Abstract
Objective
The devastating effect of traumatic brain injury (TBI) is exacerbated by an acute secondary neuroinflammatory response, clinically manifest as elevated intracranial pressure (ICP) due to cerebral edema. The treatment effect of cell based therapies in the acute post-TBI period has not been clinically studied although preclinical data demonstrate that bone marrow derived mononuclear cell (BMMNC) infusion downregulates the inflammatory response. Our study evaluates whether pediatric TBI patients receiving intravenous, autologous BMMNCs within 48 hours of injury experienced a reduction in therapeutic intensity directed towards managing elevated ICP relative to matched controls.
Design
The study was a retrospective cohort design comparing pediatric patients in a Phase I clinical trial treated with intravenous autologous BMMNCs (n=10) to a control group of age and severity matched children (n=19).
Setting
The study setting was at Children's Memorial Hermann Hospital, an American College of Surgeons Level 1 Pediatric Trauma Center and teaching hospital for the University of Texas Health Science Center at Houston from 2000-2008.
Patients
Study patients were 5-14 years with post resuscitation Glasgow Coma Scale scores of 5-8.
Interventions
The treatment group received 6 million autologous BMMNC/kg body weight intravenously within 48 hours of injury. The control group was treated in an identical fashion, per standard of care, guided by our TBI management protocol, derived from American Association of Neurological Surgeons guidelines.
Measurements
The primary measure was the Pediatric Intensity Level of Therapy (PILOT) scale, used to quantify treatment of elevated ICP. Secondary measures included the Pediatric Logistic Organ Dysfunction (PELOD) score and days of ICP monitoring as a surrogate for length of neurointensive care.
Main Results
A repeated measure mixed model with marginal linear predictions identified a significant reduction in the PILOT score beginning at 24 hours post treatment through week one (P<0.05). This divergence was also reflected in the PELOD score following the first week. The duration of ICP monitoring was 8.2±1.3 days in the treated group, and 15.6±3.5 days (p=0.03) in the time matched control group.
Conclusions
Intravenous autologous BMMNC therapy is associated with lower treatment intensity required to manage ICP, associated severity of organ injury, and duration of neurointensive care following severe TBI. This may corroborate preclinical data that autologous BMMNC therapy attenuates the effects of inflammation in the early post TBI period.
Keywords: Cell therapy, Clinical trial, Pediatrics, Stem cell, Traumatic brain injury, Intracranial pressure
INTRODUCTION
Traumatic brain injury (TBI) continues to present a profound financial and social burden to the population due to its associated morbidity and disability1,2. Despite improvements in prevention, severe pediatric traumatic brain injury, defined clinically as a Glasgow Coma Score (GCS)<8 rates remain unchanged3.
TBI causes an acute secondary neuroinflammatory response clinically manifest as elevated intracranial pressures (ICP) from cerebral edema. The pathophysiology and management of traumatic brain injury can be viewed in two stages4. The first stage is treating the primary injury and the sequela of the direct mechanical impact, and involves evacuating gross macrovascular bleeding, achieving hemostasis and debridement of non-viable tissue. The second and often long term goal is directed towards treating the secondary effects of the initial impact, which involves a neuroinflammatory response exacerbated by the breakdown of the blood brain barrier, resulting in sub-acute, life threatening cerebral edema. This second stage classically peaks approximately 48-72 hours after the initial trauma5-7. Subsequent chronic inflammation and cellular dysfunction manifests as chronic motor and cognitive disabilities.
The acute neurointensive care of TBI has unfortunately remained supportive and focuses on treating edema and escalates in intensity in a tiered fashion8,9. First tier treatments include establishing an ICP treatment threshold, Cerebral Perfusion Pressure (CPP) monitoring, sedation, neuromuscular blockade, CSF drainage and hyperosmolar therapy with mannitol or 3% saline10. Second tier recommendations include hyperventilation, barbiturates, hypothermia and decompressive craniotomy11-24. In the pediatric population, the Pediatric Intensity Level of Therapy (PILOT) scoring system for treatment intensity directed towards intracranial pressure management has been developed and validated with higher scores assigned to second tier management strategies such as hyperventilation, barbiturates, hypothermia and decompressive craniectomy (Table 1) 25-28.
Table 1.
Pediatric Intensity Level of Therapy scale
| Variable | Score | Maximum Possible Score |
|---|---|---|
| General—occurring at any time in 24-hr period | 4 | |
| Treatment of fever (temperature of °38.5°C) or spontaneous temperature of °34.5°C | 1 | |
| Sedation (e.g., narcotics, benzodiazepines: any dose) | 1 | |
| Neuromuscular blockade | 2 | |
| Ventilation—most frequently observed PaCO2 in 24-hr period | 4 | |
| Intubated/normal ventilation (PaCO2 of 35.1–40 mm Hg) | 1 | |
| Mild hyperventilation (PaCO2 of 32–35 mm Hg) | 2 | |
| Aggressive hyperventilation (PaCO2 of <32 mm Hg) | 4 | |
| Osmolar therapy—total dose in 24-hr period | 6 | |
| Mannitol, ≤1 g/kg | 1 | |
| Mannitol, 1.1–2 g/kg | 2 | |
| Mannitol, >2 g/kg | 3 | |
| or | ||
| Hypertonic saline (any dose or rate, regardless of serum [Na]) | 3 | |
| CSF drainage—number of times in 24-hr period | 3 | |
| 0–11 times | 1 | |
| 12–23 times | 2 | |
| ≥24 times or continuous | 3 | |
| Barbiturates—total dose in 24-hr period | 4 | |
| Pentobarbital, ≤36 mg/kg | 3 | |
| Pentobarbital, >36 mg/kg | 4 | |
| Surgery—at any time in 24-hr period | 9 | |
| Evacuation of hematoma | 4 | |
| Decompressive craniectomy | 5 | |
| Other—at any time during 24-hr period | 8 | |
| Induced hypothermia | ||
| Mild (≥35° to 37°C) | 2 | |
| Moderate (°35°C) | 4 | |
| Lumbar drain | 2 | |
| Induced hypertension (≥95th percentile for age) | 2 | |
Total possible score 38
CSF, cerebrospinal fluid.
ICP is monitored and used either as a therapeutic target (<25mmHg) or as a component in a cerebral perfusion pressure strategy (CPP=MAP-ICP). After excluding extra-axial bleeding and contusion expansion with imaging studies, the ICP is functionally used as a surrogate for cerebral edema. Cerebral edema has been categorized as vasogenic (interstitial) and cytotoxic (intracellular), but both are consequences of the secondary brain injury that could be exacerbated by neuroinflammation29-31. Sustained elevations of ICP suggest ongoing secondary injury. Elevated average ICP in the first 48 hours of monitoring has been shown to be independently associated with mortality32. Despite ICP-targeted therapy, one-third of pediatric patients with severe TBI have unfavorable outcomes: death, persistent vegetative state, or severe/moderate disability1,3.
Our translational research laboratory has developed a focused effort investigating preclinical and clinical applications of cell therapy33. Bone Marrow-derived Mononuclear Cells (BMMNCs) comprise a progenitor cell population that share the characteristics of unilobulated or round nuclei, absence of granules in the cytoplasm, and similar size and density which allows for easy isolation for therapeutic application34 These cells have been shown to mobilize in response to tissue damage to organs such as the heart, liver and kidneys35-37. In 2008, we completed a two year Phase I trial with ten children using autologous BMMNCs delivered intravenously within 48 hours of injury and found this approach to be safe, though a secondary finding with underlying mechanistic potential was preservation of selected brain structures on follow-up MRI38. Concurrent preclinical rodent experiments demonstrated that cell based therapies could reduce the amount of locoregional brain edema in the acute post injury period, suggesting that therapy allows for preservation or recovery of the blood brain barrier39-41. We thus questioned whether or not this preclinical acute treatment associated effect could be retrospectively demonstrated in our human trials. In this study we sought to evaluate whether our previous cohort of treated pediatric TBI patients experienced a reduction in ICP directed therapeutic intensity levels relative to time, age and severity matched control patients.
MATERIALS AND METHODS
This study was a retrospective cohort study using data obtained from a clinical trial conducted under Federal Investigational New Drug Application BB 12620 and was approved by The University of Texas Health Sciences Center at Houston Committee for the Protection of Human Subjects and approved by the Children's Memorial Hermann Office of Research.
All 10 patients that were enrolled in our Phase I trial (May 2006- October 2008) were included in this cohort study as the treatment group. These patients were reported in a previous publication.38. A control group of 19 patients was formed by applying the same inclusion/exclusion criteria (Table 2) to 156 consecutive severe TBI children (defined as GCS ≤8) that were admitted to our same institution from the years 2000 to 2008. Five of the 19 patients that were admitted during the Phase I trial period were not enrolled. Three of these patients had declined to be included in the trial and two were not enrolled because their treatment window would have conflicted with the safety monitoring review period for one of the enrolled Phase I trial patients. Age, sex, Injury Severity Score (ISS), best initial Glasgow Coma Score (GCS), Modified Marshall Score and opening ICP were used for baseline characteristics (Table 3). Opening pressure data was only available for patients from 2006-2008. Two- tailed t-Tests and Fisher's exact tests were used to compare the two groups at baseline. Means were reported with standard error of the mean (SEM).
Table 2.
Inclusion/Exclusion Criteria
| Inclusion Criteria |
| Age, 5-14 y |
| Post resuscitation GCS 5-8 |
| Injury occurring <24 h within enrollment |
| Exclusion Criteria |
| Initial ICP >40 |
| Findings on head CT/MRI suggestive of prolonged hypoxic ischemic insult |
| Hemodynamic instability |
| Uncorrected coagulopathy at the time of harvest |
| Pulmonary contusions |
| Solid or hollow visceral injury of the abdomen/pelvis |
| Spinal cord injury |
Table 3.
Baseline Demographic/Injury Data
| Variable | Control ’00-06 N=14 ± SEM | Control ’06-08 N=5 ± SEM | p-value (’00-06 vs ’06-08) | Control ’00-08 N=19 ± SEM | Phase I N=10 ± SEM | p-value (Control ’06-‘08 vs Phase I) | p-value (Control ’00-‘08 vs Phase I) |
|---|---|---|---|---|---|---|---|
| Males % | 64% (n=9) | 40% (n=2) | 0.6 | 60% (n=11) | 70% (n=7) | 0.3 | 0.7 |
| Mean Age | 8.935 ± 0.7 | 8.6 ± 1.6 | 0.8 | 8.8 ± 0.8 | 8.9 ± 0.9 | 0.9 | 1.0 |
| Injury Severity Score (ISS) | 29 ± 1.1 | 35.0 ± 6.6 | 0.03 | 30 ± 1.5 | 30 ± 1.9 | 0.2 | 0.3 |
| Best initial GCS | 6.3 ± 0.3 | 6.6 ± 1.1 | 0.7 | 6.4 ± 0.4 | 5.8 ± 0.4 | 0.3 | 0.3 |
| Modified Marshall Score | 3.7 ± 0.3 | 3.3 ± 1.7 | 0.6 | 3.6 ± 0.3 | 3.7 ± 0.5 | 0.7 | 0.8 |
| Opening* Pressure | 20 ± 2.7 (n=12) | 22 ±4.3 (n=5) | 0.7 | 22 ± 2.4 (n=17) | 21 ± 2.1 (n=10) | 0.6 | 0.7 |
| Craniectomies | 29% (n=4) | 20% (n=1) | 0.1 | 26% (n=5) | 50% (n=10) | 0.1 | 0.4 |
| EVD | 42% (n=5) | 0% | 0.2 | 29% (n=5) | 40% (n=4) | 0.1 | 0.5 |
2 patients in the 2000-2006 control group did not have ICP monitor placed
Bone marrow at 3-5 mL/kg body weight was harvested aseptically from either the posterior iliac bone or the anterior iliac crests while under both local and systemic anesthesia and with continuous physiologic monitoring. Once collected, the marrow was processed by the Center for Cell and Gene Therapy. In brief, the filtered mononuclear cell fraction was isolated using Ficoll-Paque PLUS (GE Healthcare Bio- Sciences Corp., New Jersey, USA) density gradient separation. The mononuclear cells were washed with human serum albumin in normal saline and adjusted to the appropriate concentration of 6×106 BMMNC/kg at a volume of 1cc/kg of body weight. Prior to release, the cells were tested for viability and presence of endotoxin. Quality control was conducted for bacterial and fungal cultures, presence of mycoplasma via polymerase chain reaction as well as progenitor cell colony formation and flow cytometry for cell characteristics. The product was infused intravenously using catheters no smaller than 20 gauge.
Treatment intensity - PILOT Score
The primary measure of this study was the intensity of treatment required to counter elevated intracranial pressure due to the neuroinflammatory response to injury. The PILOT score was calculated at 24 hour intervals starting from time of admission, by combining subtotals from seven different treatment modalities (Table 1). A repeated measure mixed model with marginal linear predictions was used to identify if there were any differences between treatment and control groups from time of admission through 21 days as well as from hospital day 3 – 10 (24 hours after infusion of cell product for the treatment group). An area under the curve analysis was also conducted to determine if treated patients had less intense cumulative therapy over the first and or second week compared to controls. The PILOT score does not account for different degrees of daily hypertonic saline administration, thus peak serum sodium levels were also compared between groups.
Organ dysfunction-PELOD Score
The pediatric logistic organ dysfunction (PELOD) score is a prospectively validated outcomes measure of the degree of multiple organ dysfunction in pediatric patients42-47. The PELOD score was calculated daily from 12 variables derived from 6 organ system categories: neurological, cardiovascular, renal, respiratory, hematological, and hepatic. For each variable, the most abnormal daily variable was used. A separate comparison between groups was conducted for the GCS score, a major component of the PELOD score for TBI patients.
ICP days
Our study was designed to study the effects of cell based therapy on TBI and thus the inclusion and exclusion criteria were designed to allow enrollment of patients without major organ system dysfunctions at time of admission. Rather than using the length of stay as an indicator of short term outcome, we defined ICP monitoring days as a more specific surrogate of duration of neurointensive treatment required. In the control group, one patient was excluded from ICP monitor days due to death, one patient was managed without invasive monitoring, and one patient underwent craniectomy and the neurosurgeon elected to not leave a pressure monitor. In the treated group, one patient developed hydrocephalus and underwent multiple ventriculostomies and creation of a ventriculoperitoneal shunt. All groups demonstrated normal distribution via the Shapiro-Wilk and D'Agostino & Pearson omnibus normality tests.
RESULTS
There was no statistically significant difference in baseline characteristics between the treated Phase I group and the control groups (Table 3). In the 21 days following injury, there were no deaths in the treatment group and one death in the control group.
Treatment Intensity (PILOT)
The treated group experienced a statistically significant reduction in PILOT scores beginning at 24 hours post treatment through week one (P<0.05) (Figure 1). The divergence of the PILOT scores began approximately 48 hours from time of admission, which corresponded to the period in which treated patients received autologous bone marrow mononuclear cells. Following cell therapy, the divergence in the PILOT score tracing was maintained, with the treatment group following a near linear decline through 21 days. In the first week, the control group treatment intensity remained elevated while the treated patients experienced a de-escalation in treatment intensity following cell therapy. The control group required equal or escalated therapy, and did not approach the treatment group scores until after two weeks post injury. All Phase I patients (n=10) received hypertonic therapy and reached a peak serum sodium concentration of 160 ± 3 mEq/L. This was not statistically significant when compared to the 9 of the 19 patients in the control group that underwent hypertonic saline therapy (163 ± 3.0 mEq/L, p=0.5). However, there was statistical difference between the time matched subgroup of 2006-2008 at 170 ± 3 mEq/L and the Phase I treated group p=0.02 (Figure 2).
Figure 1.
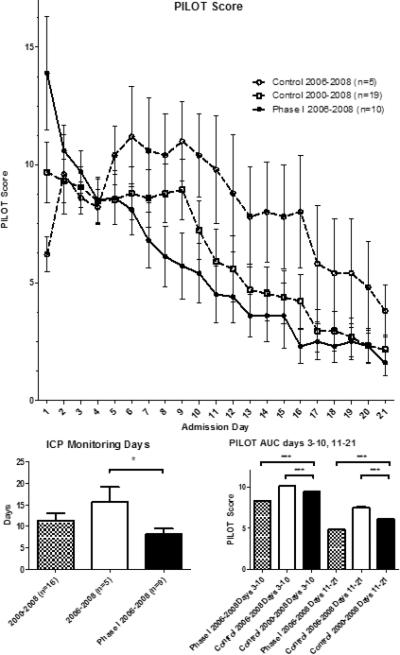
Pediatric Intensity Level of Therapy (PILOT) score calculated from time of admission with divergence seen following time of cell therapy (patients were treated within 48 hours of admission) with p<0.001 verses both 2006-2008 and 2000-2008 controls. Also displayed are the mean ICP monitoring days with SEM for each group and area under the curve analysis where cumulative PILOT scores in each group of patients were summed for days 3-10 and for days 11-21.
Figure 2.
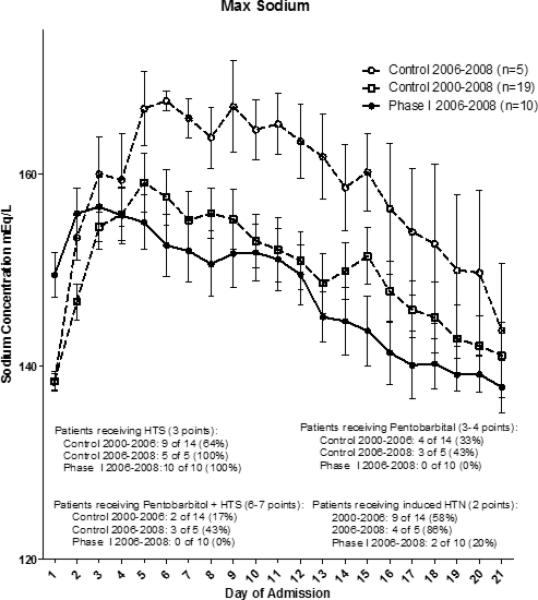
Maximum sodium levels were highest in the 2006-2008 control group, which was significantly higher than the 2006-2008 Phase I group (p<0.05).
Organ dysfunction (PELOD)
The divergence seen in the PILOT score was also reflected in the PELOD score (Figure 3) but appeared to occur at one week compared to the first 24-48 hours seen in the PILOT score. GCS scores also began to improve beginning at one week in the treated group (Figure 4).
Figure 3.
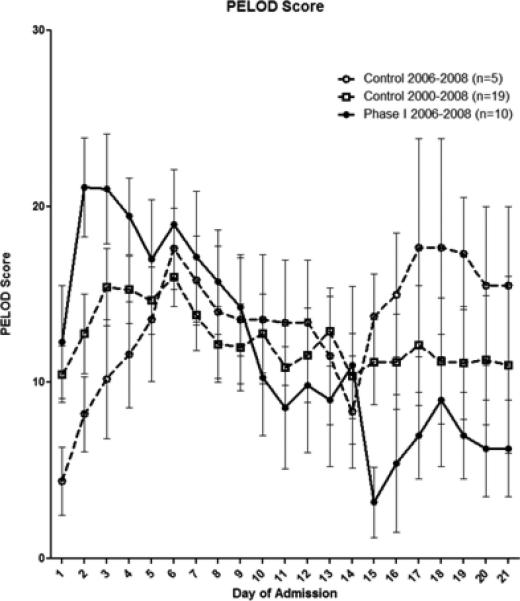
Pediatric Level of Organ Dysfunction (PELDO) score calculated from time of admission with divergence seen after one week with the Phase I 2006-2008 group verses both 2006-2008 and 2000-2008 controls p<0.001.
Figure 4.
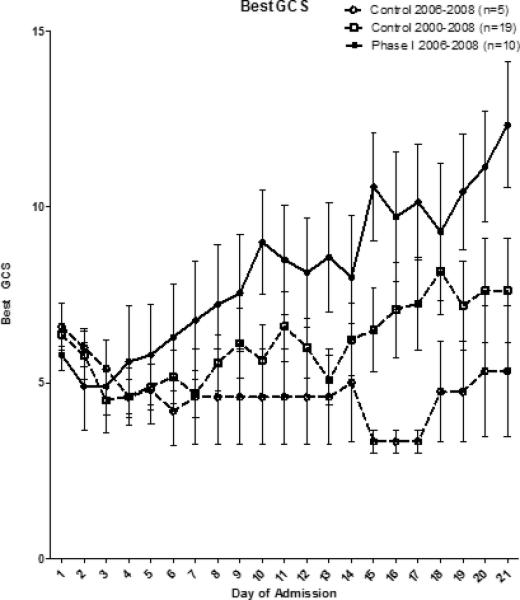
When plotting best GCS per day, there appears to be a cell therapy related improvement in GCS scores after one week in the Phase I 2006-2008 patients compared to either control group.
ICP monitoring
Maximum ICP levels per day begin to decrease approximately 48 hours after treatment in Phase I patients (Figure 5). The mean duration of ICP monitoring was 8.2 ±1.3 days in the treated group, which was significantly less compared to 15.6 ±3.5 days in the time matched control group (p=0.03), but not significantly less when compared to the entire control group 11±1.8 days (p=0.2).
Figure 5.
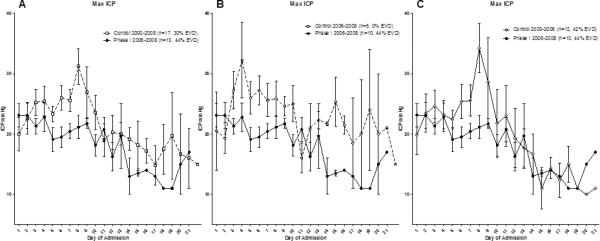
When plotting maximum ICP per day, there appears to be a cell therapy related decrease in maximum ICP values per day beginning approximately 48 hours after treatment which can be seen when the Phase I 2006-2008 group is compared with A) all controls B) time matched controls from 2006-2008 and C) controls from 2000-2006.
DISCUSSION
Our retrospective cohort study suggests that intravenous infusion of BMMNCs is associated with lower treatment intensity levels in pediatric patients with severe TBI in the early post injury period. The divergence of clinical status as defined by the PILOT for treatment intensity and PELOD score for organ dysfunction occurs at distinct time periods. For the PILOT score, the point of divergence, which occurs at time of autologous bone marrow mononuclear cell infusion, suggests that BMMNCs may be exerting an immediate down-regulatory effect on factors contributing to cerebral edema. Furthermore, the divergence is sustained, suggesting that the cellular therapy exerts a continued primary or propagative effect that is durable up to two weeks. Together with the duration of ICP monitoring, the PELOD data suggests that the BMMNCs reduce organ dysfunction past the time of neurointensive care and that the sustained elevated PELOD scores in the control group were more attributed to sustained low GCS scores from managing intracranial pressure/edema or encephalopathy rather than organ dysfunction such as known pulmonary complications associated with TBI48,49. In the BMMNC treated group, the GCS scores begin to improve at one week, the same time the PELOD scores begin to improve, suggesting that the GCS is an important contributor to the PELOD score.
The heterogeneity in TBI injury patterns make short long term and outcome trials very difficult to conduct and interpret28. Our retrospective study has several limitations. First, this study used a relatively small retrospective cohort design. The Phase I trial used in this study as the treatment group only had 10 patients. The Phase I trial was also not blinded. The control group included patients from a time period both prior to and during the safety trial. However, due to the nature of the Phase I trial, only five of the 19 control patients were admitted within the same period of the Phase I trial. Although all patients in this study were from the same institution and selected consecutively, specific treatment strategies employed by different clinicians from 2000-2008 were not captured by our data analysis. Dean et al reported in 2007 that neurointensivists have least agreement in serum osmolality thresholds for hypertonic therapy, prophylactic hyperventilation and ICP thresholds50. Secondly, the injury pattern of TBI is very heterogeneous, making the initial injury severity classification difficult. Therefore, in addition to initial GCS, we also used the Modified Marshall CT score as well as the opening CSF/intracranial pressure to estimate the severity of the initial TBI in our patients51. The transition between paper charting to electronic medical records also occurred between during the study period of 2000-2008, making data gathering such as opening pressure determination difficult. Data was not available to directly compare long term outcome data between the treatment and control groups. The Phase I trial followed each subject to six months post injury.
In this study, the PILOT score was used to describe the intensity of treatment directed towards intracranial pressure, a surrogate measure of cerebral edema. The PILOT score has limitations, one of which is in hyperosmolar therapy. The goal serum sodium concentrations are not specified in the scoring system, so patients treated with hypertonic saline to achieve a serum concentration of 148mEq/L would be scored the same as patients titrated to a serum concentration of 160mEq/L52-54. In our study, all of the ten treatment group patients underwent hypertonic saline therapy and reached a serum sodium concentration of 160 ± 3 mEq/L. This was statistically less than 170 ± 3 mEq/L in the 2006-2008 time matched control subgroup. When plotting maximum ICP per day, there appears to be a cell therapy related decrease in maximum ICP values per day beginning approximately 48 hours after treatment. Also patients in the Phase I clinical trial required pentobarbital. These differences may be associated with a therapeutic effect of the cell therapy.
Although not statistically significant, the number of patients that received external ventricular drains (EVD) was not equal across the groups. 42% of the control patients from 2000-2006 had EVDs placed, which if used for CSF drainage, may increases the PILOT score by up to 3 points. None of the control patients from 2006-2008 underwent EVD placement versus 42% in the 2000-2006 control group (p=0.4). 40% of the Phase I patients received EVD's (p=0.68 vs. 2000-2008).
Decompressive craniectomy can alter the course of neurointensive care. While fewer patients underwent decompressive craniectomy in the control group (26%) compared to treatment (50%), this was not statistically significant with a Fisher exact statistic p value of 0.42. The effect of decompression on subsequent treatment intensity may need to be further investigated. Excluding patients that underwent craniectomy from both groups did not alter the degree of divergence but did shift the start of divergence to 7 days rather than the first day. Decompressive craniectomy is a component of the PILOT score, so excluding craniectomy patients form the analysis changes the validity of the treatment intensity estimates of the scoring system used in this study.
Although we now have human clinical trial evidence from this study showing decreased treatment intensity as well was pre-clinical data demonstrating blood brain barrier permeability preservation and increased proinflammatory microglial apoptosis, the question remains whether clinical and preclinical data can be linked by neuro/systemic cytokines and biomarkers. The correlation of biomarker profiles with treatment intensity would further strengthen our clinical observations. In our current prospective Adult Phase IIA (clinicaltrials.gov NCT01575470) and Pediatric Phase IIB (clinicaltrials.gov NCT01851083) studies involving Autologous Bone Marrow Mononuclear Cell therapy, CSF and peripheral blood are being collected for biomarker analysis of IL1, 2, 4, 6, 8 and TNF-α in both treated and control patients.
Our clinical trial experience in pediatric and adult patients suggests that differences likely exist in the pathophysiology of TBI and our clinical findings of treatment intensity in the pediatric population may not be identical in our adult population. For example, children are more likely to be coagulopathic in the acute to subacute post injury period compared to adults. Both adult and pediatric patients have been observed to have an initial decline in platelet numbers followed by a rebound effect. This effect appears to be mediated by circulating endothelial progenitor cells and may be modifiable by the treatment effect exerted by our clinical trial protocol 55. In adults, the rebound effect can sometimes be dramatic, with platelet levels exceeding three times the admission level.
Lastly, this retrospective cohort study design was not able to address long-term functional and neurocognitive outcomes between the treatment and control groups. In the treatment group, 40% of patients had an improvement from a Glasgow Outcome Score range of 1-3 to 4-5 between 30 days to 180 days post injury. In the control groups, follow-up information was not always available, but at least 35% of patients from 2000-2006 (4 unknown) and at least one patient from 2006-2008 (4 unknown) were estimated to have the same outcome improvement from 30 days to beyond 180 days. The pediatric Phase IIB study has been designed to address this issue, and includes a control group with robust randomization and blinding to help detect functional and neurocognitive treatment effects.
CONCLUSIONS
Intravenous autologous BMMNC therapy was associated with a reduction in treatment intensity required to manage ICP, associated severity of organ injury, and duration of ICP monitoring following severe TBI. This corroborates preclinical data that autologous BMMNC therapy attenuates the effects of inflammation in the early post TBI period.
ACKNOWLEDGMENTS
The authors would like to thank the faculty and staff at Children's Memorial Hermann Hospital for their tireless efforts in the care and study of critically injured pediatric trauma patients. We are indebted to our patients and their families for their willingness to participate.
Copyright form disclosures: Dr. Liao, and Ms. Day received support for article research from NIH. Their institutions received grant support from NIH. Drs. Harting, Hetz, Shah, Jimenez, Tsao, Lee, and Worth received support for article research from NIH. Dr. Kosmach received support for article research from NIH. His institution received grant support from NIH/NINDS. Dr. Cox Jr consulted for CBR Inc., lectured for CBR Inc., has patents with Athersys, Inc. and EMIT Corp, and received support for article research from NIH. Dr. Cox and his institution received grant support from NIH, Athersys Inc. and CBR Inc. received royalties from EMIT Corp; and has stock with EMIT Corp. His institution received grant support from NIH.
Financial Support Used for this Study: National Institutes of Health M01 RR 02558
National Institutes of Health UL1 RR024148
National Institute of Health T32 GM 0879201
REFERENCES
- 1.Robertson BD, McConnel CE, Green S. Charges associated with pediatric head injuries: a five year retrospective review of 41 pediatric hospitals in the US. Journal of injury & violence research. 2013 Jan;5(1):51–60. doi: 10.5249/jivr.v5i1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker PA, Harting MT, Shah SK, Cox CS. Current trends in cell therapy for pediatric acquired brain injury. Minerva pediatrica. 2010 Feb;62(1):91–106. [PubMed] [Google Scholar]
- 3.Bowman SM, Bird TM, Aitken ME, Tilford JM. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics. 2008 Nov;122(5):988–993. doi: 10.1542/peds.2007-3511. [DOI] [PubMed] [Google Scholar]
- 4.Mustafa AG, Alshboul OA. Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 2013 Jul;18(3):222–234. [PubMed] [Google Scholar]
- 5.Hetz RA, Bedi SS, Olson S, Olsen A, Cox CS., Jr. Progenitor cells: therapeutic targets after traumatic brain injury. Translational stroke research. 2012 Sep;3(3):318–323. doi: 10.1007/s12975-012-0192-7. [DOI] [PubMed] [Google Scholar]
- 6.Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. Journal of neurotrauma. 1997 Nov;14(11):839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- 7.Stocchetti N, Colombo A, Ortolano F, et al. Time course of intracranial hypertension after traumatic brain injury. Journal of neurotrauma. 2007 Aug;24(8):1339–1346. doi: 10.1089/neu.2007.0300. [DOI] [PubMed] [Google Scholar]
- 8.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012 Jan;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 9.Clausen T, Bullock R. Medical treatment and neuroprotection in traumatic brain injury. Current pharmaceutical design. 2001 Oct;7(15):1517–1532. doi: 10.2174/1381612013397267. [DOI] [PubMed] [Google Scholar]
- 10.Huh JW, Raghupathi R. New concepts in treatment of pediatric traumatic brain injury. Anesthesiology clinics. 2009 Jun;27(2):213–240. doi: 10.1016/j.anclin.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 15. Surgical treatment of pediatric intracranial hypertension. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S56–59. [PubMed] [Google Scholar]
- 12.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 14. The role of temperature control following severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S53–55. [PubMed] [Google Scholar]
- 13.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 13. The use of barbiturates in the control of intracranial hypertension in severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S49–52. [PubMed] [Google Scholar]
- 14.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 12. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S45–48. [PubMed] [Google Scholar]
- 15.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 11. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S40–44. [PubMed] [Google Scholar]
- 16.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 10. The role of cerebrospinal fluid drainage in the treatment of severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S38–39. [PubMed] [Google Scholar]
- 17.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 9. Use of sedation and neuromuscular blockade in the treatment of severe pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S34–37. [PubMed] [Google Scholar]
- 18.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S31–33. [PubMed] [Google Scholar]
- 19.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 7. Intracranial pressure monitoring technology. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S28–30. [PubMed] [Google Scholar]
- 20.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S25–27. [PubMed] [Google Scholar]
- 21.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S19–24. [PubMed] [Google Scholar]
- 22.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 2: Trauma systems, pediatric trauma centers, and the neurosurgeon. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S5–8. [PubMed] [Google Scholar]
- 23.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S2–4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 24.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Jul;4(3 Suppl):S65–67. [PubMed] [Google Scholar]
- 25.Shore PM, Hand LL, Roy L, Trivedi P, Kochanek PM, Adelson PD. Reliability and validity of the Pediatric Intensity Level of Therapy (PILOT) scale: a measure of the use of intracranial pressure-directed therapies. Critical care medicine. 2006 Jul;34(7):1981–1987. doi: 10.1097/01.CCM.0000220765.22184.ED. [DOI] [PubMed] [Google Scholar]
- 26.Maas AI, Harrison-Felix CL, Menon D, et al. Standardizing data collection in traumatic brain injury. Journal of neurotrauma. 2011 Feb;28(2):177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamat P, Kunde S, Vos M, et al. Invasive intracranial pressure monitoring is a useful adjunct in the management of severe hepatic encephalopathy associated with pediatric acute liver failure. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012 Jan;13(1):e33–38. doi: 10.1097/PCC.0b013e31820ac08f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Archives of physical medicine and rehabilitation. 2010 Nov;91(11):1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- 29.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurgical focus. 2007;22(5):E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- 30.Marmarou A, Signoretti S, Aygok G, Fatouros P, Portella G. Traumatic brain edema in diffuse and focal injury: cellular or vasogenic? Acta neurochirurgica. Supplement. 2006;96:24–29. doi: 10.1007/3-211-30714-1_6. [DOI] [PubMed] [Google Scholar]
- 31.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. Journal of neurosurgery. 2006 May;104(5):720–730. doi: 10.3171/jns.2006.104.5.720. [DOI] [PubMed] [Google Scholar]
- 32.Badri S, Chen J, Barber J, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive care medicine. 2012 Nov;38(11):1800–1809. doi: 10.1007/s00134-012-2655-4. [DOI] [PubMed] [Google Scholar]
- 33.Walker PA, Harting MT, Baumgartner JE, Fletcher S, Strobel N, Cox CS., Jr. Modern approaches to pediatric brain injury therapy. The Journal of trauma. 2009 Aug;67(2 Suppl):S120–127. doi: 10.1097/TA.0b013e3181ad323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem cells translational medicine. 2012 May;1(5):403–408. doi: 10.5966/sctm.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maltais S, Perrault LP, Ly HQ. The bone marrow-cardiac axis: role of endothelial progenitor cells in heart failure. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Mar;39(3):368–374. doi: 10.1016/j.ejcts.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. The Journal of clinical investigation. 2003 Jul;112(2):160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. The Journal of pathology. 2001 Sep;195(2):229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 38.Cox CS, Jr., Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011 Mar;68(3):588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. Journal of neuroinflammation. 2013;10(1):106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. Journal of neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker PA, Shah SK, Jimenez F, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Experimental neurology. 2010 Oct;225(2):341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia PC, Eulmesekian P, Branco RG, et al. External validation of the paediatric logistic organ dysfunction score. Intensive care medicine. 2010 Jan;36(1):116–122. doi: 10.1007/s00134-009-1489-1. [DOI] [PubMed] [Google Scholar]
- 43.Thukral A, Kohli U, Lodha R, Kabra SK, Kabra NK. Validation of the PELOD score for multiple organ dysfunction in children. Indian pediatrics. 2007 Sep;44(9):683–686. [PubMed] [Google Scholar]
- 44.Leteurtre S, Duhamel A, Grandbastien B, Lacroix J, Leclerc F. Paediatric logistic organ dysfunction (PELOD) score. Lancet. 2006 Mar 18;367(9514):897. doi: 10.1016/S0140-6736(06)68371-2. author reply 900-892. [DOI] [PubMed] [Google Scholar]
- 45.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Critical care medicine. 2005 Mar;33(3):654–660. doi: 10.1097/01.ccm.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
- 46.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003 Jul 19;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 47.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Medical decision making : an international journal of the Society for Medical Decision Making. 1999 Oct-Dec;19(4):399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 48.Kalsotra A, Zhao J, Anakk S, Dash PK, Strobel HW. Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007 May;27(5):963–974. doi: 10.1038/sj.jcbfm.9600396. [DOI] [PubMed] [Google Scholar]
- 49.Zygun DA, Zuege DJ, Boiteau PJ, et al. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocritical care. 2006;5(2):108–114. doi: 10.1385/ncc:5:2:108. [DOI] [PubMed] [Google Scholar]
- 50.Dean NP, Boslaugh S, Adelson PD, Pineda JA, Leonard JR. Physician agreement with evidence-based recommendations for the treatment of severe traumatic brain injury in children. Journal of neurosurgery. 2007 Nov;107(5 Suppl):387–391. doi: 10.3171/PED-07/11/387. [DOI] [PubMed] [Google Scholar]
- 51.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. Journal of neurotrauma. 2008 Jul;25(7):719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker PA, Jimenez F, Cox CS., Jr Progenitor cell therapy for traumatic brain injury: effect of serum osmolarity on cell viability and cytokine production. Regenerative medicine. 2010 Jan;5(1):65–71. doi: 10.2217/rme.09.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zygun DA. Sodium and brain injury: do we know what we are doing? Crit Care. 2009;13(5):184. doi: 10.1186/cc8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oddo M, Levine JM, Frangos S, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. Journal of neurology, neurosurgery, and psychiatry. 2009 Aug;80(8):916–920. doi: 10.1136/jnnp.2008.156596. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Liu H, Jiao J, Bergeron A, Dong JF, Zhang J. Changes in circulating human endothelial progenitor cells after brain injury. Journal of neurotrauma. 2007 Jun;24(6):936–943. doi: 10.1089/neu.2006.0250. [DOI] [PubMed] [Google Scholar]


