Abstract
Background/Aims
Although normal endoscopic findings are, as a rule, part of the diagnosis of microscopic colitis, several cases of macroscopic lesions (MLs) have been reported in collagenous colitis, but hardly in lymphocytic colitis (LC). The aim of this study was to investigate the endoscopic, clinical, and histopathologic features of LC with MLs.
Methods
A total of 14 patients with LC who were diagnosed between 2005 and 2010 were enrolled in the study. Endoscopic, clinical, and histopathologic findings were compared retrospectively according to the presence or absence of MLs.
Results
MLs were observed in seven of the 14 LC cases. Six of the MLs exhibited hypervascularity, three exhibited exudative bleeding and one exhibited edema. The patients with MLs had more severe diarrhea and were taking aspirin or proton pump inhibitors. More intraepithelial lymphocytes were observed during histologic examination in the patients with MLs compared to the patients without MLs, although this difference was not significant. The numbers of mononuclear cells and neutrophils in the lamina propria were independent of the presence or absence of MLs.
Conclusions
LC does not always present with normal endoscopic findings. Hypervascularity and exudative bleeding are frequent endoscopic findings in patients with MLs.
Keywords: Lymphocytic colitis, Hypervascularity, Exudative, Hemorrhage
INTRODUCTION
Lymphocytic colitis (LC) together with collagenous colitis (CC) is a type of microscopic colitis (MC) characterized by chronic diarrhea and increased intraepithelial lymphocyte (IEL) infiltration.1,2 Most cases of LC have a grossly normal-appearing colonic mucosa, sometimes accompanied by nonspecific findings such as mild erythema or edema. However, several types of macroscopic lesion (ML) have been noted in association with CC, such as longitudinal ulcers,3,4 hypervascularity,5 loss of normal vascularity,6 and exudative bleeding.6 Although the etiology and pathogenesis of MLs of MC is unknown, several kinds of medications, such as proton pump inhibitors (PPIs) and nonsteroidal anti-inflammatory drugs (NSAIDs), have been suggested to be associated with such endoscopic abnormalities.3,7 However, the endoscopic features of LC have not been well described. The aim of this study was to investigate the endoscopic, clinical, and histopathologic features of LC associated with MLs.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Hanyang University Guri Hospital and was performed in accordance with the Declaration of Helsinki as revised in 1989. We reviewed the medical records of 120 patients with chronic diarrhea in Hanyang University Guri Hospital between January 2005 and December 2010. Of these patients, 14 cases were diagnosed with lymphocytic colitis.
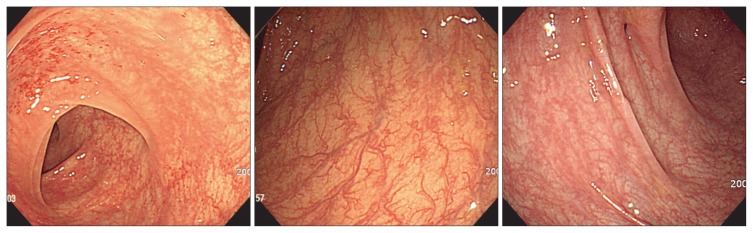
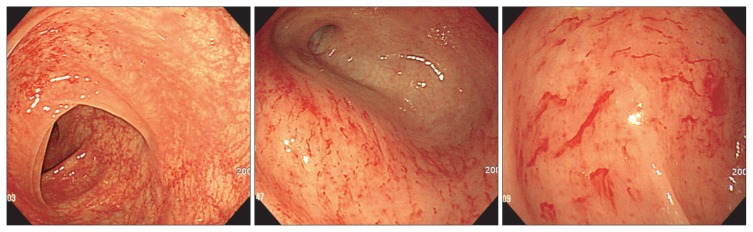
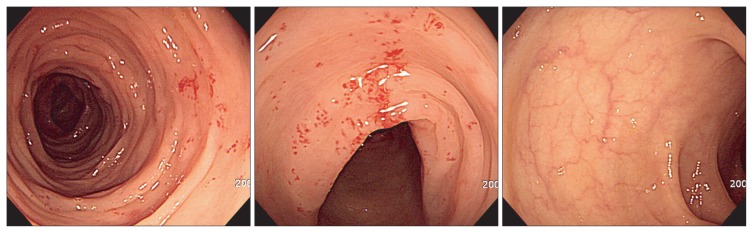
Each patient underwent total colonoscopy with multiple biopsies, in most cases from both right and left colon, and macroscopic appearance during endoscopy was registered. Endoscopic abnormalities were categorized into hypervascularity (Fig. 1), exudative bleeding (Fig. 2), longitudinal ulcer, and loss of normal vascularity (Fig. 3), according to findings in collagenous colitis.3–6 To evaluate the distribution of these findings, we divided the mucosal lesions into those in the right colon (including cecum, ascending colon, and transverse colon) and those in the left colon (including splenic flexure, descending colon, sigmoid colon, and rectum).
Fig. 1.
A crowded vascular pattern, defined as “hypervascularity” is evident (patients 8, 13).
Fig. 2.
Multiple areas of hemorrhage or oozing, defined as “exudative bleeding” can be seen (patient 8).
Fig. 3.
Loss of normal vascular marking is evident (patient 9).
Two expert pathologists reviewed 66 biopsy slides obtained from the colonic segments of the patients with LC. A diagnosis of LC was established if the following two conditions were fulfilled: presence of IEL ≥20 per 100 surface epithelial cells, and lamina propria with chronic inflammatory infiltration of cells such as lymphocytes. Inflammation of the lamina propria was classified and scored as 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe) according to the extent of mononuclear cell and neutrophil infiltration.1,8
Demographic data, medical history, family history, drug use, severity of diarrhea (frequency/day), duration of diarrhea (days) and routine laboratory tests were collected, and patients were excluded if diagnoses other than LC were given, such as infection, graft-versus-host disease, and autoimmune disease.
Data are expressed as numbers (percentages) of patients, and means±standard deviations of variables. Categorical variables were analyzed with Fisher exact test or the chi-square test. For continuous variables, Student t-test was used where appropriate. All statistical analyses were performed with SPSS statistical software version 13.0 (SPSS Inc., Chicago, IL, USA). p-values <0.05 were considered to be statistically significant.
RESULTS
Table 1 presents the clinical, colonoscopic, and histologic findings for patients with and without ML. There were no significant differences of age or gender between two groups. All the patients complained of watery diarrhea, and the average number of diarrheal events was significantly higher in the patients with ML (11.1±6.3 times/day vs 3.6±1.2 times/day, p=0.019). In addition, the duration from diagnosis to symptom improvement was not significantly different in the two groups; however, there was a tendency for it to be slightly longer in the patients with ML (3.1±2.5 weeks vs 1.8±1.6 weeks, p=0.301). There was no difference in the duration of diarrhea before diagnosis between the two groups (p=0.605) (Table 2). In this study, there were no patients with a history of smoking or autoimmune disease such as Graves disease and rheumatoid arthritis. On the other hand, there were no diagnostic differences between right and left colon on the pathologic review.
Table 1.
Characteristics of Patients with Lymphocytic Colitis
| Patients | Age, yr | Sex | Symptom | Symptom frequency (per day) | Symptom duration (wk) | Concomitant drugs (duration, mo) | Concurrent disease | Treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| PPI | Aspirin | NSAIDs | Statin | BSP | Ticlopidine | ||||||||
| Patients without mucosal lesions | |||||||||||||
| 1 | 77 | M | Diarrhea | 4 | 4 | O (48) | HLD | 5-ASA | |||||
| 2 | 25 | F | Diarrhea | 3 | 12 | None | 5-ASA | ||||||
| 3 | 66 | F | Diarrhea | 5 | 4 | Thyroiditis | None | ||||||
| 4 | 64 | F | Diarrhea | 2 | 48 | O (4) | O (24) | HLD, HL | 5-ASA | ||||
| 5 | 68 | F | Diarrhea | 3 | 4 | None | None | ||||||
| 6 | 76 | F | Diarrhea | 5 | 4 | O (48) | O (24) | HTN, OA, HL | 5-ASA | ||||
| 7 | 51 | M | Diarrhea | 3 | 8 | TB | None | ||||||
| Patients with mucosal lesions | |||||||||||||
| 8 | 75 | M | Diarrhea | 20 | 12 | LPZ (12) | DM, GERD | Steroid | |||||
| 9 | 63 | M | Diarrhea | 20 | 5 | LPZ (8) | O (2) | DM, HTN, GERD | Steroid | ||||
| 10 | 55 | F | Diarrhea | 10 | 12 | None | 5-ASA | ||||||
| 11 | 77 | F | Diarrhea | 7 | 8 | O (12) | HTN | None | |||||
| 12 | 74 | F | Diarrhea | 10 | 8 | None | Steroid | ||||||
| 13 | 78 | F | Diarrhea | 5 | 8 | LPZ (4) | O (20) | O (20) | O (20) | HTN, GERD | None | ||
| 14 | 72 | F | Diarrhea | 6 | 8 | O (2) | OA | 5-ASA | |||||
PPI, proton pump inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs; BSP, bisphosphonate; M, male; HLD, herniated lumbar disc; 5-ASA, 5-aminosalicylic acid; F, female; HL, hyperlipidemia; HTN, hypertension; OA, osteoarthritis; TB, pulmonary tuberculosis; LPZ, lansoprazole; DM, diabetes mellitus; GERD, gastroesophageal reflux disease.
Table 2.
Comparison of Clinical and Pathologic Findings in Lymphocytic Colitis with and without Mucosal Lesions
| LC without mucosal lesions (n=7) | LC with mucosal lesions (n=7) | p-value | |
|---|---|---|---|
| Clinical findings | |||
| Age, yr | 61.0±18.1 | 70±8.5 | NS |
| Male:female | 2:5 | 2:5 | NS |
| Severity of diarrhea, frequency/day | 3.6±1.2 | 11.1±6.3 | 0.019 |
| Diarrhea duration, day | 12.0±6.17 | 8.71±2.50 | 0.605 |
| Duration from diagnosis to symptom improvement, wk | 1.8±1.6 | 3.1±2.5 | 0.301 |
| Pathologic findings | |||
| IEL, % | 37.86±14.09 | 62.86±25.63 | 0.885 |
| Mononuclear cell infiltration | 1.86±0.69 | 2.14±0.38 | 0.356 |
| Neutrophil infiltration | 1.14±0.69 | 1.00±0.82 | 0.730 |
Data are presented as mean±SD.
LC, lymphocytic colitis; NS, not significant; IEL, intraepithelial lymphocyte infiltration.
Of the LC-associated drugs reported, NSAIDs were the most frequently used (four of 14 cases, 28.6%), followed by PPI and aspirin (three cases each, 21.4%), statins (two of 14 cases, 14.2%), ticlopidine (one of 14 cases, 7.1%), bisphosphonate (one of 14 cases, 7.1%). Of these medications, aspirin and PPI were taken only by patients with ML. As treatment, discontinuing the use of drugs that might have caused the diarrhea was effective. If diarrhea persisted, 5-aminosalicylic acid (5-ASA) or steroids were prescribed. In three of the patients with ML, diarrhea improved after taking steroids (Table 1).
On colonoscopic examination, hypervascularity was seen in six of the seven patients with ML (86%). ML was mainly observed in the descending colon, and it was characterized by crowded, tortuous vascularity of the colonic mucosa. Exudative bleeding was found in three patients (43%), mostly in the transverse colon. However, the presentation of ML did not differ significantly between right and left colon (Table 3).
Table 3.
Frequencies of Abnormal Endoscopic Findings in the Right and Left Colon
| Endoscopic finding | Right colon (n=7) | Left colon (n=7) | p-value |
|---|---|---|---|
| Hypervascularity | 3 (43) | 6 (86) | 0.266 |
| Exudative bleeding | 3 (43) | 1 (14) | 1.000 |
| Loss of vascular marking | 1 (14) | 1 (14) | 1.000 |
| Longitudinal ulcer | 0 | 0 | 1.000 |
Data are presented as number (%).
On histologic examination, numbers of IEL infiltrated were higher in the patients with ML than in those without ML (62.86±25.63 per 100 epithelial cells vs 47.85±26.74 per 100 epithelial cells, p=0.885), although the difference did not reach statistical significance. Numbers of mononuclear cells and neutrophils infiltrated into the lamina propria did not differ significantly between two groups (mononuclear cell, p=0.356; neutrophils, p=0.730) (Table 2).
DISCUSSION
In this study, abnormal endoscopic findings including hypervasculary, exudative bleeding, and loss of normal vascularity were observed in half (seven of 14 cases) of all LC patients. The pathogenesis of CC is similar to that of LC, and in several studies of CC about 30% of cases were accompanied by MLs, with characteristic longitudinal ulcers associated with the use of lansoprazole.3,4,6,7 However, in the present study there was no evidence of longitudinal ulcers, and neovasulcarization was the most common and prominent finding, occurring in six of the seven LCs with MLs. The frequency of endoscopic abnormalities was somewhat higher than in previous studies.7 The reason for this may be strict application in the previous studies of the diagnostic criterion stating that normal or nonsignificant endoscopic findings are required for a diagnosis of LC. However, chronic diarrhea that has persisted for over a month and pathologic findings of IEL ≥20 per 100 surface epithelial cells must be present in order to diagnose LC,9 and recently several groups have suggested that LC can be accompanied by a variety of different endoscopic findings.7 In addition, because we excluded conditions that may increase IEL infiltration or be accompanied by endoscopic abnormalities such as hypervascularity or exudative bleeding, LC is less likely to have been misdiagnosed.
Although there was no significant difference in the frequency of endoscopic abnormalities between the right and left colons (hypervascularity, p=0.266; exudative bleeding, p=1.000), hypervascularity was mostly seen in the descending or transverse colon while exudative bleeding were more prominent in the cecum, ascending and transverse colon. In view of this variation, colonoscopy of the entire colon is essential in order to diagnose LC in clinical practice.
The mean age of LC patients enrolled in this study was 65.8 years, and females predominated with a male to female ratio of 4:10. These findings are similar to previous reports in which the mean age of LC patients was between 53 and 59 years, and the male to female ratio was between 3:1 and 9:1.10–12 In the present study, the incidence of diarrhea was three times higher in the LC with ML than without ML (p=0.019). Although the difference was not statistically significant, the duration from onset of therapy to symptom improvement was longer in the LC with ML than in the LC without ML. Again there was also a tendency for there to be more IEL infiltration in the LC with ML.
The diarrhea that develops in LC is due to reduced absorption or increased secretion of electrolytes, which is caused by increased epithelial permeability due to inflammation of the epithelial cells or weakening of intraepithelial cell tight junctions. All of these effects are known to be related to the degree of inflammation of the lamina propria.10,13,14 In the present study, the score for mononuclear cell infiltration in the lamina propria of the LC with ML was 2.14±0.38. Although the difference was not significant, it was relatively higher compared to the score (1.86±0.69) for the LC with ML (p=0.356). The increase in the severity of diarrhea in the LC with ML is understandable since the degree of intraepithelial cell inflammation was more severe.
Another possible reason may be use of PPIs. In our study, the patients who complained of persistent diarrhea and who also had evidence of mucosal lesions (patients 8 and 9) were taking PPIs. Since diarrhea manifests as a side effect in 2.9% to 7.6% of patients taking PPIs, it is possible that the medication may have caused the severe clinical symptoms.15,16 However, medications such as PPIs, NSAIDs, and aspirin are reported to be associated with CC with abnormal endoscopic findings.3,17 In this study, too, there were a number of patients taking PPIs or aspirin who also had endoscopic abnormalities. It should be noted that the number of patients enrolled was too small and a sizable proportion of the patients were taking multiple medications that could cause LC, and therefore exactly which medication had how much effect cannot be determined. However, as in the case of CC, it is possible that some mucosal changes are related to use of medication and a large scale study would be desirable to examine this matter.
The limitations of the study include the following: first, the study was conducted in a single center and involved only a small number of patients. In the present study, 14 out of 120 chronic diarrhea patients (11.7%) were diagnosed with LC, which showed a higher diagnostic percentage of LC compared to previous published studies.10 This study was a retrospective study performed in a single university hospital with a relatively small number of patients enrolled, which might have been the reason for a higher diagnostic rate of LC. Secondly, there were no specific diagnostic criteria for the LC cases in which endoscopic abnormalities were evaluated, and third, subjective assessment by the endoscopist cannot be completely excluded. However, the endoscopic abnormalities were assessed based on the findings of CC,3–6 and two endoscopists both with more than 10 years of experience in this field assessed and interpreted the findings. Therefore, these limitations may not be a problem. Finally, there was no endoscopic follow up in the LC with ML. However, all but one of the patients attended the outpatient clinic regularly for at least 6 months and their symptoms improved after discontinuation of their medication so that none required antidiarrheal drugs or 5-ASA, etc. These points suggest that even though endoscopic follow-up was not performed, the possibility of patients with inflammatory bowel disease or other forms of colitis having been included in the study is low.
In conclusion, atypical features may be seen in some cases although endoscopic findings in LC are generally normal. This study is unique in that it is the first to suggest that in older diarrheal patients with MLs such as hypervascularity or exudative bleeding who have a history of taking medication such as PPIs or aspirin, the endoscopic findings may be suggestive of LC as well as CC. Since more and more elderly patients are taking medications that can induce LC, and the incidence of LC is ever-increasing, it is important to recognize the existence of LC with ML. We suggest that a large-scale prospective study should be carried out to establish the clinical characteristics and risk factors of LC.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120176).
Footnotes
See editorial on page 137.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Lymphocytic (“microscopic”) colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18–28. doi: 10.1016/0046-8177(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 2.Tremaine WJ. Diagnosing collagenous colitis: does it make a difference? Eur J Gastroenterol Hepatol. 1999;11:477–479. doi: 10.1097/00042737-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nomura E, Kagaya H, Uchimi K, et al. Linear mucosal defects: a characteristic endoscopic finding of lansoprazole-associated collagenous colitis. Endoscopy. 2010;42(Suppl 2):E9–E10. doi: 10.1055/s-0029-1214795. [DOI] [PubMed] [Google Scholar]
- 4.Couto G, Bispo M, Barreiro P, Monteiro L, Matos L. Unique endoscopy findings in collagenous colitis. Gastrointest Endosc. 2009;69:1186–1188. doi: 10.1016/j.gie.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Matsui T, Tsuda S, et al. Endosocopic abnormalities in a Japanese patient with collagenous colitis. J Gastroenterol. 2003;38:812–813. doi: 10.1007/s00535-003-1151-6. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello FM, Bayless TM, Yardley JH. Collagenous colitis. Compr Ther. 1989;15:49–54. [PubMed] [Google Scholar]
- 7.Capurso G, Marignani M, Attilia F, et al. Lansoprazole-induced microscopic colitis: an increasing problem? Results of a prospecive case-series and systematic review of the literature. Dig Liver Dis. 2011;43:380–385. doi: 10.1016/j.dld.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Veress B, Löfberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995;36:880–886. doi: 10.1136/gut.36.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read NW, Krejs GJ, Read MG, Santa Ana CA, Morawski SG, Fordtran JS. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264–71. [PubMed] [Google Scholar]
- 10.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–1165. doi: 10.1053/j.gastro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Pardi DS, Smyrk TC, Tremaine WJ, Sandborn WJ. Microscopic colitis: a review. Am J Gastroenterol. 2002;97:794–802. doi: 10.1111/j.1572-0241.2002.05595.x. [DOI] [PubMed] [Google Scholar]
- 12.Pardi DS, Loftus EV, Jr, Smyrk TC, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504–508. doi: 10.1136/gut.2006.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443. doi: 10.1053/gast.2002.34784. [DOI] [PubMed] [Google Scholar]
- 14.Protic M, Jojic N, Bojic D, et al. Mechanism of diarrhea in microscopic colitis. World J Gastroenterol. 2005;11:5535–5539. doi: 10.3748/wjg.v11.i35.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colin-Jones DG. Safety of lansoprazole. Aliment Pharmacol Ther. 1993;7(Suppl 1):56–60. doi: 10.1111/j.1365-2036.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 16.Freston JW. Long-term acid control and proton pump inhibitors: interactions and safety issues in perspective. Am J Gastroenterol. 1997;92:51S–55S. [PubMed] [Google Scholar]
- 17.Kakar S, Pardi DS, Burgart LJ. Colonic ulcers accompanying collagenous colitis: implication of nonsteroidal anti-inflammatory drugs. Am J Gastroenterol. 2003;98:1834–1837. doi: 10.1111/j.1572-0241.2003.07579.x. [DOI] [PubMed] [Google Scholar]