Abstract
Background
The benefit of routinely measuring autoimmune biomarkers to evaluate patients with interstitial lung disease (ILD) remains debated outside specific contexts such as connective tissue disease (CTD). This study aimed at evaluating the influence of biomarkers on outcome on patients with ILD in a case-control study at a tertiary referral center. We hypothesized that patients with positive autoimmune biomarkers have increased odds of developing ILD even in the absence of CTD.
Methods
We reviewed the medical records of 3573 patients seen at the ILD clinic in Mayo Clinic Rochester between September 2001 and September 2006. We assessed their clinical course through June 25, 2013. We included patients with patterns of ILD most often associated with CTD (n=1256) while excluding patients with other known causes of ILD. Controls (n=2317) included cases seen at the ILD clinic without evidence of ILD.
Results
We identified 930 (26%) cases of ILD alone, 124 (3%) CTD alone, 326 (9%) ILD combined with CTD, and 2193 (61%) with no ILD or CTD. Positive antinuclear antibodies (ANA), rheumatoid factor and aldolase were associated with ILD. After adjustment for age, gender, race, smoking history and CTD, ANA remained an independent risk factor for ILD (OR 1.70, 95% CI 1.33–2.17). Among patients with ILD, the presence of CTD but not biomarker alone was associated with a better survival.
Conclusion
In this study, the presence of positive biomarkers was associated with increased odds of ILD, even in the absence of overt CTD, but was not associated with a better outcome.
Introduction
Interstitial lung disease (ILD) can be defined by the presence of diffuse parenchymal opacities on chest imaging and restrictive physiology not attributable to cardiac disease, infection, exposures or other identifiable cause.1 ILD is frequently associated with connective tissue disease (CTD), its frequency varying with the specific type of CTD, and the diagnostic criteria used.2 For example, ILD was present in 58% of 36 rheumatoid arthritis (RA) patients with early joint disease but clinically significant in only 14% of patients.3 ILD was present in 65% of cases of newly diagnosed dermatomyositis and polymyositis,4 and had a major impact on morbidity and mortality.5 ILD may affect up to 70% of patient with systemic sclerosis (SSc) where it represents the most common cause of death.6 It may be less frequent in systemic lupus erythematosus (SLE) but is associated with increase in mortality.7 Interestingly, ILD may be the first manifestation of CTD in 15% of the cases and may precede the diagnosis of CTD by up to two years.8 Therefore, screening for CTD is not only recommended whenever systemic complaints suggestive of CTD are present, but it may also be considered with each new diagnosis of ILD. This is particularly true in case of rapidly progressive or chronic ILD, where polymyositis (PM), dermatomyositis DM), SLE, and RA are among the possible differential diagnoses.9
Since diagnostic manifestations of CTD are not always present at the time of diagnosis of ILD,10 the clinician may wonder whether the ILD is the first11 or isolated manifestation of autoimmune-featured ILD12 or lung-dominant form of CTD.13 Laboratory data may include antinuclear antibody (ANA), autoantibodies to extractable nuclear antigens (ENA), rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (CCP), anti-Jo-1 antibody, creatinine kinase, aldolase, erythrocyte sedimentation rate and C-reactive protein.10 However, the benefit of routinely measuring autoimmune biomarkers to evaluate ILD remains controversial.14 Although it is recommended to assess for biochemical tests and/or serology markers in order not to overlook an underlying CTD,15 there are limited data about the influence of such findings on the occurrence, severity and prognosis of ILD16, 17. Therefore, the goal of this study was to determine if positive autoimmune biomarkers were associated with ILD and how their presence would influence outcome, using a case-control study design in a large tertiary referral center. Our hypothesis was that patients with positive autoimmune biomarkers had increased odds of developing ILD, even in the absence of overt CTD. Identification of autoimmune biomarkers strongly associated with ILD could identify a subgroup of patients that deserve specific focus of research on diagnosis, management and outcome.
Methods
a. Study design
This was a case-control study, using the database of the first five years of the ILD clinic Mayo Clinic Rochester registry. All adult patients, including women and racial/ethnic minorities, were included, some with a diagnosis of ILD, some to rule out ILD and some with nonspecific pulmonary issues, between September 26, 2001 and September 26, 2006. We only included patients who had given permission for their medical records to be used for research.
b. Definition of ILD
ILD was diagnosed by a pulmonologist based on a combination of clinical history, physical examination, chest imaging, pulmonary function testing, and pathology findings during the initial and/or subsequent visits. ILD was defined, as previously described,18 by the presence of diffuse parenchymal opacities on chest imaging (chest radiographs or computerized tomography) and restrictive physiology, not attributable to congestive heart failure, infection (pneumonia), cancer or other non-ILD causes. We included patients with patterns of ILD associated with CTD:19, 20, 21 usual interstitial pneumonia (UIP), suspected (computerized tomography findings only) or confirmed (biopsy), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), lymphoid interstitial pneumonia (LIP) and pulmonary fibrosis not-otherwise specified or unclassifiable (from isolated strand of fibrosis to undetermined fibrosis after extensive evaluation sometimes including lung biopsy). Cases of diffuse alveolar damage were excluded. After a careful history and physical examination, focusing on comorbidities, medication use, environmental exposures, and family history, and using a standardized approach, we excluded patients with ILDs of other known causes or other distinct types of ILD: hypersensitivity pneumonitis, drug toxicity, smoking-related ILD, radiation pneumonitis, sarcoidosis, eosinophilic pneumonias, vasculitis, and rare forms of ILD (e.g., lymphangioleiomyomatosis, pulmonary alveolar proteinosis). All individual medical records were then reviewed through June 25, 2013.
c. Definition of biomarkers
Predictor variables sought included ANA, ENA (including anti-SSA/Ro, anti-SSB/La, anti-RNP, anti-Sm, anti-Jo1, and anti-Scl70), RF, CCP and aldolase measured at any time. Quantitative determination of ANA (negative <1 U) and ENA (negative <25 U) were measured by enzyme immunoassay, RF (negative <15 U) by particle-enhanced immunoturbidimetric assay, CCP (negative <20 U) by binding to the wells of a commercial microtiter plate coated with synthetic CCP (Quanta Lite CCP3 IgG ELISA, INOVA Diagnostics) and aldolase from the rate at which NADH is oxidized to NAD measured photometrically by a decrease in absorbance (negative <7.4 U).
Controls were defined as patients who were seen in the ILD clinic, but did not have ILD.
d. Definition of CTD
CTD, a strong potential confounding variable, was diagnosed by a rheumatologist, according to established criteria,10 based on the combination of clinical history, physical examination, and laboratory and radiologic findings during the initial and/or subsequent visits in the rheumatology clinic of the same institution, at any time. For ILD, the date of first symptoms and/or abnormal chest imaging were collected based on clinician evaluation as reported in the chart (i.e. persistent dyspnea, or cough or evidence or ‘rales’ or ‘crackles’) or first abnormal chest radiographic finding, whichever came first. For CTD, the date of first symptoms and/or abnormal laboratory finding was collected based on clinician evaluation as reported in the chart. CTD included rheumatoid arthritis, polymyositis/dermatomyositis including anti-synthetase syndrome, systemic sclerosis, systemic lupus erythematosus, mixed connective tissue disease, Sjögren syndrome, and undifferentiated connective tissue diseases. Undifferentiated CTD was defined as a condition characterized by the presence of signs and symptoms suggestive of a systemic autoimmune disease that did not satisfy the classic criteria for defined CTD.22 Patients seen in the ILD clinic during the study period but with no diagnosis of ILD or CTD, served as controls (non-cases from the same source). Potential effect modifiers included age, gender, race, and smoking status. Date of death and last follow up was ascertained until June 25, 2013 when it was censored.
e. Sample size
Sample size for this unmatched case-control study was estimated at 516 in each group, with a probability of type I error of 0.05, a power of 0.8, expected proportion in control cases of 0.05 and a relative risk of 2 (2 sided test).23 For a sample size of 774 the power was estimated at 0.93 with the same parameters, otherwise.
f. Bias
In order to limit sampling biases, all patients seen at the same clinic, during the study period, were considered for the study and only patients who had declined to have their charts reviewed for research purpose were excluded (n=316). To limit measurement biases, variables were collected through electronic records available since 1996. Study data were collected and managed using JMP® Pro (version 9.0.1, SAS Institute Inc., Cary, NC).
g. Statistical methods
Demographics are expressed as median with interquartile range (IQR) for continuous variables and as number with percentage for categorical variables. Comparisons between groups used Wilcoxon’s test and odd ratio with confidence interval and Chi-square test as appropriate. The association of demographics and autoimmune biomarkers with ILD was assessed using logistic regression. Given the known association of CTD with ILD, these analyses were performed separately for patients with and without CTD. Among patients with ILD, survival following onset of ILD symptoms was assessed using left truncated Kaplan–Meier and Cox proportional-hazards regression analyses. Since, for some patients, the diagnosis of CTD occurred after the onset of ILD symptoms, this variable was included in the Cox model using a binary time dependent covariate and supplemental analyses were performed which excluded patients whose CTD diagnosis occurred following their initial ILD clinic visit. Analyses were performed using SAS software (SAS version 9, SAS Institute Inc, Cary, NC) (ACH and DRS). Sensitivity analyses evaluated separately patients coming from the USA only and those from Minnesota only. A subgroup of ILD without clear evidence of CTD was independently reviewed by a pulmonologist (SK) and a rheumatologist (TGO).
This study was conducted in accordance with the amended Declaration of Helsinki and approved by the Mayo Clinic (13-001307) institutional review board.
Results
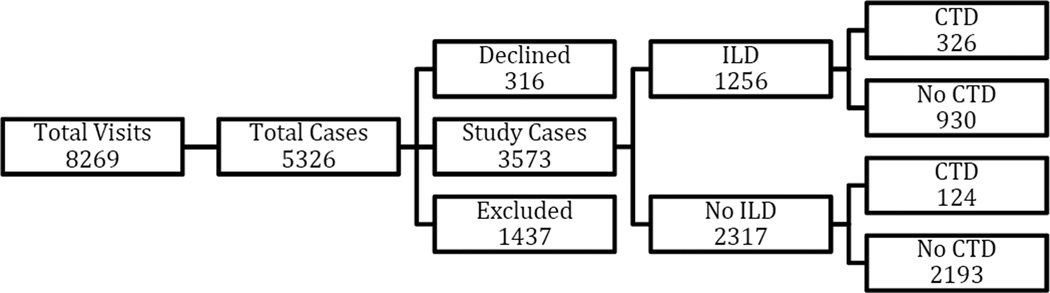
During the 5-year recruitment period, a total of 5326 patients were available for review. After excluding 316 patients who declined to have their medical records reviewed for research, and 1437 ILD patients with other known causes or distinct types of ILDs, 3573 cases were included for further study (Figure 1). There were 1256 (13%) cases with ILD alone, and 2317 (65%) without ILD. We further identified 930 (26%) cases with ILD alone, 326 (9%) cases with combined ILD and CTD (CTD-ILD), 124 (3%) cases with CTD alone, and 2193 (61%) cases with no ILD or CTD (Figure 1). Rheumatology consultation occurred in 121 (34%) cases with ILD alone, 302 (93%) cases with combined ILD and CTD, 1 (100%) case with CTD alone (diagnosis established by Rheumatology elsewhere prior to the visit to the ILD clinic) and 10 (16%) cases with no ILD or CTD. Among ILD cases, 326 (26%) had CTD and among non-ILD cases, 124 (5%) had CTD (OR 6.20, 95% CI 4.97–7.73) (Tables 1 and 2). Most cases of CTD were associated with ILD (72%). Overall, patients with ILD (69 years, 60–76) were older than patients without ILD (65 years, 54–74). Patients with CTD-ILD (59 years, 50–68) were younger than those without CTD (72 years, 65–77). Although the percentage of current smokers was lower in patients with ILD vs. no ILD (2.3% vs. 9.2%), the percentage of previous smokers was higher (54.6% vs. 48.6%). Male gender, race, older age, smoking status and presence of CTD were all independent predictors of ILD.
Figure 1.
Flow diagram: number of cases per category. The group ‘Declined’ corresponds to those who did not consent. ‘Excluded’ represents other forms of ILD (ILD, interstitial lung disease; CTD, connective tissue disease)
Table 1.
Types and distribution of interstitial lung disease and connective tissue disease†
| Characteristics | ILD (n=1256) |
No ILD (n=2317) |
||||
|---|---|---|---|---|---|---|
| UIP 551 (44%) |
NSIP 74 (6%) |
OP 70 (6%) |
LIP 4 (<1%) |
PF-NOS 557 (44%) |
||
| CTD (n=450) | 29 (9%) | 35 (11%) | 19 (6%) | 2 (<1%) | 241 (74%) | 124 (5%) |
| RA | 8 (28%) | 6 (17%) | 3 (16%) | 0 (0%) | 64 (27%) | 62 (50%) |
| PM/DM | 4 (14%) | 7 (20%) | 5 (26%) | 0 (0%) | 27 (11%) | 6 (5%) |
| SSc | 3 (10%) | 6 (17%) | 2 (11%) | 0 (0%) | 59 (24%) | 13 (10%) |
| SLE | 0 (0%) | 2 (6%) | 0 (0%) | 1 (50%) | 13 (5%) | 6 (5%) |
| MCTD | 7 (24%) | 7 (20%) | 2 (11%) | 1 (50%) | 48 (20%) | 14 (11%) |
| Sjögren | 2 (7%) | 3 (9%) | 1 (5%) | 0 (0%) | 9 (4%) | 11 (9%) |
| UCTD | 5 (17%) | 4 (11%) | 6 (31%) | 0 (0%) | 21 (9%) | 12 (10%) |
| No CTD (n=3123) | 522 (56%) | 39 (4%) | 51 (6%) | 2 (<1%) | 316 (34%) | 2193 (95%) |
ILD, interstitial lung disease; CTD, connective tissue disease; UIP, usual interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; LIP, lymphoid interstitial pneumonia; PF-NOS, pulmonary fibrosis non-otherwise specified or unclassifiable; RA, rheumatoid arthritis; PM/DM, poly-/dermatomyositis; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; MCTD, mixed connective tissue disease; UCTD, undifferentiated connective tissue disease
Table 2.
characteristics of predictors of ILD†
| Characteristics | ILD | No ILD | OR (95% CI)* | P value** |
|---|---|---|---|---|
| CTD | ||||
| Overall | n=326 | n=124 | ||
| Age (year)§ | 59 (50–68) | 63 (53–70) | 0.94 (0.87–1.02) | ns |
| Female | 223 (68) | 93 (75) | 0.72 (0.45–1.15) | ns |
| White | 262 (80) | 111 (90) | 0.48 (0.25–0.91) | 0.024 |
| Smoking | 0.002 | |||
| Never | 176 (54) | 55 (44) | Ref. | |
| Previous | 141 (43) | 55 (44) | 0.80 (0.52–1.24) | |
| Current | 9 (3) | 14 (11) | 0.20 (0.08–0.49) | |
| No CTD | ||||
| Overall | n=930 | n=2193 | ||
| Age (year)§ | 72 (65–77) | 66 (54–74) | 1.24 (1.20–1.28) | <0.001 |
| Female | 368 (40) | 1077 (49) | 0.68 (0.58–0.79) | <0.001 |
| White | 763 (82) | 1857 (85) | 0.83 (0.67–1.01) | 0.067 |
| Smoking | <0.001 | |||
| Never | 365 (39) | 921 (42) | Ref. | |
| Previous | 545 (59) | 1072 (49) | 1.28 (1.09–1.50) | |
| Current | 20 (2) | 200 (9) | 0.25 (0.16–0.41) |
ILD, interstitial lung disease; CTD, connective tissue disease; OR, odds ratio; CI, confidence interval;
Values are median (IQR) or number (percentage);
odds ratios represents 5 year increase in age;
P-values are from logistic regression; ns, not significant
The most frequent tests ordered ANA (47% of all cases), RF (34%), and ENA (30%). ILD was more frequently observed in 326 (72%) cases with CTD and in 261 (56%) cases with positive test but no evidence of CTD, than in 669 (25%) cases with no CTD (p<0.0001). Overall, positive testing for ANA, ENA, RF and aldolase were independent variables associated with ILD (Table 3a and 3b).
Table 3.
| a: Values of biomarkers† | |||||
|---|---|---|---|---|---|
| ILD | No ILD | ||||
| Characteristics | N* | Value, median (IQR) | N* | Value, median (IQR) | P value** |
| CTD | |||||
| ANA (<1U) | 258 | 3.05 (1.075–8.325) | 89 | 1.4 (0.5–5.2) | 0.0011 |
| ENA (<25 U) | 267 | 10.3 (3.4–148.5) | 66 | 6.1 (2.5–130.6) | ns |
| RF (<15 U) | 233 | 0 (0–125) | 91 | 28 (0–190) | 0.0375 |
| CCP (<20 U) | 100 | 0 (0–72) | 44 | 2.5 (0–100) | ns |
| Aldolase (<7.4 U) | 120 | 7.8 (5.5–16.05) | 23 | 5.7 (4.2–8.1) | 0.0098 |
| No CTD | |||||
| ANA (<1U) | 602 | 0.6 (0.3–1.1) | 656 | 0.5 (0.3–0.8) | <0.0001 |
| ENA (<25 U) | 416 | 3.7 (2.2–6.675) | 320 | 3.4 (2.025–5.9) | ns |
| RF (<15 U) | 466 | 0 (0–16) | 417 | 0 (0–0) | <0.0001 |
| CCP (<20 U) | 171 | 0 (0–0) | 97 | 0 (0–0) | ns |
| Aldolase (<7.4 U) | 49 | 5.1 (4.35–7.05) | 111 | 5.3 (4.4–6.9) | ns |
| b: Positive biomarkers as predictors of ILD† | ||||||
|---|---|---|---|---|---|---|
| ILD | No ILD | |||||
| Characteristics | N* | n (%) positive | N* | n (%) positive | OR (95% CI) | P value** |
| CTD | ||||||
| ANA | 285 | 227 (80) | 99 | 62 (63) | 2.34 (1.42–3.85) | 0.0007 |
| ENA | 270 | 116 (43) | 66 | 25 (38) | 1.24 (0.71–2.15) | ns |
| RF | 235 | 111 (47) | 91 | 58 (64) | 0.51 (0.31–0.84) | 0.0075 |
| CCP | 100 | 37 (37) | 44 | 21 (48) | 0.64 (0.31–1.32) | ns |
| Aldolase | 120 | 66 (55) | 23 | 7 (30) | 2.79 (1.07–7.28) | 0.0309 |
| No CTD | ||||||
| ANA | 611 | 169 (28) | 681 | 137 (20) | 1.52 (1.17–1.96) | 0.002 |
| ENA | 421 | 23 (6) | 321 | 14 (4) | 1.26 (0.64–2.50) | ns |
| RF | 467 | 120 (26) | 418 | 60 (14) | 2.06 (1.46–2.91) | <0.0001 |
| CCP | 171 | 4 (2) | 97 | 1 (1) | 2.30 (0.25–20.87) | ns |
| Aldolase | 49 | 12 (24) | 111 | 26 (23) | 1.06 (0.48–2.33) | ns |
ILD, interstitial lung disease, CTD, connective tissue disease; ANA, antinuclear antibodies; ENA: extractable nuclear antigens; RF, rheumatoid factor; CCP; anti-cyclic citrullinated peptide antibodies; U, units representing cutoff values; IQR, interquartile range;
Number of patients with biomarker value available;
P-value from Wilcoxon Test; ns, not significant
(*when available; IQR, interquartile range)
OR, odds ratio; CI, confidence interval;
Number of patients with the given biomarker assessed;
P-value from logistic regression; ns, not significant
Among patients with ILD but no overt CTD, positive serologies were seen in different subtypes of ILD, mainly with UIP and unclassifiable pulmonary fibrosis and less often with organizing pneumonia or NSIP (Table 4). A restrictive pattern appeared more pronounced in case of UIP and NSIP than with OP or unclassifiable pulmonary fibrosis. On high resolution computed tomography, honeycombing and traction bronchiectasis were more frequent with a UIP pattern than with unclassifiable pulmonary fibrosis.
Table 4.
Subtypes of ILD with positive serologies in the absence of overt CTD†
| Characteristics | ILD (n=261) |
P value** | |||||
|---|---|---|---|---|---|---|---|
| N* | UIP 135 (52) |
NSIP 14 (5) |
OP 15 (6) |
LIP 1 (<1) |
PF-NOS 96 (37) |
||
| Demographics | |||||||
| Age (year)§ | 261 | 71 (65–77) | 68 (55–74) | 68 (64–78) | 27 (27–27) | 73 (66–78) | |
| Female | 121 | 57 (42) | 9 (64) | 7 (47) | 1 (100) | 47 (49) | ns |
| White | 213 | 107 (79) | 10 (71) | 12 (80) | 0 (0) | 84 (88) | ns |
| Biomarkers, n (%) | |||||||
| ANA | 253 | 81 (63) | 6 (43) | 12 (80) | 1 (100) | 69 (73) | ns |
| ENA | 190 | 13 (13) | 1 (8) | 2 (22) | 0 (0) | 7 (10) | ns |
| RF | 219 | 63 (54) | 10 (91) | 6 (60) | 1 (100) | 40 (50) | ns |
| CCP | 65 | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 2 (91) | ns |
| Aldolase | 29 | 6 (43) | 3 (60) | 0 (0) | 0 (0) | 3 (30) | ns |
| PFT§ | |||||||
| TLC (%) | 229 | 67 (59–75) | 61 (49–66) | 73 (59–92) | - | 72 (62–80) | 0.002 |
| FVC (%) | 254 | 65 (53–76) | 52 (42–60) | 74 (56–95) | 97 | 69 (51–79) | 0.0231 |
| FEV1 (%) | 254 | 71 (60–80) | 54 (44–67) | 75 (58–80) | 85 | 71 (59–82) | 0.0446 |
| FEV1/FVC | 254 | 84 (81–89) | 82 (76–95) | 80 (73–82) | 76 | 82 (76–88) | 0.0021 |
| DLCO (%) | 238 | 47 (34–57) | 37 (27–52) | 47 (22–68) | 94 | 50 (39–59) | ns |
| DLCO adj (%) | 174 | 47 (35–55) | 36 (29–58) | 49 (33–60) | 92 | 49 (39–56) | ns |
| HRCT | |||||||
| Honeycombing | 196 | 102 (89) | 2 (18) | 3 (43) | 0 (0) | 39 (62) | <0.0001 |
| Bronchiectasis | 195 | 101 (93) | 9 (82) | 5 (63) | 0 (0) | 55 (82) | 0.0277 |
| Reticularity | 240 | 127 (100) | 12 (92) | 11 (85) | 0 (0) | 85 (98) | 0.0011 |
| GGO | 115 | 31 (70) | 9 (82) | 7 (88) | 1 (100) | 46 (90) | ns |
ILD, interstitial lung disease, CTD, connective tissue disease; ANA, antinuclear antibodies; ENA: extractable nuclear antigens; RF, rheumatoid factor; CCP; anti-cyclic citrullinated peptide antibodies;
Values are median (IQR);
Number and percentage of patients with the given characteristic assessed;
p value from Chi-square or Wilcoxon as appropriate; ns, not significant, PFT, pulmonary function test; TLC, total lung capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity for carbon monoxide; DLCO adj, diffusing capacity for carbone monoxide adjusted for hemoglobin; HRCT, high resolution computed tomography; GGO, ground glass opacities
In the presence of CTD, a positive ANA alone (OR 2.34, 95% CI 1.42–3.85) or aldolase alone (OR 2.79, 95% CI 1.07–7.28), were each predictors of ILD. In the absence of CTD, both positive ANA (OR 1.52, 95% CI 1.17–1.96) and RF (OR 2.06, 95% CI 1.46–2.91) were associated with an increased likelihood of ILD. These findings were not affected by restricting the study to the US population or to the residents of Minnesota alone. After adjustment for age, gender, race, smoking history and connective tissue disease, ANA remained an independent risk factor for ILD (OR 1.70, 95% CI 1.33–2.17).
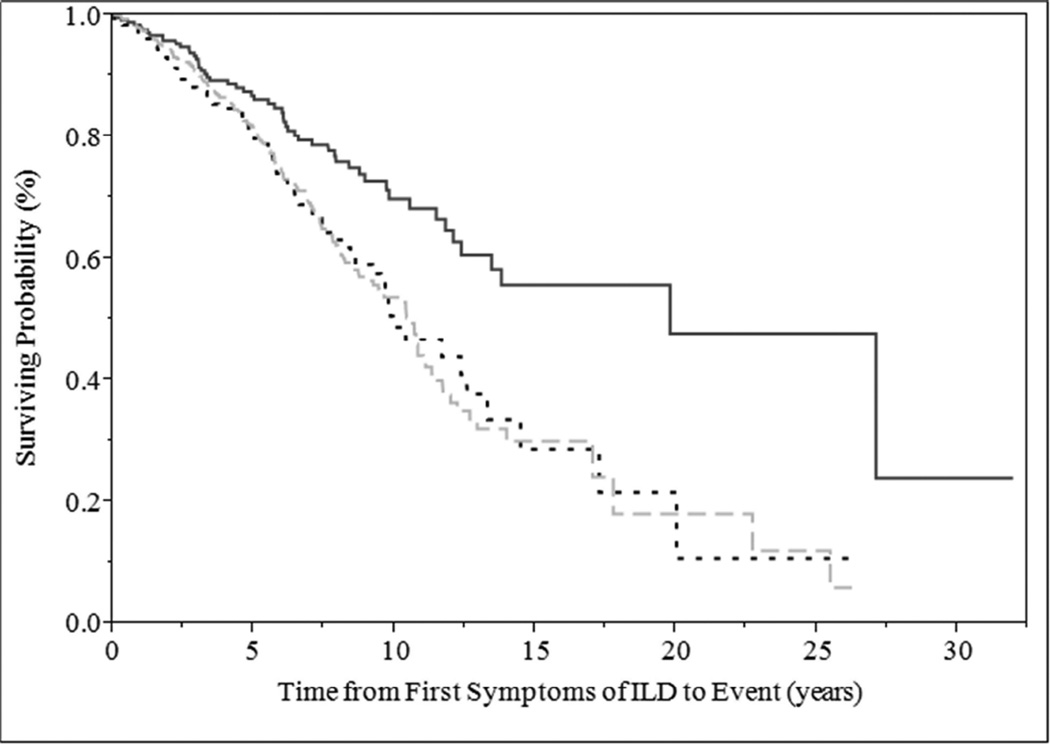
Patients with CTD-ILD had better survival than patients with ILD alone and patients with both ILD and biomarkers (p=0.001) (Figure 2). Median survival was 20 years, 10 years and 10.5 years for those with CTD-ILD, ILD with positive biomarkers only and ILD alone, respectively.
Figure 2.
Survival plot of interstitial lung disease cases from the time of first symptoms according to presence or absence of CTD at the time of initial ILD clinic visit, and positive or negative biomarkers; Log-Rank test, p=0.0001 (ILD, interstitial lung disease; CTD, connective tissue disease; solid line, ILD with CTD; dotted line, ILD with positive biomarkers only; half-dashed line, ILD with negative biomarker)
Discussion
In this case-control study of patients seen in a tertiary referral ILD center, we found significant associations between the presence of positive biomarkers, including ANA, RF and aldolase, and ILD. We also found that patients with ILD alone (with or without the presence of positive biomarkers) had worse survival than those patients with CTD-ILD. The higher percentage of UIP in those with ILD without CTD (56%), including ILD with positive biomarkers only (52%), and ILD alone (58%), compared to CTD-ILD (9%) could explain this difference in survival since a UIP pattern is associated with worse survival compared to other types24. The greater frequency of pulmonary fibrosis not otherwise specified in CTD-ILD (74%) compared with ILD alone (34%) may reflect clinical practice of not pursuing pathological diagnosis in presence of CTD, since the distinction between a fibrotic NSIP and a UIP pattern in the context of CTD is contended to not influence outcome, with the possible exception of rheumatoid arthritis where a UIP pattern may be associated with a worse outcome than fibrotic NSIP25. We found that some cases of ILD were associated with positive biomarkers but without overt CTD and postulate that they may represent an occult form of CTD.26 Isolated NSIP, which has been associated with undifferentiated CTD27, 28, was rarely present, that is 6 (1%) out of 169 cases of ILD with positive ANA but no overt CTD or 14 (5%) of 261 cases of ILD with at least one positive autoimmune biomarker. This may reflect the definition used by the clinicians to define undifferentiated CTD-associated ILD as any ILD with at least one auto-antibody and at least one extra-thoracic feature,2, 22 thus leaving those ILD with a positive serology but no apparent extra-thoracic feature of auto-immune disease in a separate category. Nevertheless, and although there is a known correlation with older age and positive ANA or RF,29 as well as an increased incidence of ILD with age,30 especially idiopathic pulmonary fibrosis,31 the presence of both positive biomarkers and ILD could, in the absence of overt CTD, suggest the possibility of isolated pulmonary form of CTD.
In presence of ILD, the clinical relevance of a positive biomarker alone, without evidence of CTD, is unknown. In a subgroup analysis, we carefully reviewed 261 cases of ILD with no evidence of CTD but with at least one positive biomarker. The proportion of observed agreement by an independent pulmonologist was 98% for the presence of ILD and, by a reviewing rheumatologist not directly involved in their care, 92% for the absence of CTD, while suggesting 20 cases of possible undiagnosed CTD. Thus, a positive biomarker alone could indicate an increased risk for ILD either associated with subclinical CTD or reflecting an immunologic process associated with idiopathic interstitial pneumonia32. However, the lack of survival benefit in this subgroup of patients when compared to isolated forms of ILD seems to indicate that, contrary to overt CTD, the natural course or response to immunosuppressive therapy may be similar to idiopathic interstitial pneumonias. Whether or not this specific group of patients requires specific treatment usually reserved for ILD associated CTD, or showed response to such treatment if given, is unknown and was not directly addressed in this study.
The field of ILD studied was restricted to UIP, NSIP, OP, LIP and other unclassifiable pulmonary fibrosis, that have all been associated with CTD.8 Other types of idiopathic interstitial pneumonias such as desquamative interstitial pneumonia and respiratory bronchiolitis interstitial lung disease, which are strongly associated with smoking33, were excluded. Acute interstitial pneumonia34 occurs in a very unique setting and was also excluded. We believe these would encompass the type of ILD one would expect even with occult CTD, without sacrificing too much sensitivity or specificity. To limit selection biases, the presence of ILD and CTD were assessed separately and based on the opinion of the clinician who had evaluated the patient. However, cases of CTD may have had ILD but not been referred to the ILD clinic and cases of ILD may have developed CTD later without follow up or recognition by us.
The first limitation is the retrospective design of this study, as attested by an overall relatively low proportion of patients tested for ANA (47%) and even less for other biomarkers (i.e. aldolase), which may represent a sampling bias. Overall, ANA testing was performed in 71% of all ILD, which is consistent with 76% of at least one antibody assayed in ILD patients by others35. Testing was most frequently performed in presence of CTD, with or without ILD (87 versus 80%). Testing was also performed in the absence of CTD, and more often in patients with than without ILD (66 versus 31%).
The second limitation is the choice of the index date as the appointment date in the ILD clinic of a tertiary referral center, and therefore the uncertainty as to the exact inception date of ILD, as some cases were simply referred for a second opinion regarding diagnosis, etiology and prognosis of ILD previously diagnosed and evaluated elsewhere. The same holds true for the diagnosis of CTD. To address these two concerns, the outcome was evaluated from the first symptom of ILD reported by the clinician in the initial evaluation and not from the date of appointment in the ILD clinic. Although subject to limitation (recollection bias), this is probably less subjective than using the date of visit to the clinic36, 37.
As a third limitation, the selection of the population from a tertiary referral center and the predominantly white race may limit the generalizability of this study to other institutions, populations and other ethnic groups.
In summary
This study shows that the presence of positive autoimmune biomarkers, such as ANA, RF, and aldolase was associated with increased odds of ILD, even in the absence of overt CTD. After adjustment for age, gender, race, smoking history and CTD, ANA remained an independent risk factor for ILD. In case of ILD most commonly encountered with CTD, the presence of CTD was associated with a better outcome than either ILD with positive antibodies or ILD alone. Whether or not the group of ILD with positive autoimmune biomarker but not extra-thoracic manifestation of CTD represents a different category of patients warrants further study.
Acknowledgments
Funding covered the cost for statistical analyses.
Philippe R. Bauer was the recipient of the 2013 Department of Medicine Career Development Enhancement Award, and the 2013 Small Grant award from the Division of Pulmonary Medicine, Mayo Clinic, Rochester.
Funding:
This publication was made possible by the Center for Clinical and Translational Science (CCaTS) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was funded by the 2013 Small Grant award from the Division of Pulmonary Medicine, Mayo Clinic, Rochester.
Abbreviation List
- ANA
antinuclear antibodies
- CCP
anti-cyclic citrullinated peptide antibodies
- CI
confidence interval
- CTD
connective tissue disease
- CTD-ILD
interstitial lung disease associated with connective tissue disease
- ENA
autoantibodies to extractable nuclear antigens
- ILD
interstitial lung disease
- LIP
lymphoid interstitial pneumonia
- MCTD
mixed connective tissue disease
- NSIP
nonspecific interstitial pneumonia
- OP
organizing pneumonia
- OR
odds ratio
- PF-NOS
pulmonary fibrosis non-otherwise specified or unclassifiable
- PM/DM
polymyositis/dermatomyositis including anti-Jo1 antibody disease
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SLE
systemic lupus erythematosus
- SSc
systemic sclerosis
- UCTD
undifferentiated connective tissue disease
- UIP
usual interstitial pneumonia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution at which the work was performed: Mayo Clinic, Rochester, Minnesota, USA
The content of this work was presented at the 2014 ATS International Conference in San Diego, California on May 18, 2014.
Conflict of interest disclosure:
The remaining authors have declared no conflict of interest to disclose.
Philippe R. Bauer contributed to conception, design, collection, analysis, and interpretation of data, review, and final approval of the manuscript.
Sanjay Kalra contributed to interpretation of data, and drafting, review, and final approval of the manuscript.
Thomas G. Osborn contributed to interpretation of data, and drafting, review, and final approval of the manuscript.
Jennifer St. Sauver contributed to conception, design, analysis, and interpretation of data, review, and final approval of the manuscript.
Andrew C. Hanson and Darrell R. Schroeder contributed to statistical analysis, and interpretation of data, review, and final approval of the manuscript.
Jay H. Ryu contributed to interpretation of the data, review and final approval of the manuscript.
Contributor Information
Philippe R. Bauer, Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, bauer.philippe@mayo.edu
Sanjay Kalra, Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, kalra.sanjay@mayo.edu
Thomas G. Osborn, Rheumatology, Mayo Clinic, Rochester, MN, osborn.thomas@mayo.edu
Jennifer St. Sauver, Health Sciences Research – Epidemiology, Mayo Clinic, Rochester, MN, stsauver.jennifer@mayo.edu
Andrew C. Hanson, Health Sciences Research - Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN, hanson.andrew@mayo.edu
Darrell R. Schroeder, Health Sciences Research - Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN, schroedd@mayo.edu
Jay H. Ryu, Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, ryu.jay@mayo.edu
References
- 1.Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82(8):976–986. doi: 10.4065/82.8.976. [DOI] [PubMed] [Google Scholar]
- 2.Cottin V. Significance of connective tissue diseases features in pulmonary fibrosis. Eur Respir Rev. 2013;22(129):273–280. doi: 10.1183/09059180.00003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156:528–535. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 4.Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathi M, Lundberg IE. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2005;17:701–706. doi: 10.1097/01.bor.0000179949.65895.53. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Valentini G, Cozzi F, et al. Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc) Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Murin S, Wiedemann HP, Matthay RA. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med. 1998;19:641–665. doi: 10.1016/s0272-5231(05)70108-8. [DOI] [PubMed] [Google Scholar]
- 8.Strange C, Highland KB. Interstitial lung disease in the patient who has connective tissue disease. Clin Chest Med. 2004;25(3):549–559. doi: 10.1016/j.ccm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Ryu JH, Olson EJ, Midthun DE, Swensen SJ. Diagnostic approach to the patient with diffuse lung disease. Mayo Clin Proc. 2002;77:1221–1227. doi: 10.4065/77.11.1221. [DOI] [PubMed] [Google Scholar]
- 10.Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143(3):814–824. doi: 10.1378/chest.12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzelepis GE, Toya SP, Moutsopoulos HM. Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur Respir J. 2008;31(1):11–20. doi: 10.1183/09031936.00060107. [DOI] [PubMed] [Google Scholar]
- 12.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest. 2011;140(5):1292–1299. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010;138(2):251–256. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007;176(7):691–697. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 16.Papiris SA, Kagouridis K, Bouros D. Serologic evaluation in idiopathic interstitial pneumonias. Curr Opin Pulm Med. 2012;18(5):433–440. doi: 10.1097/MCP.0b013e3283560840. [DOI] [PubMed] [Google Scholar]
- 17.Alhamad EH, Al-Kassimi FA, Alboukai AA, et al. Comparison of three groups of patients with usual interstitial pneumonia. Respir Med. 2012;106(11):1575–1585. doi: 10.1016/j.rmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Bauer PR, Schiavo DN, Osborn TG, et al. Influence of interstitial lung disease on outcome in systemic sclerosis: a population-based historical cohort study. Chest. 2013;144(2):571–577. doi: 10.1378/chest.12-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Costabel U, Hansell DM, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutsche M, Rosen GD, Swigris JJ. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr Respir Care Rep. 2012;1:224–232. doi: 10.1007/s13665-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosca M, Tani C, Neri C, Baldini C, Bombardieri S. Undifferentiated connective tissue diseases (UCTD) Autoimmun Rev. 2006;6:1–4. doi: 10.1016/j.autrev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Rollin Brant. Unmatched Case/Control Studies. [Accessed online October 28, 2013]; http://www.stat.ubc.ca/~rollin/stats/ssize/caco.html. [Google Scholar]
- 24.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58(2):143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lauretis A, Veeraraghavan S, Renzoni E. Review series: Aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011;8(1):53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 26.Kinder BW, Shariat C, Collard HR, et al. Undifferentiated connective tissue disease-associated interstitial lung disease: changes in lung function. Lung. 2010;188(2):143–149. doi: 10.1007/s00408-009-9226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suda T, Kono M, Nakamura Y, et al. Distinct prognosis of idiopathic nonspecific interstitial pneumonia (NSIP) fulfilling criteria for undifferentiated connective tissue disease (UCTD) Respir Med. 2010;104(10):1527–1534. doi: 10.1016/j.rmed.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Corte TJ, Copley SJ, Desai SR, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J. 2012;39(3):661–668. doi: 10.1183/09031936.00174910. [DOI] [PubMed] [Google Scholar]
- 29.Schuller E, Allinquant B, Reboul J, Fournier C, Dardenne M, Bach JF. Immunological studies in human ageing. II. Associated increase in anti-RNA and anti-DNA antibodies. J. Clin Lab Immunol. 1981;6(2):107–110. [PubMed] [Google Scholar]
- 30.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 31.Moua T, Maldonado F, Decker PA, Daniels CE, Ryu JH. Frequency and implication of autoimmune serologies in idiopathic pulmonary fibrosis. Mayo Clin Proc. 2014;89(3):319–326. doi: 10.1016/j.mayocp.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Tzouvelekis A, Zacharis G, Oikonomou A, et al. Increased incidence of autoimmune markers in patients with combined pulmonary fibrosis and emphysema. BMC Pulm Med. 2013;13:31. doi: 10.1186/1471-2466-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells AU, Nicholson AG, Hansell DM. Challenges in pulmonary fibrosis. 4: smoking-induced diffuse interstitial lung diseases. Thorax. 2007;62(10):904–910. doi: 10.1136/thx.2004.031021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay S, Parambil JG. Acute interstitial pneumonia (AIP): relationship to Hamman-Rich syndrome, diffuse alveolar damage (DAD), and acute respiratory distress syndrome (ARDS) Semin Respir Crit Care Med. 2012;33(5):476–485. doi: 10.1055/s-0032-1325158. [DOI] [PubMed] [Google Scholar]
- 35.Mittoo S, Gelber AC, Christopher-Stine L, Horton MR, Lechtzin N, Danoff SK. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir Med. 2009;103(8):1152–1158. doi: 10.1016/j.rmed.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Kocheril SV, Appleton BE, Somers EC, et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 2005;53(4):549–557. doi: 10.1002/art.21322. [DOI] [PubMed] [Google Scholar]
- 37.Su R, Bennett M, Jacobs S, et al. An analysis of connective tissue disease-associated interstitial lung disease at a US Tertiary Care Center: better survival in patients with systemic sclerosis. J Rheumatol. 2011;38(4):693–701. doi: 10.3899/jrheum.100675. [DOI] [PubMed] [Google Scholar]