Abstract
Neonatal hypoxic-ischemic (HI) brain injury is a leading cause of mortality and morbidity in infants and children for which there is no promising therapy at present. Previously, we reported induction of neuronal pentraxin 1 (NP1), a novel neuronal protein of the long-pentraxin family, following HI injury in neonatal brain. Here, we report that genetic deletion of NP1 expression prevents HI injury in neonatal brain. Elevated expression of NP1 was observed in neurons, not in astrocytes, of the ipsilateral cortical layers (I–IV) and in the hippocampal CA1 and CA3 areas of WT brains following hypoxia-ischemia; brain areas that developed infarcts (at 24–48 h), showed significantly increased numbers of TUNEL-(+) cells and tissue loss (at 7 d). In contrast, NP1-KO mice showed no evidence of brain infarction and tissue loss after HI. The immunofluorescence staining of brain sections with mitochondrial protein COX IV and subcellular fractionation analysis showed increased accumulation of NP1 in mitochondria, pro-death protein Bax activation and NP1 co-localization with activated caspase-3 in WT, but not in the NP1-KO brains; corroborating NP1 interactions with the mitochondria-derived pro-death pathways. Disruption of NP1 translocation to mitochondria by NP1-siRNA in primary cortical cultures significantly reduced ischemic neuronal death. NP1 was immunoprecipitated with activated Bax[6A7] proteins; HI caused increased interactions of NP1 with Bax, thereby, facilitating Bax translocation to mitochondrial and neuronal death. To further delineate the specificity of NPs, we found that NP1 but not the NP2 induction is specifically involved in brain injury mechanisms and that knockdown of NP1 only results in neuroprotection. Furthermore, live in vivo T2-weighted magnetic resonance imaging (MRI) including fractional anisotropy (FA) mapping showed no sign of delayed brain injury or tissue loss in the NP1-KO mice as compared to the WT at different post-HI periods (4–24 weeks), examined; indicating a long-term neuroprotective efficacy of NP1 gene deletion. Collectively, our results demonstrate a novel mechanism of neuronal death and predict that inhibition of NP1 expression is a promising strategy to prevent hypoxic-ischemic injury in immature brain.
Keywords: Neuronal pentraxin 1, neuronal pentraxin 2, hypoxia-ischemia, neonatal brain injury, oxygen glucose deprivation, mitochondria, Bax, caspase-3, T2-weighted magnetic resonance imaging
Introduction
Perinatal/neonatal hypoxic-ischemic brain injury is the leading cause of long-term disabilities and neurological disorders (Johnston, 1997; Lorenz et al., 1998; Northington et al., 2011). Neonatal brain injury following hypoxia-ischemia (HI) occurs at a rate of about three per thousand live-born infants (Johnston, 2005; Johnston et al., 2011); affecting the life of all ages and population including patient, care-takers and the family. However, current treatment regimens are not optimal. A number of clinical trials have focused on new therapies, however, very few trials have documented limited clinical benefits due to lack of understanding of the discrete cellular and molecular factors and signaling pathways involved in brain injury mechanisms (Blomgren and Hagberg, 2006; Kalenderian et al., 2009; Pezzini and Padovani, 2009).
Discoveries of the role of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) glutamate receptor-mediated excitotoxicity, oxidative stress, and cell-death signaling pathways have provided a platform for understanding neonatal brain injury (Barks and Silverstein, 1992; Choi and Rothman, 1990; Hossain et al., 1998; Portera-Cailliau et al., 1997). Among the candidate target molecules considered to act as early mediators of AMPAR-recruitment and clustering at synaptic sites include members of the neuronal pentraxin family; neuronal pentraxin 1(NP1), neuronal activity-regulated pentraxin (Narp; also called NP2), and neuronal pentraxin receptor (NPR), and NPs are exclusively expressed in neurons (Goodman et al., 1996; Schlimgen et al., 1995; Tsui et al., 1996). O’Brien et al (1998, 1999) have shown that Narp regulates AMPA glutamate receptor clustering at synaptic sites (O’Brien et al., 1998; O’Brien et al., 1999). We found that NP1 co-localizes with GluR1 and enhanced GluR1 membrane insertion at the synaptic site in OGD exposed hippocampal neurons, whereas, knockdown of NP1 resulted in decrease in the surface GluR1 cluster density, synaptic localization, and neuronal death after OGD (Al Rahim and Hossain, 2013). Thus, it is possible that NP1 may disrupt inter-neuronal synaptic activity, and the suppression of which possibly contributes to the recovery function after HI brain injury.
Proposed functions of NPs include modulation of synaptic uptake, synapse formation, and synaptic remodeling (Kirkpatrick et al., 2000; Schlimgen et al., 1995). Previously, we have shown induction of NP1 in neonatal brain injury following HI (Hossain, 2008; Hossain et al., 2004) that is regulated via a pro-death glycogen synthase kinase 3α/β-dependent pathway (Russell et al., 2011); indicating a novel regulatory link between NP1 induction and neonatal death. Neuronal cell death signaling pathways in mitochondria have been demonstrated in the ischemic brain (Chan, 2004; Perez-Pinzon et al., 1999) that are often triggered by the translocation of Bad and Bax, two proapoptotic Bcl-2 family members, to mitochondria (Armstrong and Jones, 2002; Zong et al., 2001). In addition, various cell death mechanisms require de novo synthesis of both RNA and lethal proteins to trigger the apoptotic cell death (D’Mello et al., 1993). However, how the induction of NP1 expression leads to the propagation of neuronal death or its inhibition contributes to neuroprotection against hypoxic-ischemic cerebral injury in neonatal brain is not completely understood. Here, we report that that genetic deletion of NP1 expression attenuated mitochondria-mediated HI-induced brain injury and showed persistent long-term neuroprotection in neonatal brain.
Materials and Methods
Neonatal mice model of HI
Neonatal (P8) wild type (WT) and NP1 knockout (NP1-KO) mice (C57BL/6 background) originated from separate WT and NP1-KO breeding colonies were used for the HI study. These mice are of congenic C57BL/6 background, constitutive knockouts; viable and fertile as mutant and can be bred as such, maintain normal body weight and appear grossly normal (Bjartmar et al., 2006; Kirkpatrick et al., 2000). NP1-KO mice were kindly provided by Dr. Paul Worley, Dept. of Neuroscience, School of medicine, Johns Hopkins University, Baltimore, MD, USA. Animals were anesthetized and subjected to unilateral common carotid artery occlusion (CCAO) combined with controlled hypoxia exposure in humidified 10 % oxygen balanced nitrogen similar to that described previously (Hossain et al., 2004). Briefly, pups were separated from their dams and placed in a temperature-controlled incubator set to an ambient temperature at 35 °C. Under deep inhalant anesthesia (isoflurane in an oxygen/nitrous oxide mixture delivered via a Drager vaporizer) a 5–6 mm incision was made on the neck and the right common carotid artery was isolated, double ligated, and cut between the ligatures. Hypoxia was induced by continuous flow of warm, humidified gas (10%, balanced nitrogen) for 30 min. After hypoxic exposure, the animals were placed in the temperature controlled incubator for 2 h prior to returning to their dams in order to eliminate post-insult temperature variability that can influence the extent of injury. A subset of control animals were mock-treated with a small incision on their necks without CCA occlusion and placed in the Plexiglass chamber at normal air (Sham control). We used 1 pup/litter as each litter is considered as n=1 for comparison between control and HI (WT vs. KO). Animals were sacrificed at 24, 48, 72 h and 7 d after HI. All procedures involving animals were carried out in accordance with the NIH guide for the Care and Use of Laboratory Animals and approved by The Johns Hopkins University Animal Care and Use Committee.
Histochemistry
Injury to different brain areas was determined by triphenyltetrazolium chloride (TTC, Sigma, St. Louis, MO, USA) staining of brain sections (2 mm) collected at 24 and 48 h post-HI and from Nissl-stained brain sections and obtained at 7 d post HI. Quantitative morphometric analysis of injury volumes was determined using the computer-assisted image analysis software package Microcomputer Imaging Device (MCID) of Imaging Research (Brock University, St. Catharines, Ontario, Canada) according to our established methods (Hossain et al., 1998). For Nissl-stained sections, brain injury was assessed by histologic analysis of striatum and dorsal hippocampus. Serial 25 μm coronal sections were cut with a Cryostat (Microm, Heidelberg, Germany) and stained with 0.5% Cresyl violet. For each animal, the cross-sectional areas of straitum and hippocampus at both ipsilateral and contralateral sides were measured using MCID image analysis. For striatum, six coronal sections selected at 250 μm intervals, rostral to the anterior commissure were analyzed. For hippocampus, six coronal sections caudal to the rostral most edge of hippocampus were analyzed. For each section the percent damage was calculated by comparing the cross-sectional area of the injury (I) and the contralateral (C) side using the formula: 100 X (C-I/C) as described previously (Hossain et al., 1998). For TTC staining, a subset of animals from the sham control and HI groups were sacrificed by decapitation at indicated times post-HI to determine cerebral infarctions as described previously (Hossain et al., 2004). The areas of infarction were visualized with a digital scanner (HP Scanjet G4010) and quantified by MCID analysis to determine infarct volumes. The average values of percent damage were determined for each structure and animal.
TUNEL staining
The Dead End Fluorometric TUNEL System (Promega, Madison, WI, USA) was used to detect degenerated cells in brain sections following HI as described previously (Hossain et al., 2004). Briefly, brain sections were processed according to the manufacturers’ instructions. Negative controls were performed under identical conditions except for the omission of terminal deoxynucleotidyl transferase (TdT) from the reaction buffer and sections were counter stained with DAPI. Fluorescein fluorescence was visualized under a fluorescence microscope (Carl Zeiss Axioplan 1) with an excitation at 485 nm and an emission at 535 nm. DAPI fluorescence (blue) was visualized with an excitation and emission filters at 365 nm and 450 nm, respectively. The number of TUNEL (+) cells was quantified by using MCID image analysis as described previously (Russell et al., 2006).
Immunofluorescence analysis
To assess the distribution of NP1 protein in different brain regions and sub-regions (e.g. hippocampal CA1, CA3 and dentate gyrus) representative coronal brain sections (20 μm) were subjected to immune-staining and analyzed by fluorescence microscopy as described previously (Hossain et al., 2004). Mouse pups were sacrificed at the indicated time-points after HI by perfusion fixation with ice-cold 4% paraformaldehyde in 1X PBS and cryoprotected sequentially in 15% and 30% sucrose solutions for overnight followed by freezing on dry ice. Representative coronal brain sections (20 μm) from control and HI animals were immunostained as described previously (Al Rahim et al., 2013; Hossain et al., 2004). Mouse monoclonal anti-NP1 (1:200) (BD Transduction Laboratories, Temecula, CA, USA), GFAP (1:1000), COXIV (1:250) and cleaved caspase-3 (1:200; Cell Signaling, Beverly, MA, USA) was used as the primary antibody, while donkey anti-mouse, anti-rabbit Alexa fluor 488 (green) and 568 (red) are the secondary antibodies used (Invitrogen, Carlsbad, CA, USA). Immunofluorescence was visualized using an inverted fluorescence microscope (Olympus IX51 fitted with DP2-DSW-V3.2 application software) at 10 X and ZEISS Axioimager M2 (AxioVision SE64 Rel. 4.8.1 application software) at 100 X magnification.
Preparation of cytosolic and mitochondrial fractions
Subcellular fractionation was performed using cortical tissues from sham control and HI animals (WT and NP1-KO) as described previously (Al Rahim et al., 2013; Russell et al., 2011). Briefly, the cerebral cortices were collected and placed in 10 volumes of ice-cold homogenization buffer (250 mM sucrose, 10 mM HEPES, 1mg/ml BSA, 0.5 mM EDTA, 0.5 mM EGTA) supplemented with protease and phosphatase inhibitor cocktails (Calbiochem, San Diego, CA, USA). Tissues were homogenized using a hand-held homogenizer by eight strokes and centrifuged at 2000 g for 3 min. The pellet (P1) is the nuclear fraction. The post-nuclear supernatant (S1) was centrifuged at 12,000 g for 10 min at 4°C for the cytosolic and mitochondrial fractions. The supernatant was used as the crude cytosolic fraction. The pellet was washed with sucrose buffer (in mM: 320 sucrose, 3 CaCl2, 2 Mg-acetate, 0.1 EDTA, 10 Tris-HCl), and further centrifuged at 12,000 g for 10 min. The pellet containing the mitochondrial fraction was resuspended in the sucrose buffer supplemented with protease and phosphatase inhibitor cocktails. To confirm the separation of cytosolic and mitochondrial fractions, blots were stripped and incubated with β-actin (Sigma) and prohibitin (Abcam, Cambridge, MA, USA), respectively, which also served as loading controls.
Embryonic cortical neuronal culture
Primary cortical neuronal cultures were prepared from embryonic day 16 (E16) wild-type (WT) and NP1-knockout (NP1-KO) mice as described previously (Hossain et al., 2004). Briefly, primary cortical neurons were grown in a culture medium consisting of Neurobasal™ medium (Invitrogen, Carlsbad, CA, USA), 2% B27 supplement (Invitrogen), 2-mM L-glutamine, and 1% penicillin-streptomycin as described previously (Hossain et al., 2004). At 3 days in vitro (DIV), one-third of the media was replaced with fresh medium (without L-glutamine) containing cytosine arabinofuranoside (AraC, 5 μM; Sigma, St. Louis, MO, USA) to arrest the growth of non-neuronal cells. Experiments were conducted at DIV 12, when cultures consisted primarily of neurons (>95% MAP-2 immunoreactive cells) (MAP-2; Chemicon, Temecula, CA, USA).
Short interference RNA (siRNA) directed against NP1 mRNA (NP1-siRNA)
For NP1 gene silencing experiments, we transfected primary cortical neurons with Ntpx1 specific siRNA constructs (5′-AATTCTTCCAGCCAAACCAAC-3′) (construct #7) (5-AAGAACGACACAGAGGAAAGG-3′) (construct #9) generated using a Silencer™ siRNA construction kit (Cat # 1620) (Ambion, Inc. Austin, TX, USA) and the commercially available control scramble siRNA (SsiRNA) following the methods described previously (Russell et al., 2011). The oligodeoxyribonucleotide sequences exhibited no similarity to any other known mammalian genes as determined by BLAST. Experimental treatments were initiated ~ 48 h after transfection. Using siRNA specific for NP1, we have achieved >90% reduction in NP1 protein levels compared to control SsiRNA. To mechanistically demonstrate the mitochondrial localization of NP1, primary cortical neurons were co-transfected with either DsRed2 control or pDSRed2-Mito vector (Clontech) DNA that targets to the host cell’s mitochondria (Rizzuto et al., 1995) in the presence of NP1-siRNA, and exposed to OGD as described above (Al Rahim et al., 2013).
Induction of oxygen glucose deprivation (OGD), modeled in vitro, using cultured primary cortical neurons
To induce oxygen glucose deprived conditions, cultured cortical neurons at DIV 12 were exposed to OGD as described previously (Al Rahim and Hossain, 2013; Al Rahim et al., 2013; Sharma et al., 2014). Briefly, neurons were placed in glucose-free Earl’s balanced salt solution (EBSS) and then exposed to humidified 95% N2/5% CO2 using anaerobic modular incubator chambers (Billups-Rothenberg, Del Mar, CA, USA) for different time periods (2–8 h). Control cultures were incubated with EBSS with glucose and incubated in humidified 95 % air/5% CO2 for the same duration.
Assessment of cell cytotoxicity
Lactate dehydrogenase (LDH) activity released in the media after ODG exposure was measured using the CytoTox96 Non-radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA) as described previously (Hossain et al., 2004; Russell et al., 2007). Percent cell death was determined using the formula: % cytotoxicity = hypoxic LDH release (OD490)/maximum LDH release (OD490) after correcting for baseline absorbance of LDH release at 490 nm.
Co-immunoprecipitation of NP1 with Bax
Cortical tissue homogenate from sham control and HI animals was prepared as described above. The crude cytosolic fraction was further centrifuged at 12,000 g for 10 min and the pellet was suspended in immunoprecipitation buffer (10 mM sodium phosphate, 100 mM NaCl, 1% Triton X-100) containing 1X protease inhibitor cocktail set I, and phosphatase inhibitor cocktail II (Calbiochem) following the method as described previously (Hossain et al., 2004 ). One hundred micrograms of total proteins were subjected to immunoprecipitation by incubating overnight with activated Bax[6A7] antibody (1:100, Cell Signaling) at 4 °C with constant shaking. Then, 20 μl of protein A/G-agarose conjugated beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added to each sample and incubated for 2–4 h at 4°C. The beads were collected, washed, and boiled for 3 min in 50 μl of 1 X electrophoresis sample buffer followed by Western blot analysis.
SDS-PAGE and Western blot analysis
SDS-PAGE and immunoblotting were performed according to the method of Laemmli (Laemmli, 1970) as described previously (Hossain et al., 2002). Crude homogenates (250–300 μg) from the ipsi- and contralateral cerebral cortices were subjected to SDS-PAGE and immunoblotting using antibodies specific for NP1, COXIV (1:1000; Cell Signaling), Bax (1: 1000, Cell Signaling), activated Bax[6A7] (1:500, Abcam), procaspase-3 and cleaved caspase-3 (1:1000; Cell Signaling), prohibitin and β-actin (1:5000, Sigma) antibodies according to the method described previously (Hossain et al., 2002; Hossain et al., 2004). Horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ, USA) were used at 1:5000 dilutions for 1 h at RT. HRP reaction product was then visualized by enhanced chemiluminescence using an ECL Western blotting detection kit (SuperSignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA ). Digitized images were quantified using NIH ImageJ software.
Quantification of NP1 expression by real-time PCR
Total RNA was extracted from hippocampal and cortical brain areas at different times after HI using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. cDNA was synthesized from 1 μg of purified total RNA using iScript™ cDNA Synthesis Kit (Bio-Rad laboratories, Richmond, CA, USA), following the manufacturer’s instructions. Quantitative real-time PCR was performed in triplicate by using iQ SYBR Green Supermix on the CFX96™ Real-Time System (Bio-Rad) as described previously (Al Rahim et al., 2013). The mRNA level was normalized by housekeeping gene HPRT (Semenza, 2001). The primers sets used for NP1 (Acc. No. NM_008730.2) were 5′-GCT GCG AGA GCC AGA GCA CC-3′ (sense) and 5′-TTG CCC GAG TTG GCT GAG CG-3′ (anti-sense), and for HPRT were 5′-CCT GGC GTC GTG ATT AGT GAT G-3′ (sense) and 5′-CAG AGG GCT ACA ATG TGA TGG C-3′ (anti-sense).
In vivo magnetic resonance imaging (MRI)
In vivo MRI experiments were performed on a horizontal bore 11.7 Tesla MR scanner (Bruker Biospin, Ballerica, MA, USA) equipped with a triple-axis gradient unit (maximum strength of 70 Gauss/cm), using a volume excitation coil and a 4-channel phased array receiver coil designed for mouse brain imaging as described previously (Aggarwal et al., 2010). Briefly, animals were anesthetized with 1% to 1.5% isoflurane in a mix of oxygen and air at 1:3 ratios and placed in an animal holder. During imaging, the animal’s respiration rate was monitored using a pressure sensor (SAII, Stony Brook, NY, USA) and maintained at 50–60 breaths per minute by adjusting the concentration of isoflurane. Body temperature was monitored at 35 ~ 37°C via a thermocouple placed under the body. Multi-slice T2-weighted images were performed utilizing a rapid acquisition with refocused echo (RARE) sequence with the following parameters: echo time (TE)/repetition time (TR) = 60/3800 ms, RARE-factor = 8, four signal averages, field of view (FOV) = 15 mm x 15 mm, 28 slices with 0.5 mm slice thickness, in-plane resolution of 0.08 mm x 0.08 mm, and an imaging time of 12 minutes. Multi-slice in-vivo DTI was performed using an four-segment diffusion-weighted EPI sequence with the following parameters: TE/TR = 24/14000ms, one signal average, 30 diffusion directions, b = 1500 s/mm2, an in-plane resolution of 0.12 mm x 0.12 mm with a partial Fourier factor of 1.4 in the phase encoding direction, and the same FOV, slice thickness and number of slices as the T2-weighted images. With respiratory gating, the total imaging time was approximately 30 min. All animals recovered in 5 minutes after imaging. Diffusion tensor was reconstructed at each pixel along with apparent diffusion coefficient (ADC) and fractional anisotropy (FA) using the log-linear fitting method implemented in DTIStudio (http://www.Mristudio.org). The percentage of ipsilateral volume relative to contralateral volume was determined by ipsi/contra X 100 in each animals (n=6)
Statistical analysis
Statistics were performed using GraphPad Prism software, Version 5.01. For one experimental and one control group, two-tailed Student’s t-test was used to determine if differences exist between means. Comparisons involving multiple groups were done by ANOVA, followed by Bonferroni/Dunn post-hoc test where appropriate. Significance level was assigned at P<0.05.
Results
Morphometric quantification of HI brain injury and neuroprotection in neonatal brain
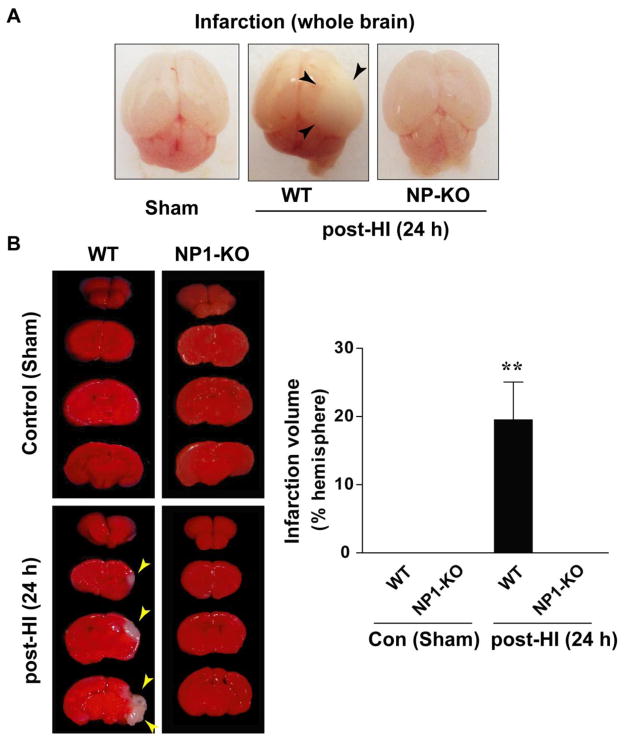
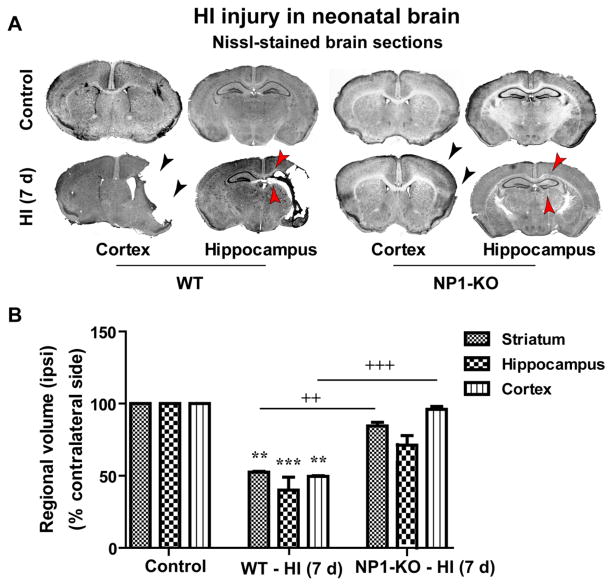
We have examined whether knockdown of the NP1 gene (Ntpx1) expression protects against HI-induced injury in neonatal brain (Figure 1). Brain injury was assessed by visual observation of postmortem brains (Figure 1A), from TTC- stained brain sections (2.0 mm) (Figure 1B), and by measuring ipsi- and contralateral cortical and hippocampal volumes at 7 d post-HI Nissl-stained coronal brain sections (WT vs. NP1-KO) by using MCID Elite and NIH Image J software (Figure 2). Although the severity of brain injury varied, the WT HI brain at 24 h appeared edematous and developed infarction (white areas) visible in the ipsi-lateral side of the whole brain (shown by arrows) as compared to sham controls. Whereas, NP1-KO HI brain appeared normal and no sign of infarction (no injury) similar to that observed in sham control brains (Figure 1A). Morphometric quantification of TTC-stained brain sections for assessment of injury in WT animals showed significantly increased brain infarction area (p<0.01), which was completely absent in the NP1-KO brains (Figure 1B). Quantitative morphometry of brain injury from Nissl-stained brain sections for assessment of tissue damage showed significant decrease in the ipsilateral-cortical (50%) and -hippocampal (~60%) volumes at 7 d post-HI compared to the sham controls (Figure 2 A–B). In contrast, volumetric quantification of Nissl-stained brain sections from NP1-KO animals revealed no cortical and hippocampal tissue loss compared to that observed in WT brains under similar conditions. Our results suggest the involvement of NP1 in the brain injury mechanisms.
Figure 1.
Cerebral HI-induced brain injury in neonatal brain. The right CCA of neonatal rats was ligated and then subjected to 30 min of hypoxia as described in the Materials and Methods. A) Representative whole brain morphology of WT and NP1-KO animals shown at 24 h after HI. The hemisphere ipsilateral to the ligation appeared edematous and show apparent sign of liquefaction area (black arrows) in WT brains but not in the NP1-KO brain. B) TTC-staining of brain sections (2 mm) showed marked infarction (white areas) and quantification of infarction volume showed significantly increased (>20%) infarcted area in the right cerebral hemisphere as compared to the sham controls. In contrast, the infarction was almost absent in the NP1-KO brains following HI and appeared similar to sham controls. Data represent mean ± SEM (n=10; **p<0.01). Representative images are shown.
Figure 2.
Cerebral HI caused brain injury in WT neonatal mice but not in the NP1-KO mice brain. A) Nissl-stained brain sections of sham control (upper panels) and HI (lower panels) mice showed injury in ipsilateral-cortical and-hippocampal areas of WT brains, but no injury/tissue loss (neuroprotection) in the NP1-KO brains as compared to that in WT brain at 7d post-HI. Representative images are shown. B) Brain injury was quantified 7 days after HI by measuring cortical, striatal and hippocampal volumes from Nissl-stained coronal sections (20 μm) using the Microcomputer Imaging Device (MCID Elite) software as previously described (Hossain et al., 1998). Morphometric quantification of representative coronal sections revealed significant loss of ipsilateral striatum, cortex and hippocampus volumes of WT brains, but not in NP1-KO animals, compared to the contralateral side and that of sham controls. Representative coronal brain sections are shown. Data represent mean ± SEM (n=10). **p<0.01, ***p<0.001, compared to sham controls. ++p<0.01, +++p<0.001 compared to WT HI animals).
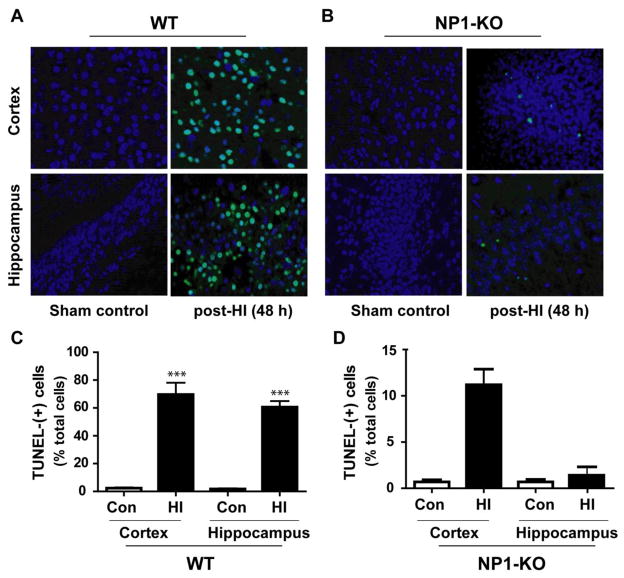
To further assess the effects of NP1 induction in brain injury and neuroprotection, we performed TUNEL-histochemistry for degenerated neurons in adjacent brain sections collected from WT (Figure 3A) and NP1-KO animals (Figure 3B) at 48h post-HI. Intensely stained TUNEL (+) cells were detected in the WT ipsilateral cortex (Figure 3A, upper panels) and hippocampus (lower panels), whereas, TUNEL- staining was almost absent in the NP1-KO ipsilateral-cortex and-hippocampus (Figure 3B). These TUNEL (+) cells showed characteristics of both apoptotic (darkly stained round chromatin) and necrotic (diffusely stained chromatin) morphologies, which are consistent with our previous findings (Hossain et al., 2004; Russell et al., 2006). Quantification of the number of TUNEL (+) cells showed significantly increased number of TUNEL (+) cells (p<0.001) were observed in the WT ipsilateral frontal and parietal cortex and in the pyramidal layer of the CA1 and CA3, but not in the dentate gyrus, hippocampal areas at 48 h post-HI as compared to the sham controls (Figure 3C). Whereas, TUNEL (+) cells were almost absent in the respective brain areas of NP1-KO brains (Figure 3D); suggesting neuroprotection in NP1-KO animals against HI.
Figure 3.
Fluorometric TUNEL histochemistry of brain section obtained from sham controls and 7 days post-HI WT and NP1-KO animals were counterstained with DAPI (dark blue) after TUNEL staining (green). Fluorescence microscopic analysis of TUNEL-(+) cells showed increased fluorescence of TUNEL (+) staining (green) with darkly stained round chromatin (apoptotic cells) in the ipsilateral-cortex (upper panels) and-hippocampus (lower panels) of WT animals (A), but less TUNEL (+ve) staining were observed in NP1-KO brains (B). Quantification of TUNEL (+ve) cells by MCID image analysis revealed significantly increased number of TUNEL (+ve) cells in the ipsilateral cortex (~70%) and hippocampus (~60%) as compared to sham controls (C). In contrast, the numbers of TUNEL (+) cells were almost absent or significantly less (<5%, non-significant) in the ipsilateral-cortex and -hippocampus of NP1-KO brain compared to corresponding sham controls. Values are mean ± SEM (n=10; ***p<0.001 with respect to sham controls). Representative images are shown.
NP1 is induced in neurons following HI and NP1 gene deletion exerts neuroprotection against HI
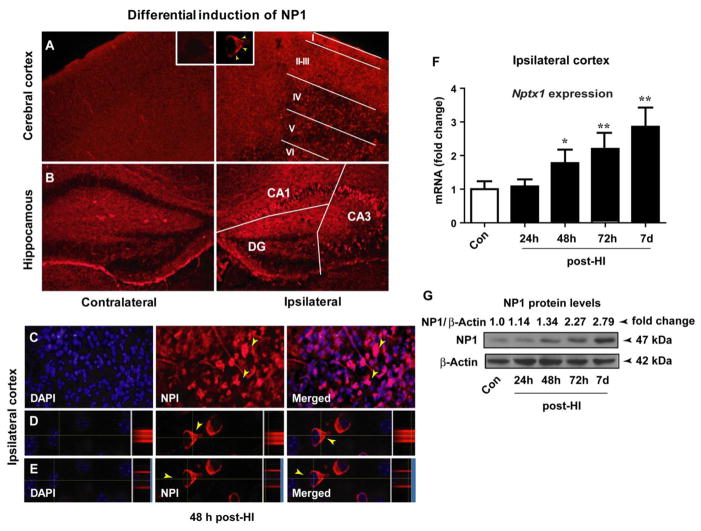
First, we examined NP1 induction following HI by multiple approaches. Immunofluorescence analysis of brain sections collected at 48h post-HI from WT animals showed differential levels of NP1 induction in different cortical layers and hippocampal regions (Figure 4). Ipsilateral-cortical layers I–III (Figure 4A) and hippocampal CA1 and CA3 areas (Figure 4B) showed higher levels of NP1 expression compared to the respective contralateral sides and sham controls. Further analysis of NP1-specific immunoreactivity (viewed at 60 X oil objective) and orthogonal projection of fluorescence images showed increased levels of NP1 localized mainly in the perinuclear region of the cytoplasm (Figure 4D) but not in the nucleus that was stained with DAPI (Figure 4E). No difference in NP1-immunoreactivity was observed in the contralateral side of the cortical and hippocampal regions as compared to sham controls.
Figure 4.
NP1 inductions in the cerebral cortex and hippocampal areas of WT animals following HI. Immunostaining of WT brain sections (20 μm) with NP1-specific antibody showed intense NP1 immunofluorescence in different layers of the ipsilateral cortex as compared to the contralateral side and sham controls. The negative control for NP1 immunostaining was performed by replacing NP1 primary antibody with nonimmune IgG. A) NP1-specific immunoreactivity showed differential distribution of NP1 in the different cortical layers (I–VI) with more intense immunoreactivity was observed in layers I–III as compared to the deeper layers (IV–VI). NP1-specific immunofluorescence in individual neurons is shown at 100 X magnification (inset). Similarly, a high level of NP1 immunoreactivity was observed in the hippocampal pyramidal layer of the ipsilateral-CA3 and -CA1, but not in DG, areas relative to the contralateral hemisphere and sham controls (B). C) NP1 induction was more pronounced at 24 h which persisted up to 7 days post-HI, examined. D, E) Orthogonal view, NP1 cellular localization is more pronounced in the perinuclear region of the cytoplasm, but not in the nucleus. Representative images are shown. F) Induction of NP1 expression in cerebral cortex at different time periods following HI. Total RNA was extracted from cortical tissue and NP1 mRNA expression levels were analyzed by quantitative real-time PCR. Fold induction is the ratio of NP1 to internal control HPRT. Values are mean ± SEM (n=5; *p<0.05, **p<0.01, ***p<0.001). G) Total proteins from cortical tissue were analyzed by SGS-PAGE and immunoblotted for NP1 protein. The NP1 antibody detected a distinct single band of NP1-immunoreactive protein of molecular mass 47 kDa. The β-actin serves as loading control. Values are NP1/β-actin ratio and expressed as fold changed over controls. Representative bands are shown (n=5).
To further validate the induction of NP1 in HI brain, we examined expression of NP1 mRNA and protein levels in the ipsilateral cerebral cortex. Quantification of NP1 mRNA levels by real-time RT-qPCR showed a >2-fold induction of NP1 mRNA at 72 h (p<0.01) which remained increased up to 7 d post-HI (p<0.001), examined, in the ipsilateral cortex as compared to the contralateral side (Figure 4 F). Western blot analysis of the cortical tissue homogenate also confirmed a HI time-dependent increase in NP1 protein levels (>2-fold) after HI compared to the sham controls (Figure 4G); corroborating the induction of NP1 in the cerebral cortex and hippocampus with HI injury. Our results clearly demonstrate a temporal relationship of NP1 induction with HI brain injury in WT animals. In contrast, NP1 protein was completely absent in the NP1-KO brain, validating the NP1 knockout status in NP1-KO animals (Supplemental Figure 1). Our results further confirm neuroprotection against HI in the absence of NP1 expression in the neonatal brain.
NP1 induction is neuronal specific
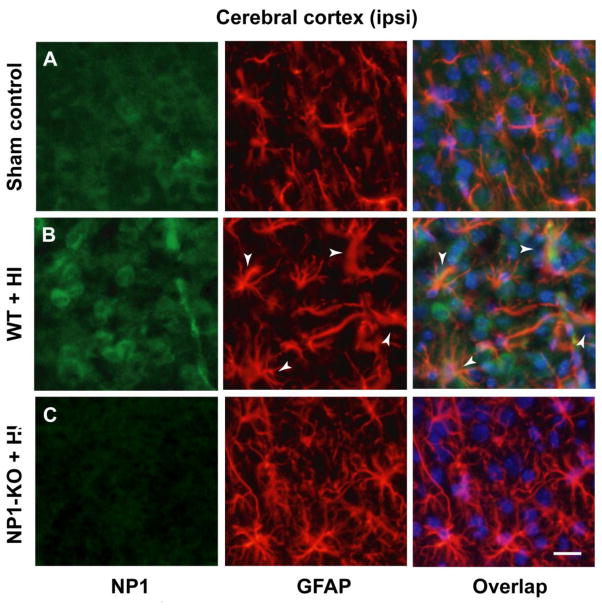
Glial cells especially astrocytes (activated astrocytes) play an active role in ischemic neuropathology (Norris et al., 2005; Rogers et al., 1996) by secreting inflammatory cytokines (Mojsilovic-Petrovic et al., 2007). We asked if NP1 is induced in non-neuronal cells in the brain following HI. Brain sections from control and HI animals (WT and NP1-KO) were double immunostained with NP1 and astrocyte marker GFAP (Figure 5). Fluorescence microscopy revealed activated phenotype of astrocytes (hypertrophy of soma and processes, Figure 5 B, middle row) in brain areas where we also observed intense NP1 induction as compared to sham controls (Figure 5A, upper row). However, merged images revealed that NP1 is not expressed/induced in astrocyte cells following HI as evident in the absence of co-localization between NP1 and GFAP; confirming neuron-specific expression of NP1 (Hossain, 2008; Hossain et al., 2004). It is possible that NP1, though exclusively expressed in neurons (Dodds et al., 1997; Goodman et al., 1996; Hossain, 2008; O’Brien et al., 2002; Tsui et al., 1996), the secreted NP1 protein is readily taken up by surrounding glial cells (Schlimgen et al., 1995) leading to glial activation. In contrast, brain sections from NP1-KO brain showed no astrocyte reactivity and retained normal astrocyte morphology (Figure 5C, bottom row) similar to those observed in brain sections from sham controls (Figure 5A). Our results suggest that NP1 induction in neurons may play a causative role in orchestrating astrocyte activation and neuronal death.
Figure 5.
WT and NP1-KO brain sections from sham controls and HI animals were double immuno-stained with GFAP- (astrocytes marker) and NP1-specific antibodies. Fluorescence microscopy revealed activated phenotype of astrocytes as characterized by larger cell bodies and substantially thicker processes (white arrows) and NP1 induction (B, middle row) in WT HI brain sections as compared to sham control (A, upper row). Merged images showed no co-localization between NP1 and GFAP. Whereas, brain sections from NP1-KO brain showed no astrocytes reactivity and retained normal astrocytes morphology (C, bottom row) similar to those observed in brain sections from sham controls (A). Representative images are shown. Scale bar, 20 μm.
Pro-death effects of NP1 induction depends on the mitochondrial localization of NP1 following HI
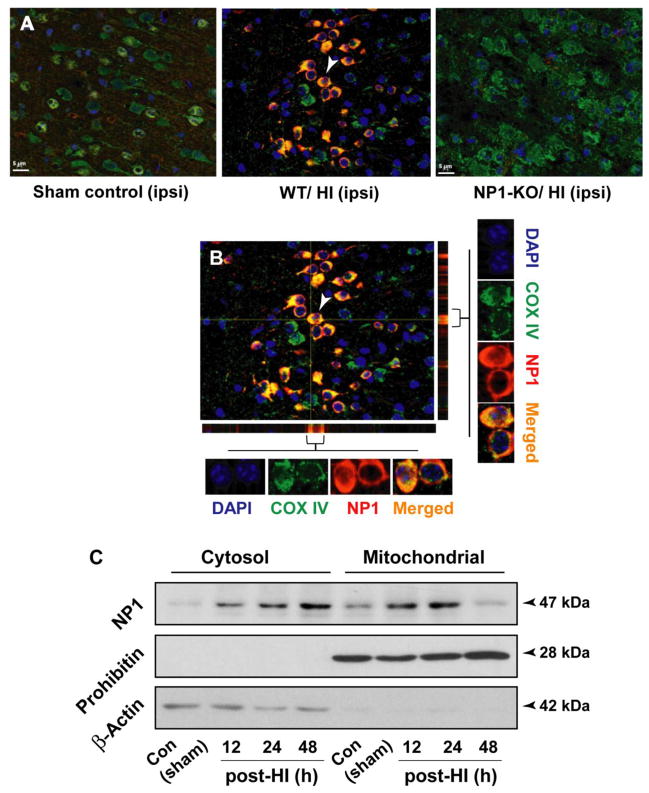
To ascertain the cellular localization of NP1 protein following induction and to gain an in-depth insight into the effects of NP1 accumulation in mitochondria, we performed dual immunofluorescence analysis using the mitochondrial specific marker COX IV and NP1, and subcellular fractionation of control and HI cortical tissues from WT brain (Figure 6). NP1 was co-localized with COX IV, a mitochondrial membrane bound protein which serve as a mitochondrial marker, in the WT HI brain, but this co-localization was completely absent in the NP1-KO brain suggesting specific localization of NP1 in the mitochondria following HI (Figure 6 A–B). An orthogonal view of merger images revealed that most of mitochondria exhibited NP1 immunofluorescence that overlapped (yellow) with mitochondrial COXIV (Figure 6 B).
Figure 6.
HI-induced NP1 protein is preferentially localized in mitochondria. Brain sections from WT and NP1-KO animals were immunostained with COX IV, a mitochondrial marker and NP1-specific antibodies. A) Fluorescence microscopy and merged digitized images showed intense NP1- and COX IV immunostaining in the WT HI brain and their colocalization (yellow), but this co-localization was completely absent in the NP1-KO brain sections. Scale bar, 5μm. B) Orthogonal sectioning shows co-localization of NP1 (red) and COX IV (green) in mitochondria seen as yellow. Representative images are shown. C) Subcellular fractionation of cortical tissues from WT brains followed by Western blot analysis showed translocation of NP1 protein from cytoplasm to mitochondrial fraction. Prohibitin and actin were used as control for purity of the mitochondrial and cytosolic fraction, respectively, which also served as loading controls. Representative bands are shown (n=5). Representative bands are shown.
Subcellular fractionation of control and HI cortical tissues (12–48 h post HI) separating mitochondrial and cytoplasm fractions followed by Western blotting using NP1-specirfic antibody showed HI time-dependent increase of NP1 protein with a molecular mass 47 kDa localization in the mitochondrial fraction as compared to the respective control (Figure 6 C). To confirm the separation of cytosolic and mitochondrial fractions, membranes were stripped and incubated with cytoplasmic (β-actin) and mitochondrial (prohibitin) markers, respectively, which also serve as loading control.
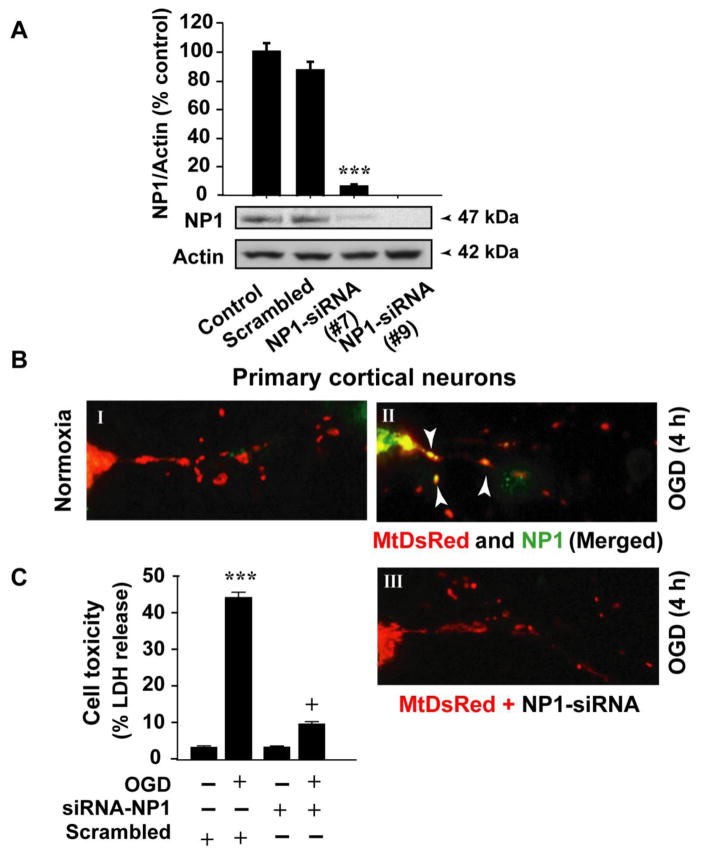
To mechanistically demonstrate the localization of NP1 in mitochondria, we transfected primary cortical neurons with DsRed2 control vector and pDSRed2-Mito vector DNA, and cortical cultures were exposed to oxygen glucose deprivation (OGD) at 12 days in vitro (DIV) (Figure 7). This vector is designed for red fluorescence labeling of mitochondria with the mitochondrial targeting sequence from subunit VIII of cytochrome c oxidase (Mito) (Rizzuto et al., 1995) that targets to the host cell’s mitochondria. Overlapped immunofluorescence images of MtDsRed (red) and NP1 (green) further confirmed a marked increase in NP1 localization into the mitochondria (yellow) following OGD as compared to normoxia control neurons (Figure 7 BI, BII). Next, we asked if NP1 localization to mitochondria caused neuronal death, and if disruption of NP1 translocation to mitochondria might reduce OGD-induced neuronal death. To directly demonstrate the involvement of mitochondrial localization of NP1 in neuronal death, a subset of cortical cultures were co-transfected with either control scramble siRNA or NP1-siRNA to knockdown NP1 protein (Figure 7A) and pDSRed2-Mito vector, and exposed to OGD as above. Fluorescence images show absence of NP1 protein in mitochondria as evident in the absence of NP1 co-localization with MitoDsRed (Figure 7 BIII). LDH release cytotoxicity assay under similar conditions revealed significant reduction in OGD-induced neuronal injury (+p<0.01) in NP1-siRNA transfected cells compared to cells transfected with control scramble siRNA (*p<0.01 vs. normoxia control cells) (Figure 7 C). Our results provide direct evidence that pro-death effects of NP1 depend on its mitochondrial localization following HI.
Figure 7.
Disruption of NP1 localization to mitochondria following HI. A) Primary cortical neurons were transfected with NP-1 specific siRNA constructs that achieved >95% knockdown of NP1 protein in as compared to cells transfected with control scramble siRNA. B) Primary cortical neurons were transfected with control and pDSRed2-Mito vector DNA (2 μg) and exposed to 4 h of OGD at DIV 12. Mitochondria labeled with MtDsRed (red) and NP1 (green) and merged images (yellow) showed mitochondrial localization of NP1 (shown by white arrows) (B I, II). A subset of cortical cultures was co-transfected with either control scramble siRNA or NP1-siRNA and pDSRed2-Mito vector and exposed to OGD. Fluorescence images show absence of NP1 immunostaining in mitochondria (B III). Representative images are shown. LDH release cytotoxicity assay revealed significant reduction in OGD-induced neuronal injury in NP1-siRNA transfected cells compared to cells transfected with control scramble siRNA (C). Results are shown mean ± SEM (n=8; **p<0.01 compared to normoxia control; +p<0.01, compared to OGD exposed cells transfected with scramble siRNA).
NP1 mitochondrial localization facilitates Bax activation
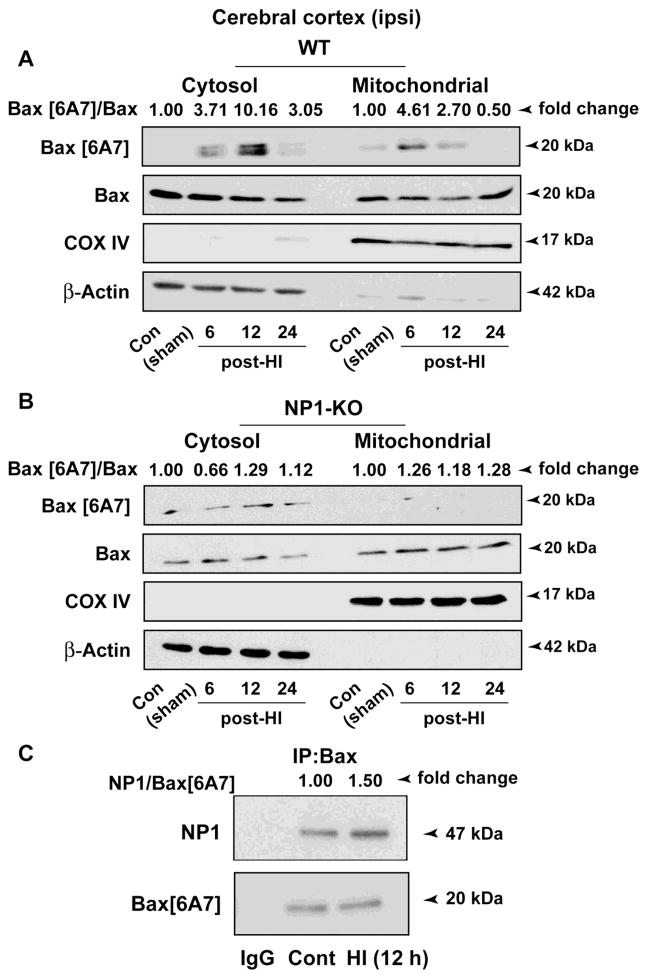
Previously we found that NP1 interacts with pro-death Bax (Al Rahim et al., 2013). We asked whether NP1 induction is associated with Bax activation and Bax localization to mitochondria leading to initiation of mitochondrial death programs. Here, we examined Bax activation in the cytoplasm and mitochondrial fractions from WT and NP1-KO cerebral cortices collected at 6–24 h post-HI (Figure 8). Western immunoblots using antibody specific for the Bax [6A7] activated form (Hsu and Youle, 1997) showed increased Bax [6A7] immunoreactive protein bands in the mitochondrial fraction at 6 h (>4-fold, P<0.01) and 12 h (>2-fold, P<0.05) post-HI from the WT cortex (Figure 8A). Whereas, activated Bax [6A7] protein bands were negligible or absent in mitochondrial fraction from NP1-KO cortical tissues (Figure 8B). Results, expressed as fold changes (Bax6A7/Bax ratio) from four separate experiments (n=4), clearly show that NP1 induction following HI and NP1’s mitochondrial localization facilitates the accumulation of active Bax to mitochondria leading to the activation of mitochondrial death pathways.
Figure 8.
Effects of HI-induced NP1 on pro-death Bax activation and its translocation to mitochondria. Subcellular fractionation of cortical lysates from WT and NP1-KO brains followed by Western blot analysis showed differential distribution of active Bax [6A7]-specific immunoreactive bands in the cytoplasm and mitochondrial fractions at different time post-HI. A) Increase in active Bax [6A7] protein levels was apparent in mitochondrial fraction at 6 and 12 h post-HI. In contrast, the Bax [6A7] specific bands were absent or remained unchanged in the absence of NP1 expression in NP1-KO brains similar to that observed in respective controls (B). COXIV and actin were used as control for purity of the mitochondrial and cytosolic fraction, respectively, which also served as loading controls. Values are shown as ratio of Bax[6A7]/Bax and expressed as fold change over sham controls from four separate experiments (n=4). Representative bands are shown. C) Interactions of NP1 with the active Bax. SDS-PAGE and immunoblotting of Bax[6A7] immunoprecipitates with NP1- and Bax[6A7]-specific antibodies revealed HI-induced increase of NP1 co-precipitation (1.5-fold) relative to sham control. IgG immunoprecipitates showed no evidence of NP1- and Bax-specific bands. Representative blots are shown.
To directly demonstrate the interaction of NP1 with active Bax protein, we performed co-immunoprecipitation experiments using Bax[6A7]-specific primary antibodies (Figure 8 C). Our results showed that NP1 was co-precipitated with active Bax protein, and Western immunoblotting showed increased precipitation of NP1 protein (>1.5-fold, p<0.01) with active Bax after HI. Our results clearly demonstrate that NP1 forms protein complexes with Bax, and that HI enhances NP1 interactions with active Bax to facilitate their mitochondrial localization.
NP1 causes neuronal injury by activating mechanisms downstream of Bax activation and NP1 gene deletion blocked activation of caspase proteases initiated following HI
It is known that activation of Bax induces cytochrome C release from mitochondria that triggers caspase-3 cleavage and apoptosis (Galluzzi et al., 2009). To further ascertain the contribution of NP1 to cell death cascades and neuroprotection in the absence of its expression, we examined the caspase-signaling pathways. Immunofluorescence analysis of activated caspase-3 in brain sections from WT animals revealed the appearance of intense cleaved caspase-3 (i.e. activated) specific immunoreactive proteins in different layers of the WT ipsilateral cortex following HI (Figure 9 A–B). Further analysis revealed that caspase-3 activation occurred in cells showing NP1 induction as evident in the intense co-localization of NP1 with cleaved caspase-3 (Figure 9 B), suggesting neuron-specific NP1-dependent activation of caspase-3 death pathway. Brain sections from sham controls or hemispheres contralateral to the CCAO showed no evidence of cleaved caspase-3-specific immunoreaction in the cerebral cortex. In contrast, brain sections from NPI-KO animals showed no evidence of cleaved caspase-3-specific immunoreactivity in the ipsilateral cerebral cortex following HI. Western blot analyses of cortical tissue homogenate further confirmed the activation of caspase-3 as evident in the 17 kDa cleaved caspase-3-specific immunoreactive protein bands, whereas, no cleaved caspase-3-specific band was visible in NP1-KO cortex homogenate (Figure 9 C). These results suggest that the caspse-3 activation did not occur in the absence of NP1 induction, implying a direct causative link between NP1 induction and caspase-3 activation in the death signaling pathways.
Figure 9.
NP1 induction in brain following HI showed co-localization with pro-apoptotic activated caspase-3. A) Immunofluorescence analysis of activated caspase-3 in brain sections from WT and NP1-KO brains revealed the appearance of intense cleaved caspase-3 (i.e. activated) specific immunoreactive proteins in the WT ipsilateral cortex following HI. Whereas, caspase-3 specific immunofluorescence was almost absent in NP1-KO brains. B) Orthogonal sectioning shows co-localization of NP1 (red) and cleaved caspase-3 (green) in WT brain sections (seen as yellow). Scale bar, 5μm. C) Western blot analysis of cortical tissues from WT and NP1-KO brains collected at different times (6–48 h) post-HI showed cleaved caspase-3 immunoreactive protein bands (17 kDa) in the WT cortical tissue lysates but not in the NP1-KO cortical lysates. Representative bands are shown.
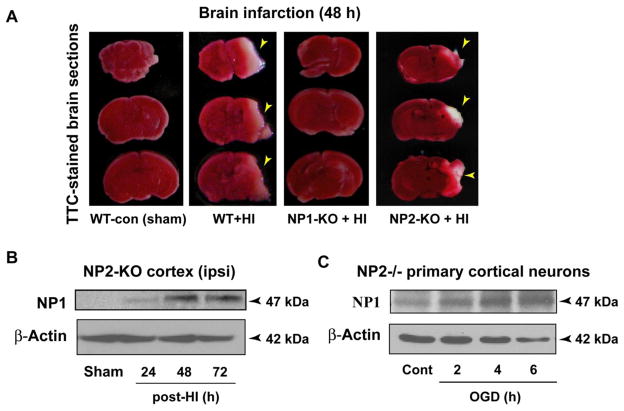
NP1 knockout, but not the NP2 knockout, animals are protected against HI-induced brain injury
Next, we asked if the induction of NP1 and its family members NP2 and NPR are specifically involved in neonatal brain injury, then there will be no injury (i.e. neuroprotection) in absence of their expression. To address this question, neonatal (P8) WT, NP1-KO, NP2-KO and NP-triple knockout (NP-TKO; in which NP1, NP2 and NP receptor genes are all knocked out) mice were subjected to HI and sacrificed at the indicated time post-HI. Quantification of infract volume by MCID image analysis of TTC-stained brain sections from WT animals showed infarction and tissue loss following 48 h post-HI (Figure 10), as observed in Figure 1B. In contrast, NP1-KO and NP-TKO HI animals showed a complete absence of infarction (i.e. no injury) at 48 h post-HI, and there was no damage (tissue loss) in the ipsilateral hemisphere of KO brains examined at 7 days post-HI compared to that in WT HI brains (Figure 10A, third panel). Most strikingly, in contrast to NP1-KO and NP-TKO brains, the TTC-stained brain sections from NP2-KO brains showed significant infarction and tissue loss following HI (Figure 10A, fourth panel). Western blot analysis of tissue homogenate from NP2-KO cortex and OGD-exposed NP2−/− cortical neurons revealed increased induction of NP1 in NP2-KO brain (Figure 10 B, C). Our results clearly delineate the involvement of NP1, but not the NP2, in hypoxic-ischemic brain injury triggered following HI, suggesting the specificity of NP1 in the brain injury mechanisms.
Figure 10.
Cerebral HI caused brain infarction in WT and NP2-KO neonatal mice but not in NP1-KO and NP-TKO brains. A) The TTC-staining of brain sections (2 mm) at 48 h post-HI from WT and NP2-KO animals revealed marked infarction (white areas) in the right cerebral cortex and hippocampal CA1 and CA3 areas, which was completely absent in brain sections from NP1-KO and NP-TKO animals under identical HI conditions. Representative images of TTC-stained sections are shown, experiments were repeated twice with n=5 each time. B, C) Western blot analyses of the ipsilateral cortical tissue from NP2-KO brain at different time post-HI showed NP1-specific immunoreactive bands of molecular mass 47 kDa. Similar induction of NP1 was observed in NP2−/− primary cortical neurons following exposure to OGD for different time periods. Representative bands are shown.
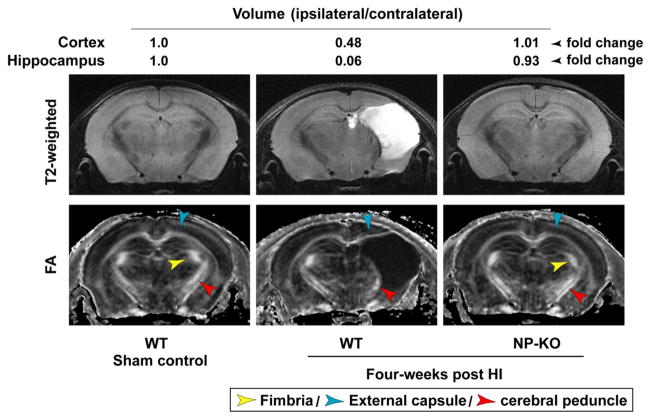
Long-term neuroprotection observed in NP1-KO and NP-TKO animals following HI
The extent of neuroprotection observed in NP1- and NP-TKO brain following HI was further confirmed by in vivo T2-weighted magnetic resonance imaging (MRI), diffusion tensor MRI (DTI) at 4 weeks post-HI (Figure 11). The T2-weighted images give an overview of the brain anatomy, and the fractional anisotropy (FA) images show the white matter tracts inside. We found that WT brain of each animal was severely injured with fluid filled cavity (hydrocephalus ex vacuo) occupies almost half of the brain at 4 weeks post-HI (Figure 11, middle panel). The fimbria (yellow arrow) and external capsule (blue arrow) were almost disappeared and the cerebral peduncle (red arrow) was partially damaged. Quantitative determination of damaged brain regions based on T2-weighted analyses showed that ipsilateral-cortical and -hippocampal volumes were reduced to 48% and 6% (ipsi/contra x 100), respectively, compared to the contralateral side (100%)(Figure 11). Most strikingly, the NP1-KO (shown) and NP-TKO mice brain showed no apparent sign of injury/damage to these structures in all the brains examined, and appeared quite similar to that of sham controls at 4 weeks post-HI (Figure 11, third column). Next, we asked if the observed neuroprotection in NP1- and NP-TKO brains persisted for longer periods of time. To address this, we examined the same group of controls and HI animals from WT and NP1-KO mice at 24 weeks post HI. The T2-weighted and DTI images showed a similar magnitude of neuroprotection in the NP1-KO and NP-TKO brains as compared to WT animals at 24 weeks post-HI (not shown) as observed at 4 weeks post-HI (Figure 11). These data are important as our results demonstrate a long-term neuroprotective efficacy and neuroanatomical integrity against HI brain injury in the absence of NP1 expression in NP1- and NP-TKO animals.
Figure 11.
In vivo magnetic resonance images of controls (WT and NP1-KO) and HI (WT and NP1-KO) brains were obtained using an 11.7T horizontal scanner at 4 weeks post-HI. The fimbria (orange arrow) and external capsule (blue arrow) were almost disappeared and the cerebral peduncle (red arrow) was partially damaged. The ipsilateral-cortical and -hippocampal volumes were reduced to 48% and 6% (ipsi/contra X 100) (middle panels), respectively, compared to percent contralateral side (100%). Most importantly, the NP1-KO mice brain showed no apparent sign of injury and appeared quite similar to the WT controls. Representative images are shown.
Discussion
In this study, we have investigated how the induction of NP-family members following HI contributes to the cerebral injury and strategies to achieve neuroprotection in neonatal brain. First, we found that the neuronal protein NP1 following induction undergoes translocation to mitochondria and initiates the activation of mitochondria-derived death cascades leading to neuronal death; providing a new evidence for intracellular function of NP1 in hypoxic-ischemic brain injury. Second, we found that NP1 induction occurs in neurons only, and is more intense in the ischemic penumbra where neuronal death is slowly developing and is more amenable to therapeutic intervention. Third, our findings further delineate that among the NP family members it is NP1, not the NP2, induction that is specifically involved in brain injury mechanisms. Most strikingly, we found that genetic deletion of NP1 ablated cerebral injury and showed complete neuroprotection in neonatal mice brain following exposure to HI. Another striking aspect of our findings is the long-term neuroprotection efficacy in the absence of NP1 expression as evident in the live MRI T2-weighted and FA brain images acquired at 4 and at 24 weeks post-HI from the same group of WT and KO animals. Together, our results show that induction of NP1 triggers mitochondria-mediated death cascades and that genetic knockdown of NP1 resulted in neuroprotection against HI, demonstrating a novel molecular insight into the mechanisms of regulating neuronal death or survival. This is the first demonstration on how inhibition of NP1 expression leads to neuroprotection against HI injury in neonatal brain.
Ample evidence suggests that mitochondria-mediated apoptotic death pathways are activated after cerebral ischemia (Johnston, 2001; Johnston and Ishiwa, 1995; Johnston et al., 2001; Martin et al., 1997). In addition, control of neuronal injury involves a balance between expression of apoptotic and anti-apoptotic proteins at post-injury periods, thus providing many potential approaches to modifying the outcome of hypoxic-ischemic insult. Here, our identification of NP1 induction and its localization to mitochondria following HI suggests that NP1 might be a new target molecule, which is possibly acting upstream in regulating the onset of the cascade of mitochondria-derived delayed events of the cell death pathways. We found post HI-time dependent increased induction of NP1 in the frontal and parietal cortices and in different hippocampal (pyramidal layers of CA3 and CA1) regions; the same brain areas that developed infarction (i.e. injury) following HI, consistent with our previous report (Hossain et al., 2004). In fact, we found that NP1 induction occurred prior to the development of the infarction (data not shown), consistent with a role of NP1 in the injury mechanisms. In contrast, NP1-KO animals showed intact brain morphology (no infarction) and neuroprotection (no tissue loss) following HI. Our findings provide clear evidence that NP1 is a pro-death protein that is induced in a greater magnitude following HI leading to neonatal brain injury.
In the present study we found that HI-induced NP1 is translocated to mitochondria as evident in our biochemical and subcellular fractionation studies. Likewise, immunofluorescence staining of brain sections confirms that NP1 initially localizes in the perinuclear region of the cytoplasm, whereas, NP1 increases markedly in mitochondria following HI. Intense co-localization of NP1 with mitochondrial marker COX IV in HI brains indicated that after induction, the majority of NP1 is targeted to mitochondria. This localization of NP1 was further confirmed in hypoxic-ischemic primary cortical neurons transfected with MtDsRed expression vector labeling mitochondria with red fluorescence. These results are consistent with the intracellular function of NP1 which is also supported by a recent report showing that majority of NP1 is intracellular under low neuronal activity (Clayton et al., 2012). Most importantly, we found that disruption of NP1 translocation by knockdown of NP1 protein in cortical neurons by NP1-specific siRNA attenuated NP1 localization to mitochondria and significantly reduced ischemic neuronal death following OGD. We performed a number of studies aimed at identifying the functional aspects of the increased accumulation of NP1 protein in mitochondria. The Bcl-2 family of proteins Bad and Bax play a crucial role in intracellular apoptotic signal transduction by regulating the permeability of mitochondrial membrane (Yuan and Yankner, 2000; Zong et al., 2001). Here, we found that the presence of NP1 in mitochondria is accompanied by increase in the Bax activation (Bax 6A7) in mitochondria. Whereas, in the absence of NP1 induction, no activated Bax[6A7] was observed in mitochondrial fraction from NP1-KO brains, suggesting a role for NP1 that acts upstream of Bax activation and mitochondrial accumulation. The majority of Bax resides in the cytoplasm and under stress conditions, undergoes a conformation change that causes Bax translocation to the mitochondrion membrane leading to the release of cytochrome C and triggering neuronal apoptosis (Youle and Strasser, 2008; Zong et al., 2001). Bax is also a direct target of GSK-3β that modulates Bax expression and functions to promote mitochondria-mediated apoptosis (Linseman et al., 2004; Watcharasit et al., 2003). Previously, we reported GSK-3α/β-dependent NP1 induction, increased NP1-Bax interaction in OGD exposed hippocampal neurons and that this interaction was reversed by GSK-3 inhibitor SB216763, confirming GSK-3 regulation of NP1 (Al Rahim et al., 2013; Russell et al., 2011), further ensuing NP1 interaction with activated Bax[6A7]. Thus it appears that NP1 forms a protein complex with Bax that facilitates Bax activation and its accumulation in mitochondria (Clayton et al., 2012), thereby contributing to mitochondrial outer membrane permeabilization following HI (Al Rahim et al., 2013).
Next, we investigated whether NP1 localization to mitochondria activates mechanisms of the apoptotic program that are downstream of Bax activation. The entry of cytochrome C from mitochondria into the cytosol plays a central role in the regulation and activation of caspase cascades of the executioner phase of apoptosis (Li et al., 1997a; Li et al., 1997b; Zou et al., 1999). We observed robust increase in the cleaved (active) form of caspase-3 in the ipsilateral cerebral cortex of WT but not in the NP1-KO brains. Most importantly, activation of caspase-3 occurred in cells showing NP1 induction following HI as evident in the intense co-localization of NP1 with cleaved caspase-3. Whereas, activation of caspase-3 was completely absent in NP1-KO brain, indicating neuroprotection against HI. Most strikingly, we found that NP1 induction, not other NP1 members, is specifically involved in HI brain injury as evidenced by the presence of infarction (injury) in NP2-KO brain, which also showed NP1 induction. It appears that the observed HI injury in NP1-KO brain is due the NP1-induction-dependent activation of caspase-3. Collectively, our results clearly demonstrate the role of NP1 in mitochondria-mediated caspase-3-dependent neuronal death in HI brain injury. It is possible that the absence of NP1 expression desensitizes neurons to HI injury by preventing mitochondrial activation of cell death cascades. Our results provide new evidence for an intracellular function of NP1.
To further demonstrate that knockdown of NP1 induction indeed specifically contributes to neuroprotection against HI, we employed high resolution T2-weighetd and FA MRI for morphometric detection of brain injury and neuroprotection over time in live animal brains (WT vs. NP-KO) at different time periods post-HI (4–24 weeks). Live T2-weighted and FA images showed brain injury and substantial tissue loss in WT ipsilateral cerebral hemisphere but no apparent sign of brain injury observed in the NP1-KO brains, further establishing the role of NP1 in the brain injury mechanisms. Furthermore, the advantage of live MRI scanning at multiple time points post-HI on the same animal both in the case of the WT and NP-KO allowed us to determine the progression of morphological changes, structural alteration/atrophy of brain. The T2-weighted and FA imaging of the same group of animals at 4 weeks and then at the 24 week post-HI time periods showed no sign of brain injury in NP-KO mice compared to that of WT mice, further demonstrating the long-term neuroprotective efficacy and neuroanatomical integrity following knockdown of NP1 expression.
In conclusion, our study clearly demonstrates a mechanism by which the induction of a pro-death neuronal protein NP1 influences mitochondria mediated neuronal death in response to hypoxic-ischemic insult, and that genetic deletion of NP1 expression ablated HI-induced brain injury. Our results suggest a novel function of NP1 in the regulation of mitochondria-mediated neuronal death program. Furthermore, live MRI revealed the long-term effects of NP1 gene deletion on neuroprotection efficacy and normal neuroanatomical integrity, pointing to a potential role of NP1 in post-injury cerebral functional recovery. Our results demonstrate that inhibition of NP1 induction is required to achieve significant neuroprotection in neonatal brain following hypoxic-ischemic insult. Together our findings identify NP1 as a novel molecular target of HI within the central neurons, and suggest that inhibition of NP1 expression is a promising strategy for therapeutic approaches for the management of neonates suffering from hypoxic-ischemic brain injury.
Supplementary Material
Highlights.
NP1 is induced in brain areas that developed infarcts and damage following HI.
NP1 facilitates Bax activation and translocation of active Bax to mitochondria.
Mitochondria localization of NP1 promotes activated caspase-3-mediated cell death.
NP1-KO animals, but not the NP2-KO, are protected against HI.
Long-term neuroprotective efficacy of NP1 deletion, but not NP2, against HI.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1 NS046030-09. We also gratefully acknowledge Dr. Paul Worley for providing the knockout mice.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal M, Mori S, Shimogori T, Blackshaw S, Zhang J. Three-dimensional diffusion tensor microimaging for anatomical characterization of the mouse brain. Magn Reson Med. 2010;64:249–61. doi: 10.1002/mrm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rahim M, Hossain MA. Genetic deletion of NP1 prevents hypoxic-ischemic neuronal death via reducing AMPA receptor synaptic localization in hippocampal neurons. J Am Heart Assoc. 2013;2:e006098. doi: 10.1161/JAHA.112.006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rahim M, Thatipamula S, Hossain MA. Critical role of neuronal pentraxin 1 in mitochondria-mediated hypoxic-ischemic neuronal injury. Neurobiol Dis. 2013;50:59–68. doi: 10.1016/j.nbd.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–5. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- Barks JD, Silverstein FS. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathol. 1992;2:235–43. doi: 10.1111/j.1750-3639.1992.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–81. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–97. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–9. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SW. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Ann Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Clayton KB, Podlesniy P, Figueiro-Silva J, Lopez-Domenech G, Benitez L, Enguita M, Abad MA, Soriano E, Trullas R. NP1 regulates neuronal activity-dependent accumulation of BAX in mitochondria and mitochondrial dynamics. J Neurosci. 2012;32:1453–66. doi: 10.1523/JNEUROSCI.4604-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A. 1993;90:10989–93. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS. Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J Biol Chem. 1997;272:21488–94. doi: 10.1074/jbc.272.34.21488. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10:481–94. doi: 10.1038/nrn2665. [DOI] [PubMed] [Google Scholar]
- Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7:191–202. doi: 10.1016/1359-6101(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Hossain MA. Hypoxic-ischemic injury in neonatal brain: involvement of a novel neuronal molecule in neuronal cell death and potential target for neuroprotection. Int J Dev Neurosci. 2008;26:93–101. doi: 10.1016/j.ijdevneu.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Bailone JC, Gomez R, Laterra J. Neuroprotection by scatter factor/hepatocyte growth factor and FGF-1 in cerebellar granule neurons is phosphatidylinositol 3-kinase/Akt-dependent and MAPK/CREB-independent. J Neurochem. 2002;81:365–378. doi: 10.1046/j.1471-4159.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Fielding KE, Trescher WH, Ho T, Wilson MA, Laterra J. Human FGF-1 gene delivery protects against quinolinate-induced striatal and hippocampal injury in neonatal rats. Eur J Neurosci. 1998;10:2490–2499. [PubMed] [Google Scholar]
- Hossain MA, Russell JC, O’Brien R, Laterra J. Neuronal pentraxin 1: a novel mediator of hypoxic-ischemic injury in neonatal brain. J Neurosci. 2004;24:4187–96. doi: 10.1523/JNEUROSCI.0347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–34. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Hypoxic and ischemic disorders of infants and children. Lecture for the 38th meeting of Japanese Society of Child Neurology, Tokyo, Japan, July 1996. Brain Dev. 1997;19:235–9. doi: 10.1016/s0387-7604(96)00561-x. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev. 2001;7:229–34. doi: 10.1002/mrdd.1032. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–40. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10:372–82. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ishiwa S. Ischemia and excitotoxins in development. Mental Retardation and Dev Disabilities Res Rev. 1995;1:193–200. [Google Scholar]
- Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–41. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Kalenderian E, Pegus C, Francis C, Goodwin N, Jacques HS, Lasa D. Cardiovascular Disease urban intervention: baseline activities and findings. J Community Health. 2009;34:282–7. doi: 10.1007/s10900-009-9159-3. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–92. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem. 1997a;272:30299–305. doi: 10.1074/jbc.272.48.30299. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997b;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152:425–35. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–85. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J Neurosci. 2005;25:4649–58. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011;69:743–58. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–98. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998;8:364–9. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–23. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Xu GP, Born J, Lorenzo J, Busto R, Rosenthal M, Sick TJ. Cytochrome C is released from mitochondria into the cytosol after cerebral anoxia or ischemia. J Cereb Blood Flow Metab. 1999;19:39–43. doi: 10.1097/00004647-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Pezzini A, Padovani A. Cerebral ischemia in young adults: pathogenic and clinical perspectives. Nova Biomedical Books; New York: 2009. [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: Further evidence for an apoptosis-necrosis continuum. J Comp Neurol. 1997;378:88–104. [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995;5:635–42. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer’s disease pathogenesis. Neurobiol Aging. 1996;17:681–6. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Russell JC, Blue ME, Johnston MV, Naidu S, Hossain MA. Enhanced cell death in MeCP2 null cerebellar granule neurons exposed to excitotoxicity and hypoxia. Neuroscience. 2007;150:563–74. doi: 10.1016/j.neuroscience.2007.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JC, Kishimoto K, O’Driscoll C, Hossain MA. Neuronal pentraxin 1 induction in hypoxic-ischemic neuronal death is regulated via a glycogen synthase kinase-3alpha/beta dependent mechanism. Cell Signal. 2011;23:673–82. doi: 10.1016/j.cellsig.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JC, Szuflita N, Khatri R, Laterra J, Hossain MA. Transgenic expression of human FGF-1 protects against hypoxic-ischemic injury in perinatal brain by intervening at caspase-XIAP signaling cascades. Neurobiol Dis. 2006;22:677–90. doi: 10.1016/j.nbd.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–26. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Sharma J, Johnston MV, Hossain MA. Sex differences in mitochondrial biogenesis determine neuronal death and survival in response to oxygen glucose deprivation and reoxygenation. BMC Neurosci. 2014;15:9. doi: 10.1186/1471-2202-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–78. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–9. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.