retrospectively reviewed a prospectively maintained database of all patients with EGC treated with ESD.
The patients were divided into two groups according to the endoscopic findings and histopathological diagnoses. The absolute criteria A group is defined as a differentiated-type adenocarcinoma without ulcerative findings, of which the depth of invasion is clinically diagnosed as T1a and the diameter is ≤2 cm. The expanded criteria E group is defined as tumors clinically diagnosed as T1a and: (a) of differentiated-type, ulcer (−), but >2 cm in diameter (b) of differentiated-type, ulcer (+), and ≤3 cm in diameter (c) of undifferentiated-type, ulcer (−), and ≤2 cm in diameter.
2. Endoscopic submucosal dissection
The ESD procedure was carried out in a standardized way. After informed consent was obtained, the ESD was performed in patients under conscious sedation with intravenous midazolam and meperidine. The procedure was performed by experienced endoscopists who performed EMR or EMR-precutting (EMR-P) over 100 cases. To determine the resection margin, chromoendoscopy with indigo carmine or narrow-band imaging (NBI) was performed in addition to conventional white light endoscopy. And then the area at about 5 mm lateral to the lesions was marked with spotty cautery with various endoscopic knives (IT knife or hook knife; Olympus, Tokyo, Japan). Then, submucosal injection of hypertonic saline mixed with epinephrine (1:10,000) or glycerol, and sodium hyaluronate was performed to lift the lesion. The endoscope was passed to the submucosa and dissection was performed under direct vision with an endoscopic knife in the caudal direction. The resected lesion was spread to mark the orientation with pins and fixed with 10% formalin solution; it was then brought to pathology for histological evaluation and diagnosis.
3. Complications
The perforations were divided into two types: macroperforation, defined as a gross defect noted during the procedure, with extraluminal organs, fatty tissues or space visualized through the lesion endoscopically, irrespective of the presence of air accumulation in the abdomen, retroperitoneum, or mediastinum; or microperforation defined as a perforation that was invisible during procedure but was recognized as free air on a plain radiography (abdomen, retroperitoneum, mediastinum) after the procedure.13
4. Histopathological evaluation
The macroscopic lesions were classified into the elevated type and the flat/depressed type. EGC location was classified into the upper, middle, and lower third of the stomach. Ulcer was defined as mucosal defect, mucosal deformity, or converging fold by endoscopic findings, or submucosal fibrosis. Resection specimens were stretched with needles and sent for histopathological assessment and sectioned perpendicularly at 2 mm intervals. The histology was divided into differentiated adenocarcinoma (well or moderately differentiated or papillary adenocarcinoma) or undifferentiated adenocarcinoma (poorly differentiated or signet ring cell carcinoma). Tumor involvement to the horizontal and deep margins, lymphatic and vascular involvement, tumor size, and presence or absence of submucosal invasion were assessed. In cases with submucosal infiltration, invasion depth was measured and described quantitatively.
En bloc resection was defined as a single resection procedure performed for a single lesion; and piecemeal resection as multiple resection procedures for a single lesion.14 The complete resection was defined as R0 resection: complete en bloc resection with vertical and horizontal margins free of neoplasia at histology. The curative resection was defined as en bloc and margin negative resection without lymphovascular involvement in the absolute and expanded criteria groups.7
5. Follow-up
The patients were followed up with an endoscopic examinations with a biopsy at 3, 6, and 12 months after ESD and then annually. To avoid case losses, we attempted to identify details by questionnaires or telephone conversation with the patients, in particular in those who delayed the follow up period. To access the presence of local recurrence or metachronous cancer, biopsy was done from the treatment-related scar or any other suspicious abnormalities. In addition, an abdominal computed tomography (CT) and/or positron emission tomography/CT was performed annually to detect lymph node and distant metastases.
The cumulative disease-specific and overall survivals were estimated.
6. Statistical analysis
The significance of differences in patients’ characteristics and clinicopathological features was determined using chi-square test, Fisher exact test, the Mann-Whitney U-test, or Student t-test. Factors associated with curability of ESD were analyzed using logistic regression analysis. Odds ratios, together with 95% confidence intervals, were calculated to estimate the relative risk of noncurative resection and their associations with various parameters. Data for the long-term outcomes were calculated using the Kaplan-Meier method and analyzed by the log rank test. p-values <0.05 were considered statistically significant.
RESULTS
1. Characteristics of patients and lesions
1) Clinical features of patients
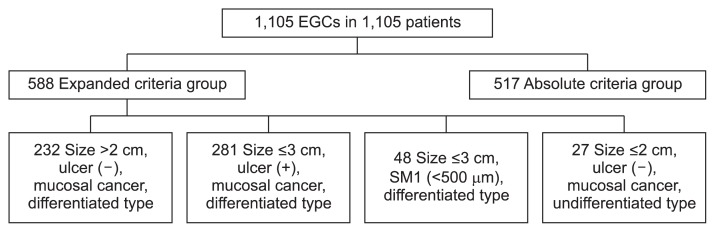
A total of 1,105 EGCs in 1,105 consecutive patients were included in our analysis and they were divided into two groups; A group (517 patients) and E group (588 patients). We targeted one EGC lesion by each person, which was the largest one or was included the highest criteria group. The E group consisted of 232 mucosal cancer without ulcer findings larger than 2 cm in tumor size, 281 mucosal cancer with ulcer findings ≤3 cm in diameter, 27 minute (<500 μm from the muscularis mucosae) submucosal invasive cancer ≤3 cm in size, and 48 undifferentiated type mucosal cancer ≤2 cm in size without ulceration (Fig. 1).
Fig. 1.
Classification of patients with early gastric cancer (EGC) according to the endoscopic and histopathological diagnoses: 517 patients were in the absolute criteria group, and 588 patients were in the expanded criteria group. SM, submucosa.
The median age of the patients was 64 years (33 to 87 years) for the A group, 66 years (27 to 87 years) for the E group. All of the groups have higher distribution of men than women (Table 1). Major comorbidity included malignancies other than EGC, cerebrovascular event, cardiopulmonary diseases, chronic kidney or hepatic diseases, and hematologic diseases which result the limitation of physical activities and need periodic treatment. Patients with major comorbidities accounted for 6.4% in the A group, and 5.3% in the E group (p=0.430).
Table 1.
Clinicopathological Characteristics Based on the Indication Criteria
| Characteristic | Absolute criteria group (n=517) | Expanded criteria group (n=588) | p-value |
|---|---|---|---|
| Age, yr | 64 (33–87) | 66 (27–87) | 0.143 |
| Gender, female/male, % | 34.6/65.4 | 30.3/69.7 | 0.123 |
| Macroscopic appearance | <0.001 | ||
| Elevated | 277 (53.6) | 251 (42.7) | |
| Flat/depressed | 240 (46.4) | 337 (57.3) | |
| Tumor size, mm | <0.001 | ||
| <20 | 517 (100.0) | 309 (52.6) | |
| 20–30 | 183 (31.1) | ||
| >30 | 96 (16.3) | ||
| Location | 0.237 | ||
| Upper | 24 (4.6) | 23 (3.9) | |
| Middle | 135 (26.1) | 180 (30.6) | |
| Lower | 358 (69.2) | 385 (65.5) | |
| Ulcer findings | <0.001 | ||
| Present | 0 | 294 (50.0) | |
| Abscent | 517 (100.0) | 294 (50.0) | |
| Invasion depth | <0.001 | ||
| M | 517 (100.0) | 540 (91.8) | |
| SM1 | 0 | 48 (8.2) | |
| SM2 | 0 | 0 | |
| Major comorbidity | 33 (6.4) | 31 (5.3) | 0.430 |
| Delayed bleeding | 17 (3.3) | 27 (4.6) | 0.269 |
| Perforation | 14 (2.7) | 18 (3.1) | 0.727 |
| Microperforation | 11 | 14 | |
| Macroperforation | 3 | 4 |
Data are presented as median (range) or number (%).
M, mucosa; SM, submucosa.
2) Characteristics of resected lesions
The most common location of the lesion was the lower third of the stomach in all groups (69.2%/65.5%, respectively). The elevated type of lesion was more common in the A group than in the E group (Table 1), and this difference was statistically significant (53.5%/42.6%, p<0.001). The mean tumor size was 12.51±0.23 mm in the A group, and 22.91±0.55 mm in the E group; The A group has significantly smaller lesion size than the E group (p<0.001). The ulcer findings by gross appearance were 50.0% in the E group.
2. Results of the ESD
1) Resectability, complete resection, and curability of ESD
The frequency of endoscopic en bloc resections was 92.9% (1,026/1,105) in all lesions; it was 93.4% in the A group, and 92.3% in the E group and there was no statistically significant difference between the groups (p=0.488) (Table 2). A complete resection rate was 97.8% (1,081/1,105) in all lesions; 98.3% in the A group, and 97.4% in the E group, however, this difference was not statistically significant (p=0.357). Of the 1,105 lesions, 1,008 (91.2%) were defined as curative; the curative resection rate was 91.1% in the A group and 91.3% in the E group, there was no statistically significant difference between the two groups (p=0.896).
Table 2.
Resectability, Completeness, and Curability of Endoscopic Submucosal Dissection for Early Gastric Cancer and the Indication Criteria
| Absolute criteria group (n=517) | Expanded criteria group (n=588) | p-value | |
|---|---|---|---|
| Resectability | 0.488 | ||
| En bloc resection | 483 | 543 | |
| Piecemeal resection | 34 | 45 | |
| En bloc resection rate, % | 93.4 | 92.3 | |
| Completeness | 0.357 | ||
| Complete | 508 | 573 | |
| Incomplete | 9 | 15 | |
| Complete resection rate, % | 98.3 | 97.4 | |
| Curability | 0.896 | ||
| Curative | 471 | 537 | |
| Noncurative | 46 | 51 | |
| Curative resection rate, % | 91.1 | 91.3 |
Data are presented as number.
Table 3 shows the association of various factors with curability of ESD. On univariate analysis, the location of the lesion, and the presence of ulcer and en bloc resection had a significant impact on ESD curability (p<0.001, p=0.020, and p<0.001, respectively). On a multivariate analysis, the tumor location: upper versus middle and lower, 2.632 (1.128–6.144) and 3.497 (1.560–7.842), and the presence of en bloc resection: 12.576 (7.442–21.250) were related to the curability.
Table 3.
Association of Clinicopathological Characteristics of the 1,105 Early Gastric Cancer Lesions with Curability of Endoscopic Submucosal Dissection
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.995 (0.973–1.017) | 0.633 | - | - |
| Gender | ||||
| Female | 1 | - | ||
| Male | 0.666 (0.413–1.076) | 0.097 | - | - |
| Tumor size, cm | ||||
| <2.0 | 1 | - | ||
| 2.0–3.0 | 0.659 (0.392–1.109) | 0.116 | - | - |
| >3.0 | 0.660 (0.335–1.299) | 0.229 | - | - |
| Tumor location | ||||
| Upper | 1 | 1 | ||
| Middle | 3.381 (1.619–7.061) | 0.001 | 2.632 (1.128–6.144) | 0.025 |
| Lower | 5.081 (2.527–10.216) | <0.001 | 3.497 (1.560–7.842) | 0.002 |
| Macroscopic appearance | ||||
| Elevated | 1 | - | ||
| Flat/depressed | 1.213 (0.795–1.853) | 0.370 | - | - |
| Ulcer findings | ||||
| Absent | 1 | 1 | ||
| Present | 1.928 (1.108–3.354) | 0.020 | 1.644 (0.917–2.947) | 0.095 |
| Resectability | ||||
| En bloc | 14.183 (8.491–23.693) | <0.001 | 12.576 (7.442–21.250) | <0.001 |
| Piecemeal | 1 | 1 | ||
OR, odds ratio; CI, confidence interval.
2) Follow-up observations and recurrence rates
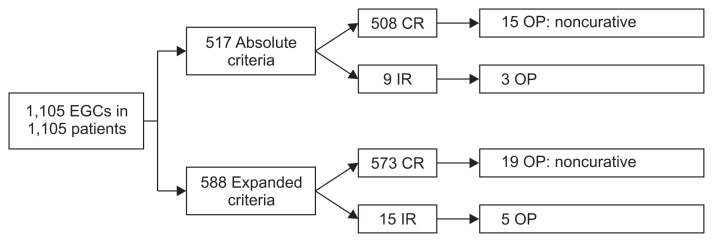
The rate of surgical treatment was 3.8% (42/1,105) in all patients; 3.5% (18/517) in the A group, and 4.1% (24/588) in the E group (Fig. 2). These 42 patients who underwent surgical treatment and the patients with a follow-up period of <1 year were excluded from the disease-free survival and overall survival analysis, and thus 1,063 patients treated by ESD were eligible for the analyses.
Fig. 2.
Flowchart of patients included in this study. A total of 1,105 lesions from 1,105 patients were included in endoscopic outcomes. EGC, early gastric cancer; CR, complete resection; OP, operation; IR, incomplete resection.
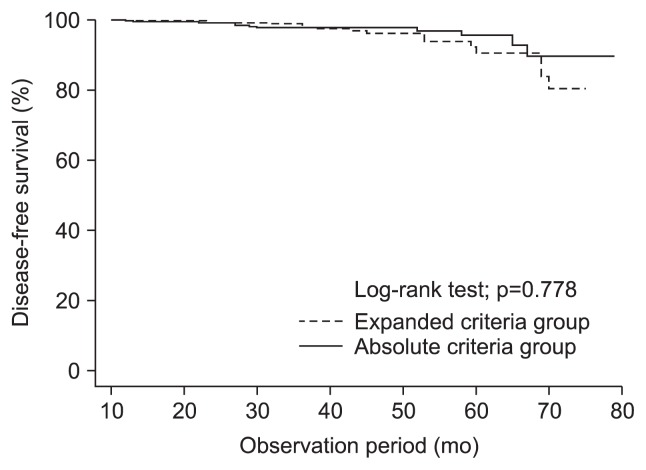
The cumulative disease-free survival rates did not significantly differ between the A group and the E group (p=0.778). The 1-year disease-free rates were 99.3% in the A group, and 99.6% in the E group, and the 3-year disease-free survival rates were 98.1%, and 97.1%, respectively (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of disease-free survival rates in the absolute and expanded criteria groups. There were no significant between-group differences (p=0.778).
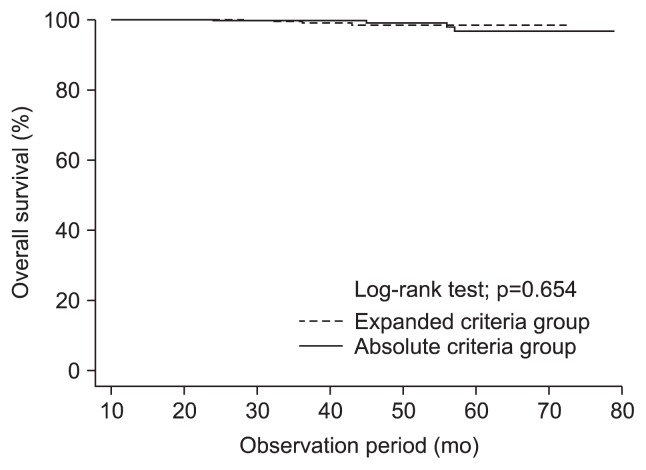
The cumulative overall survival rates did not differ significantly between the groups (Fig. 4). The 3-year overall survival rates were 99.0% in the A group, and 98.6% in the E group (p=0.654).
Fig. 4.
Kaplan-Meier estimates of overall survival rates in the absolute and expanded criteria groups. There were no significant between-group differences (p=0.654).
3) Complications
Perforation occurred in 32 out of 1,105 lesions. Macroperforations occurred during ESD in seven lesions and microperforation was identified in 25 lesions. They were successfully managed by decompression of the pneumoperitoneum with an 18-gauge puncture needle and/or hemoclipping of the perforation site and with systemic antibiotics.
Delayed bleeding occurred in 44 out of 1,105 lesions, and the mean time to bleeding was 98 hours after the procedure; all patients recovered with endoscopic intervention and conservative treatment, there was no need for surgical intervention. There was no difference in the frequency of delayed bleeding and perforation based on the criteria in this study (p=0.300 and p=0.688).
DISCUSSION
Endoscopic resection is less invasive and more cost-effective than surgery,10 and preserving the organ involved as well as patient quality of life. Thus, endoscopic resection has recently become an alternative treatment modality for EGC patients at low risk of lymph node metastasis or for whom surgery might be dangerous.6,10,15 Despite the expanding use of the criterias of ESD for EGC in clinical practice, the clinical outcomes for this new criterias have not been fully evaluated.7 Our study was designed to have an evidence for this clinical practice.
In this present study, en bloc resection rate in the E group is similar within the A group (92.3% vs 93.4%), and the reported en bloc resection rate of ESD ranges from 91.9% to 98.6%.16–18 Some recent studies reported a significant difference in the curative resection rate between the A group and the E group (97.0% vs 90.4% and 97.1% vs 91.1%, respectively).16,17 In contrast, our study showed no statistically significant difference in the curative resection rate between the A group (91.1%) and the E group (91.3%). We think that the reason of these different results among the studies might be that our definition of ulcer was distinct from other studies. We included mucosal defect as an ulcer, which was actually defined as erosion, therefore, the proportion of our E group was higher than the other studies. According to the result of our study, erosion has no influence on the outcome of ESD in EGC patient.
Another recent study evaluated the validity of expanding the criterias for ESD and reported the 1-year disease-free survival rate and the cumulative disease-free survival rate did not differ significantly between the A group and the E group.18 We also found that the cumulative disease-free survival rate did not differ significantly between the A group and E group. A recent study reported a comparable overall survival between the A group and the E group.16 Our study also found that the 3-year overall survival rates did not significantly different among the A group and the E group.
Using logistic regression analysis, we assessed the impact of various factors on the curability of ESD. The location of lesion and en bloc resection were the independent factors for curative resection. One recent study reported that the tumor size larger than 3 cm, ulceration, histological type, and piecemeal resection were the unfavorable factors of curative resection.19 Our study also revealed that piecemeal resection represented an independent factor for noncurative resection. More distal location of lesion was also the favorable factor of curative resection. Macroscopic classification of the lesions was divided into the elevated type and the flat/depressed type, and was not the independent factors for curative resection.
A recent study on the comparison of the outcomes of 1,627 cases EGCs after endoscopic resection based on the criteria20 reported that with the ESD method, the absolute criteria group has significantly higher rates of complete resection (97.8% vs 91.1%, p<0.001) and margin-negative status (98.8% vs 91.7%, p<0.001) than those in the expanded criteria group. They also reported the 3-year disease-specific, local recurrence-free rate 98.8% to 99.0% in the A group and 98.5% in the E group, and differences between criteria groups were not significant (p=0.547). Our 3-year local and metachronous recurrence-free survival rates were slightly lower than that (98.1% vs 97.1%, p=0.778). At a median follow-up period of 32 months, we observed 22 locally and metachronous recurrent tumors (2.1%). None of them was died in the follow-up period, and five were treated by surgery.
The major complications associated with the ESD were bleeding and perforation.21 The frequency of hemorrhagic complications has been reported to be 1.5% to 24%; the variation is due to the definition used as well as the type of resected lesion reported.11,21 Immediate bleeding developed in 172 cases out of the 1,105 cases (15.6%) in this study, and delayed bleeding developed in 44 cases out of the 1,105 cases (4.0%); most of them were minor bleeding that occurred during or after the procedure without changes in the vital signs. Perforation is another major complication; the frequency of perforation has been reported to be 0% to 6.7%.16,21,22 The overall frequency of perforations found in this study was 2.9% (microperforation, 2.3%; macroperforation, 0.6%), and they were diagnosed during or after ESD, managed with endoscopic procedures and conservative treatment without the need for further surgery.
There are several limitations in this study. First, this was retrospective study design, so there was potential for a selection bias. To minimize the selection bias, we included almost all patients with EGC treated by ESD who were identified within the database. Second, because the data were collected from multicenter, the ESD procedures were performed by several endoscopists. This could make differences in the indications of ESD or histopathological diagnoses. Lastly, this study has relatively short median follow-up period, so further study is needed with long-term results over 5 years and in prospective manner to establish the validity of ESD results.
In conclusion, ESD was effective for treatment of expanded criteria of EGC. The rate of E group’s en bloc resection and complete resection was comparable with A group. Further prospective investigation is needed to assess the long-term prognosis and survival.
Footnotes
See editorial on page 135.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177–182. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Estève J, Damiecki P, Arslan A, Renard H. Trends in cancer incidence and mortality. IARC Sci Publ. 1993;(121):1–806. doi: 10.3109/9780415874984-2. [DOI] [PubMed] [Google Scholar]
- 3.Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992;79:241–244. doi: 10.1002/bjs.1800790319. [DOI] [PubMed] [Google Scholar]
- 4.The Information Committee of the Korean Gastric Cancer Association. 2004 Nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007;7:47–54. [Google Scholar]
- 5.Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709–718. doi: 10.1055/s-2001-16224. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 8.Oka S, Tanaka S, Kaneko I, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
- 9.Takenaka R, Kawahara Y, Okada H, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 10.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 11.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 12.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/PL00011720. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Jeon SW, Cho KB, et al. Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc. 2013;27:1372–1378. doi: 10.1007/s00464-012-2618-4. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim DY, Hong SJ, Cho GS, et al. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver. 2014;8:519–525. doi: 10.5009/gnl13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi N, Isomoto H, Fukuda E, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173–181. doi: 10.1159/000215388. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985–1993. doi: 10.1007/s00464-010-1499-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Cho JY, Chang YW, et al. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920–926. doi: 10.1016/j.gie.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485–493. doi: 10.1016/j.gie.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]