Abstract
Hematopoietic cell transplantation (HCT) with non-myeloablative conditioning (NMA) for lymphoproliferative diseases (LD) includes fludarabine with and without low-dose total body irradiation (TBI). Transplant outcomes were compared among patients ≥40 years with LD who received a HCT with TBI (N=382) and no-TBI (N=515) NMA from 2001 to 2011. The groups were comparable except for donor, graft, prophylaxis for graft-versus-host disease (GVHD), disease status and year of HCT. Cumulative incidences of grades II–IV GVHD at 100 days, were 29% and 20% (p=0.001), and chronic GVHD at 1 year were 54% and 44% (p=0.004) for TBI and no-TBI, respectively. Multivariate analysis of progression/relapse, treatment failure and mortality showed no outcome differences by conditioning. Full donor chimerism at day 100 was observed in 82% vs. 64% in the TBI and no-TBI groups, respectively (p=0.006). Subset of four most common conditioning/ GVHD prophylaxis combinations demonstrated higher rates of grades II–IV acute (p<0.001) and chronic GVHD (p<0.001) among recipients of TBI-mycophenolate mofetil (MMF) compared to other combinations. TBI-based NMA conditioning induces faster full donor chimerism but overall survival outcomes are comparable to no-TBI regimens. Combination of TBI and MMF are associated with higher rates of GVHD without impact on survival outcomes in patients with LD.
Keywords: Nonmyeloablative, lymphoproliferative disease, allogeneic transplant, total body irradiation, chimerism
Introduction
Nonmyeloablative (NMA) conditioning regimens for allogeneic hematopoeitic cell transplantation (HCT) are mainly immunosuppressive and less toxic to the recipients’ stem cells. NMA regimens allowed options of HCTs for patients who were traditionally not eligible due to advanced age or comorbidities [1–3]. In NMA conditioning, low dose TBI (200 cGy) is utilized for immune ablation to aid achievement of donor chimerism [4]. The combinations of TBI and fludarabine (Flu) is a common NMA regimen which is most commonly combined with graft-versus-host disease (GVHD) prophylaxis using mycophenolate mofetil (MMF) and a calcineurin inhibitor (CNI) as pioneered by the Seattle group [3–4].
Transplants for lymphoproliferative diseases (LD) commonly use NMA regimens as the indolent nature of some of these diseases allow for disease control by the allograft, or graft-versus-tumor (GVT) effect. Also, NMA regimens help minimize toxicity for patients with prior autologous HCTs which are frequent in treatment of LD. Chronic lymphocytic leukemia (CLL), mantle cell lymphoma, and follicular lymphoma in particular are indications whereas NMA is the most common conditioning intensity used [5]. Since the inception of TBI-based NMA regimens, several other regimens have been used that excluded TBI and added chemotherapeutic agents commonly used to treated LD such as cyclophosphamide (Cy), rituximab, fludarabine (Flu) among others [6,7].
Nevertheless, no studies have directly addressed whether TBI-based (200 cGy) NMA conditioning regimens results in different outcomes compared to no-TBI regimens for LD [8,9]. Meta-analyses comparing different NMA regimens both with and without TBI showed conflicting results, including incidence of acute and chronic GVHD, engraftment, and survival [10–12].
The current study explored the differences in HCT outcomes using a common immune ablation method (TBI) versus disease-specific regimens (no-TBI) for LD.
Materials and Methods
Data Sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive HCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program Coordinating Center in Minneapolis [5].
Study Population
Eligibility for this study includes patients who were 40 years or older with LD and reported to the CIBMTR after the first allogeneic transplantation with NMA conditioning between 2001 and 2011 were included in analysis. Patients younger than 40 years were excluded as they represented less than 10% of the population. Regimens in the myeloablative or reduced intensity (RIC) range were excluded from this analysis. LD includes CLL or small-lymphocytic lymphoma (SLL), Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL). Recipients of cord blood grafts, ex-vivo T-cell depletion, syngeneic donors and cases with insufficient follow up (100 days, n=59) were excluded. For HCTs from unrelated donor (URD), human leukocyte antigen (HLA) matching at HLA-A, B, C, and DRB1 were included in the well matched category or 8/8 match. Recipients of at least one antigen or allele mismatched were also eligible to the study (partially matched or 7/8 match). NMA conditioning regimen was defined as TBI 200cGY [13]. Furthermore, conditioning regimens were separated in two Flu-based cohorts, with or without TBI. Overall, completeness indexes of follow-up at 3-years was 96% for TBI and 90% for no-TBI cohorts, respectively [14,15].
Diseases were grouped as low-, intermediate- grades and other diseases. Low-grade diseases included CLL, low or intermediate-grade follicular lymphoma, marginal zone lymphoma, and diffuse small cleaved cell lymphoma. Intermediate-grade diseases included high-grade follicular lymphoma, mantle cell lymphoma, diffuse large B-cell lymphoma, and T-cell lymphoma. Other diseases included HL, unknown grade follicular lymphoma, small lymphoplasmacytic lymphoma, mycosis fungoides, and other NHL.
Disease status at transplant defined groups according to disease burden, response to prior therapy and sensitivity to therapy.
Outcomes
Engraftment assessed hematopoietic recovery of neutrophils and platelets following transplant. Neutrophil engraftment was defined as neutrophil recovery 0.5×109/L for three consecutive laboratory values. Platelet engraftment was measured at platelet recovery 20 and 50×109/L without platelet transfusion for seven consecutive days on three consecutive laboratory values on different dates. The modified Glucksberg classification was used to define events of grades II–IV and III–IV acute GVHD in the first 100 days post HCT [16]. Having death without GVHD was considered a competing risk, incidences of acute and chronic GVHD were calculated. Treatment-related mortality (TRM) was defined as death without progression or relapse.
Donor chimerism analysis from peripheral blood or bone marrow source was conducted in between 21–100 days post-HCT. 90% donor chimerism was considered as full donor chimerism [17,18]. Competing risk for achieving full donor chimerism was defined as subsequent transplant, relapse or progression of primary disease, or death without achieving full donor chimerism. Conversely, loss of donor chimerism was defined as <10% donor chimerism. Donor chimerism percentages between 10% and 90% were defined as mixed chimerism. Sorted T-cell and unsorted chimerisms were analyzed separately. For the analysis of temporal achievement of full chimerism, the first day of achieving full donor chimerism was measured.
Statistical Analysis
The main comparison for this study was between TBI and no-TBI regimen. Univariate analysis of neutrophil and platelet recovery, TRM, GVHD and relapse or progression was conducted. Covariates for Cox hazard regression multivariate analysis for overall mortality, TRM, acute and chronic GVHD, disease progression and treatment failure were age, disease grouped by grade and histology, sensitivity to therapy prior to HCT, transplant year, history of prior autologous HCTs, Karnofsky Performance Score (KPS) at time of preparative regimen, use of anti-thymocyte globulin (ATG) and/or campath, use of rituximab before or after HCTs, graft source use, and donor type by HLA match status. Interaction test between the main effect and all covariates was performed and none was identified.
Donor chimerism between days 21–100 post HCT was analyzed in years 2008 and 2011, due to the completeness of the data. Median of days to achieve complete donor chimerism was assessed for groups with and without TBI. Then, proportions of patients achieving full donor chimerism were assessed for three time periods: days 21–45, 46–75, and 76–100. Median donor cell percentage for each time period was also assessed for both sorted T-lymphocyte (lymphoid lineage) and unsorted cell chimerism. If there was no chimerism tested for the time period then the available value from the previous time period were presumed for cumulative donor chimerism achievement. There were no chimerism discrepancies that could modify the chimerism status, i.e. from mixed to full donor chimerism, or from mixed chimerism to loss of chimerism among cases with multiple assessments in a single period.
Practices of NMA regimen often include the same GVHD prophylaxis as a treatment package, making it difficult to test a single component separately. To address this, the most common NMA regimen/GVHD prophylaxis combinations used for CLL, follicular lymphoma, and mantle cell lymphoma were identified and compared in a subset analysis. The method of analysis was the same as for the main comparison.
SQL developer (Oracle, Redwood City, CA) and SAS 9.2 (SAS Institute, Cary, NC) were used for the analyses.
Results
Study Population
As in Table 1, both cohorts were well balanced for age (median age 57 for TBI vs. 56 for no-TBI cohorts, p=0.13), sex (male 74 vs. 67%, p=0.05), donor sex (61 vs. 66%, p=0.16), time from diagnosis to transplant (median 51 vs. 47 months, p=0.31). Recipients of TBI based regimens were more frequently with performance score (KPS) lower than 90% compared to no-TBI (32% vs. 22%, p=0.003). 48 patients (16%) in TBI cohort and 38 patients (9%) in no-TBI cohort received autologous transplant(s) prior to the first allogeneic HCTs (p=0.005).
Table 1.
Patient characteristics according to nomyeloablative conditioning with or without use of total body irradiation prior to hematopoietic cell transplantation for lymphoproliferative diseases.
| Variable | TBI (%) | No-TBI (%)1 | P-value |
|---|---|---|---|
| Number of patients | 382 | 515 | |
| Number of centers | 53 | 80 | |
| Age, years (Median, 95% CI) | 57 (40–75) | 56 (40–74) | 0.13 |
| Male | 281 (74) | 346 (67) | 0.047 |
| Disease group2 | 0.81 | ||
| Low grade | 219 (56) | 297 (58) | |
| Intermediate grade | 135 (35) | 175 (34) | |
| Other | 28 (7) | 43 (8) | |
| Sensitive/ Resistant to therapy prior to transplant | <0.001 | ||
| Sensitive | 182 (48) | 310 (60) | |
| Resistant | 59 (15) | 70 (14) | |
| Other/Unknown | 141 (37) | 145 (26) | |
| Disease at transplant | <0.001 | ||
| CR-1 | 32 (8) | 28 (5) | |
| PR-1 | 50 (13) | 63 (12) | |
| =>CR2 | 67 (18) | 133 (26) | |
| =>PR-2 | 36 (9) | 49 (10) | |
| Relapse | 104 (27) | 159 (31) | |
| Stable disease | 16 (4) | 20 (4) | |
| Progressive disease | 17 (4) | 23 (4) | |
| CLL with unknown status | 54 (14) | 26 (5) | |
| Unknown status | 6 (2) | 14 (3) | |
| Time from diagnosis to transplant (month) | 51 (2.5–413) | 47 (2.2–325) | 0.31 |
| Year of transplant | <0.001 | ||
| 2001–2004 | 153 (40) | 228 (45) | |
| 2005–2008 | 194 (51) | 219 (43) | |
| 2009–2011 | 35 (9) | 68 (13) | |
| Bone marrow graft source | 14 (5) | 57 (14) | <0.001 |
| Donor | <0.001 | ||
| HLA-identical sibling | 121 (29) | 253 (46) | |
| URD well-matched (8/8) | 205 (49) | 212 (38) | |
| URD partially matched (7/8) | 56 (13) | 50 (9) | |
| D-R CMV match status | 0.003 | ||
| Pos/pos | 83 (22) | 167 (32) | |
| Pos/neg | 40 (10) | 55 (11) | |
| Neg/pos | 131 (34) | 137 (27) | |
| Neg/neg | 122 (32) | 142 (27) | |
| Unknown status | 6 (2) | 14 (3) | |
| Prior autologous transplant | 96 (25) | 71 (14) | <0.001 |
| Conditioning regimen | <0.001 | ||
| Flu + TBI <= 200 cGY dose | 382 | - | |
| Flu TBI (no Cy) | 341 (89) | - | |
| Flu Cy TBI | 41 (11) | - | |
| Flu + Cy | - | 493 (96) | |
| Flu + others3 | - | 22 (4) | |
| Rituximab use | 153 (40) | 320 (62) | <0.001 |
| During conditioning regimen | 26 (7) | 206 (40) | |
| GVHD prophylaxis | <0.001 | ||
| CNI + MTX +/− other | 24 (6) | 335 (65) | |
| CNI + MMF +/− other | 334 (87) | 78 (15) | |
| CNI +/− other | 10 (3) | 96 (19) | |
| Other | 14 (4) | 6 (1) | |
| Median follow-up (range) | 26 (<1–134) | 26 (<1–145) | - |
Abbreviations: CMV, cytomegalovirus serologic status; CR, complete remission; CNI, calcineurin inhibitor; Cy, cyclophosphamide; R-R; donor to recipient; Flu, fludarabine; MMF, mycophenolate mofetil; MTX, methotrexate; PR, partial remission, TBI, total body irradiation; URD, unrelated donor.
TBI dose=200 cGY.
Low-grade diseases included CLL, low or intermediate grade follicular lymphoma, marginal zone lymphoma, and diffuse small cleaved cell lymphoma. Intermediate-grade diseases included high-grade follicular lymphoma, mantle cell lymphoma, diffuse large B-cell lymphoma, and T-cell lymphoma. Other diseases included Hodgkin lymphoma (HL), unknown grade follicular lymphoma, small lymphoplasmacytic lymphoma, mycosis fungoidies, and other non-Hodgkin lymphoma (NHL)
Fludarabine + Others group consisted of 5 cases of fludarabine + ATG, 7 cases of fludarabine + campath, 3 cases of fludarabine + rituximab, 2 cases of fludarabine + anthracyclines, 1 case of fludarabine + melphalan, and 4 cases of fludarabine alone.
TBI was performed less frequently later in the period (years 2009–2011 9% vs. 11%, p<0.001), included more recipients of URD (62% vs. 47%, p<0.001), PBSC (94% vs. 85%, p<0.001), less frequently used rituximab in the conditioning (7% vs. 40%, p<0.001) and used more frequently CNI and MMF based combination of GVHD prophylaxis (87% vs. 15%, p<0.001). Furthermore, TBI cohort had more CMV mismatches (44% vs. 38%, p=0.003) and had less cases with sensitivity to prior therapy (48% vs. 60%, p<0.001) as well as less cases with complete remission at the time or preparative regimen (33% vs. 25%, p<0.001).
Hematologic Recovery
In the TBI cohort, 40% and 60% never dropped neutrophil and platelet counts below the level of engraftment compared to 24% and 43% in the no-TBI group, respectively. Neutrophil recovery by day 28 post HCT was >95% for both groups (p=0.12). Platelet recovery 20×109/L by day 100 were 84% (95% Confidence Interval [CI], 77–85%) and 92% (95% CI, 88–94%, p=0.03) for TBI and no-TBI cohorts respectively. Corresponding incidences of platelet recover 50×109/L by day 100 were 79% (95% CI, 70–85%) and 92% (95% CI, 88–95%, p<0.001).
Acute and Chronic GVHD
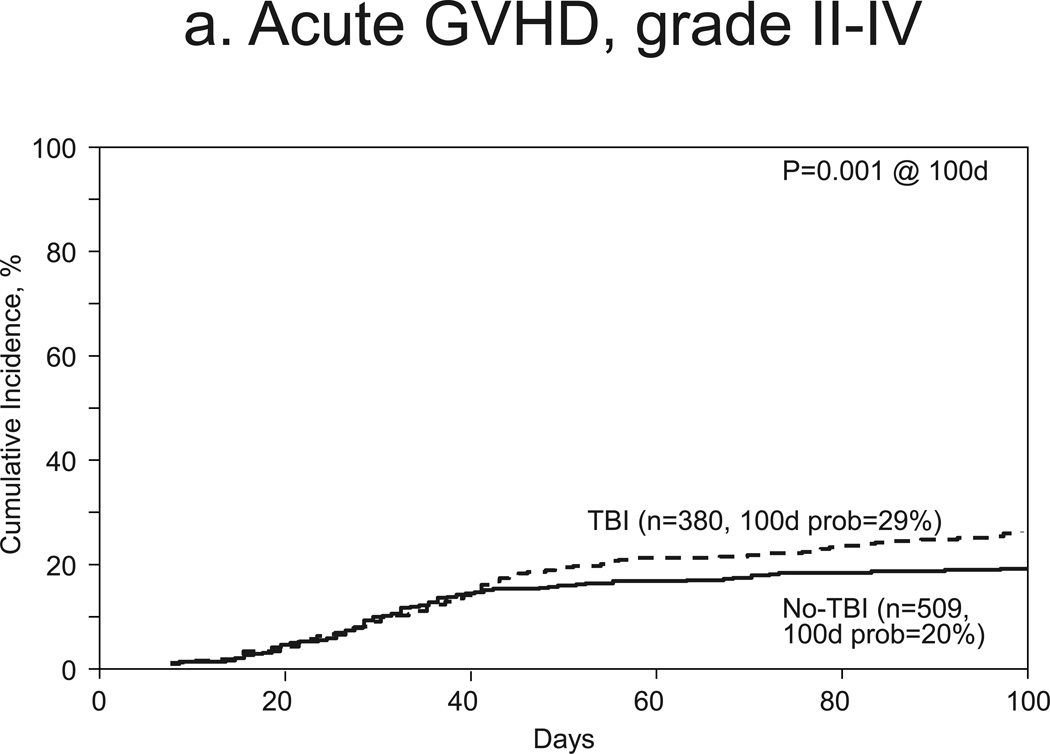
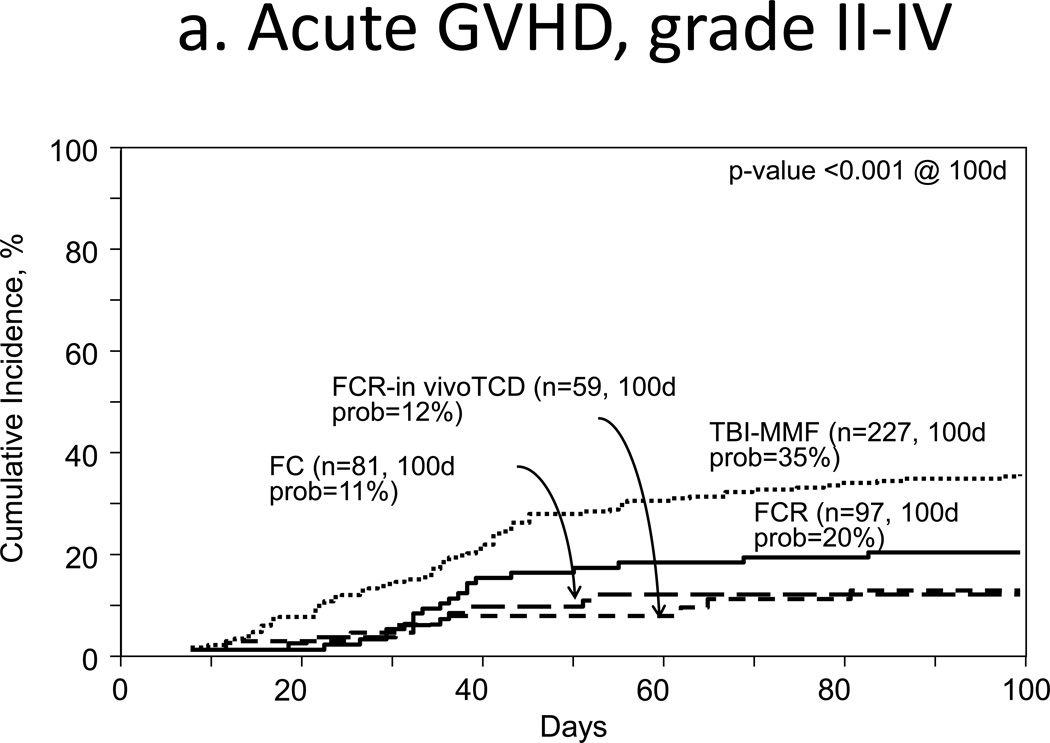
Incidences for grades II–IV acute GVHD at 100 days were 29% (95% CI, 25–34%) and 20% (95% CI, 16–23%, p=0.001) for TBI and no-TBI groups, respectively (Fig 1a). Corresponding incidences of grade III–IV acute GVHD were 13% (95% CI, 10–17%) and 7% (95% CI, 5–9% p=0.002). Multivariate analysis of grade II–IV acute GVHD confirmed a higher incidence with TBI (HR 1.51, 95% CI 1.19–2.00, p=0.001, Table 2). Other covariates associated with this outcome included no prior history of autologous transplant (HR 12.11, 95% CI 4.95–29.63, p<0.001) and resistance to (HR 2.01, 95% CI, 1.35–2.99, p<0.001) or no known prior treatment (HR 1.933, 95% CI 1.47–2.55, p<0.001, Table 2).
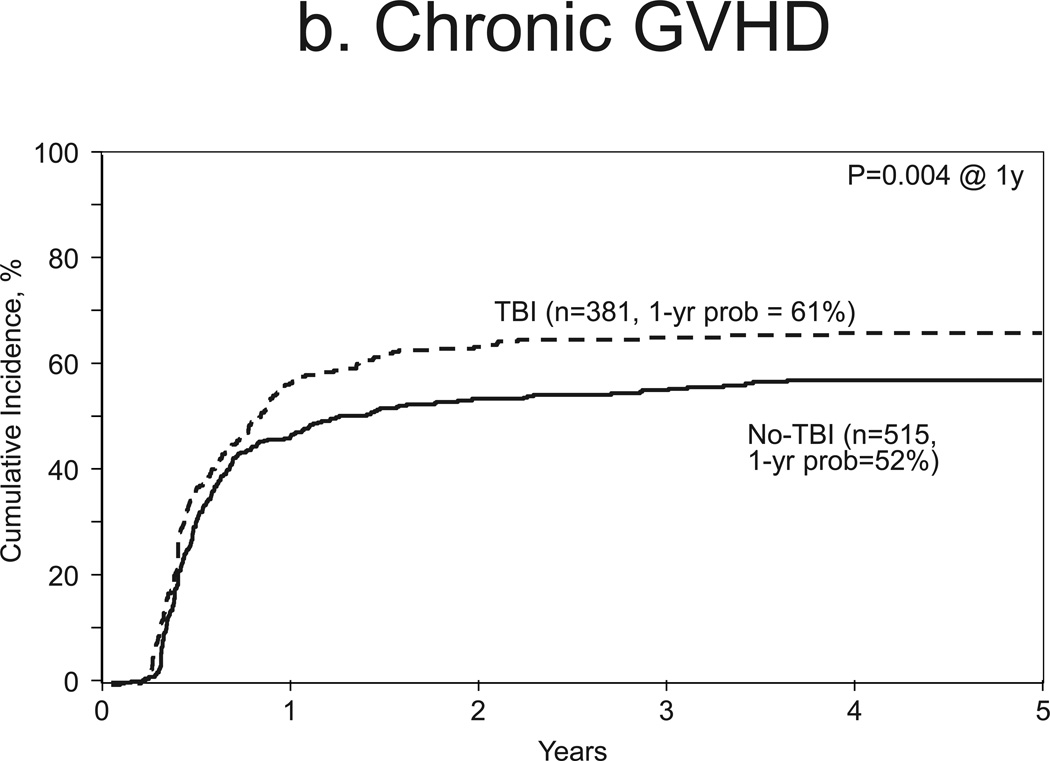
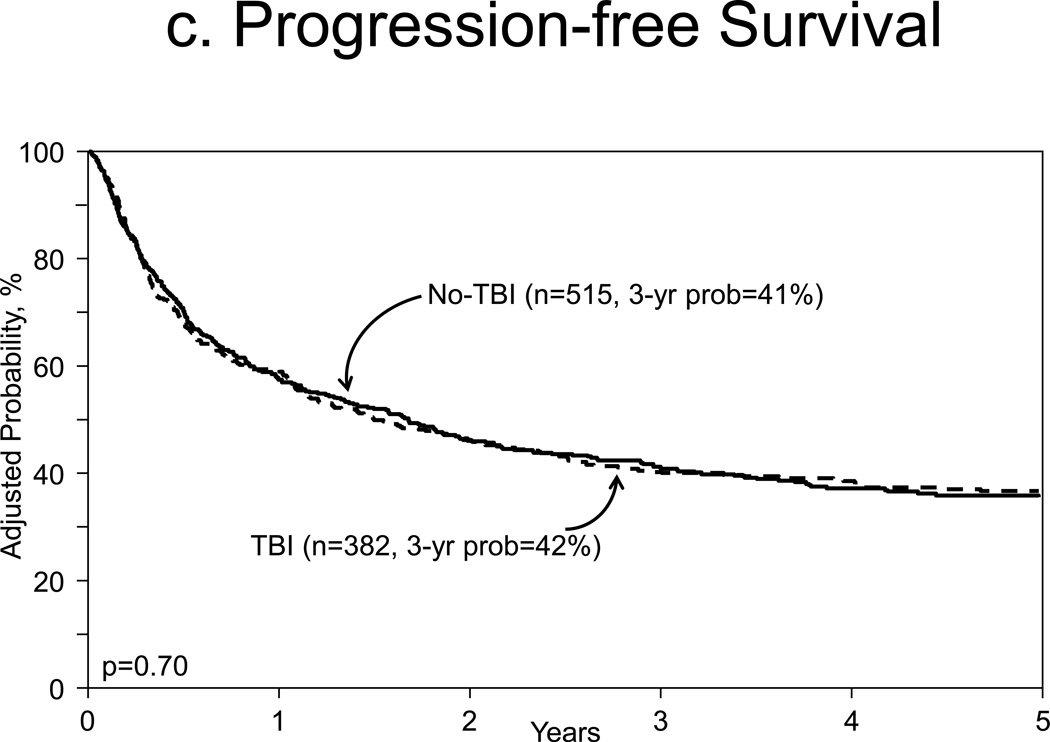
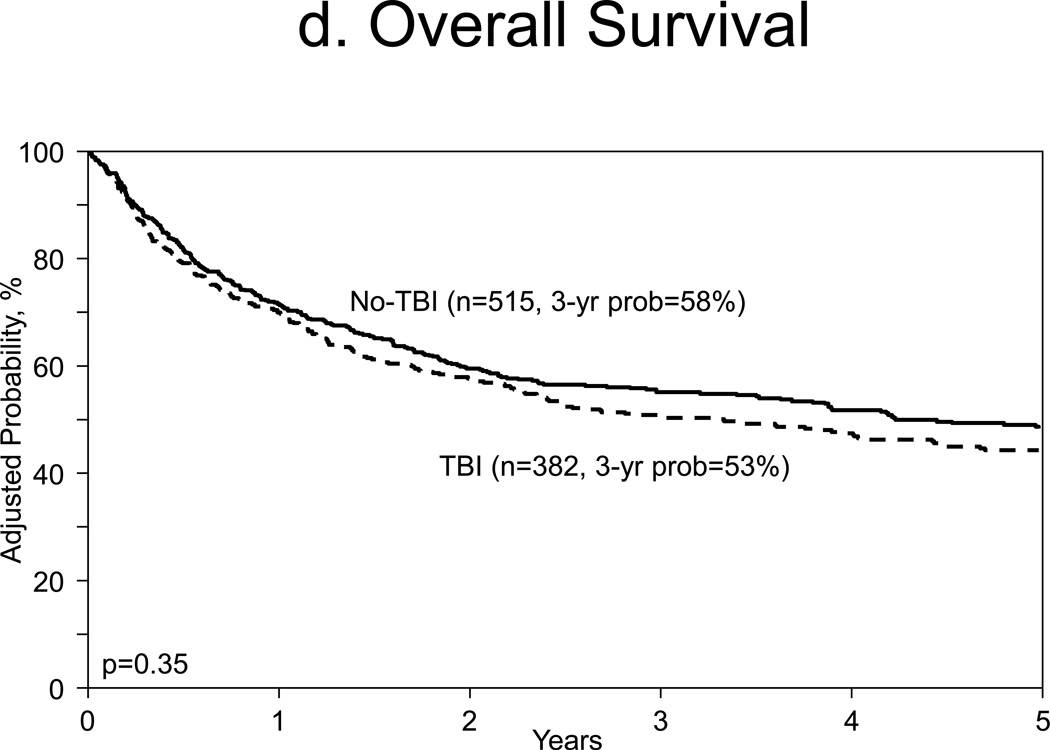
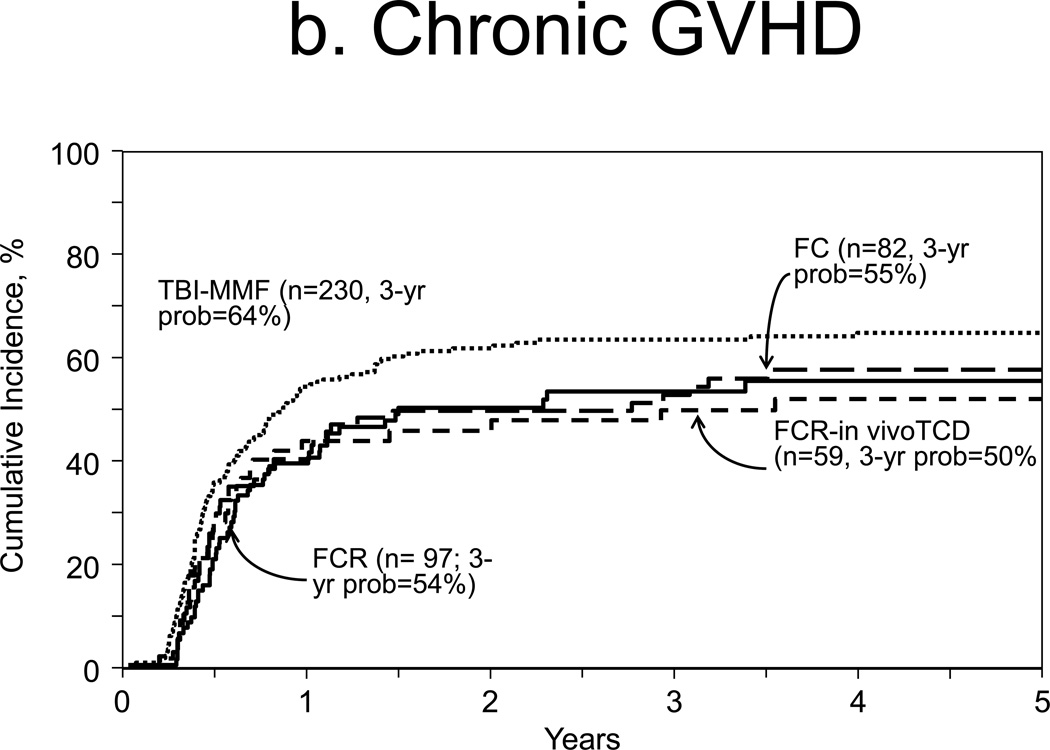
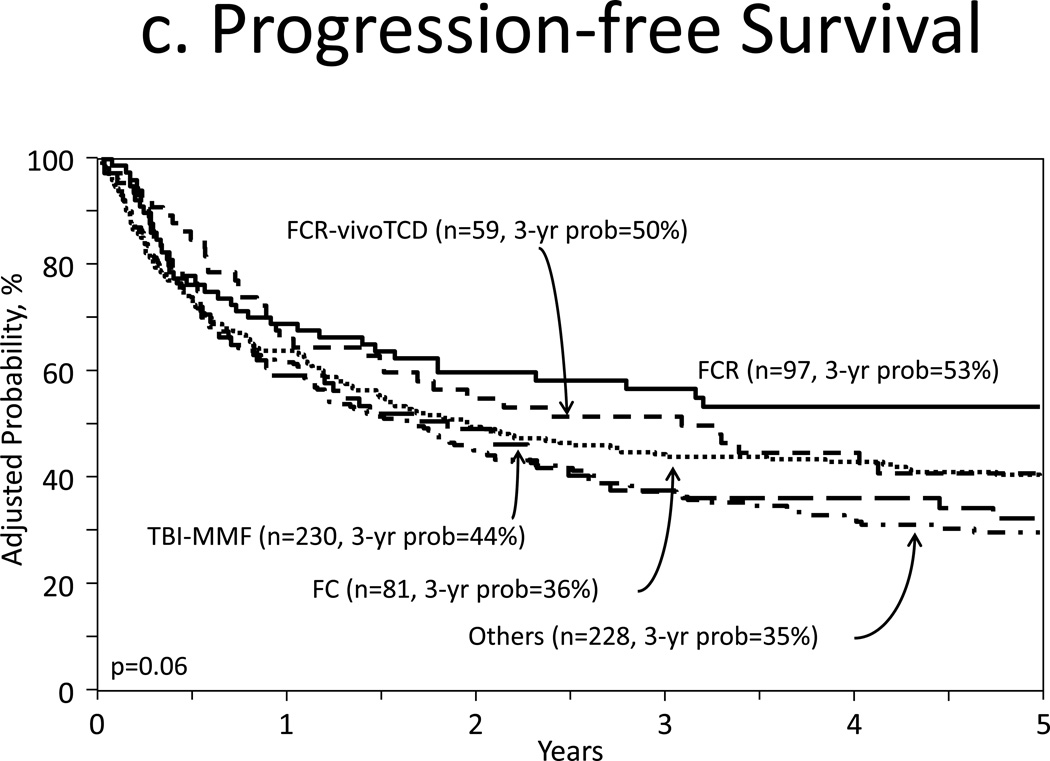
Figure 1.
Outcomes
a. Acute GVHD, grades II–IV
b. Chronic GVHD
c. Adjusted progression free survival
d. Adjusted overall survival
Table 2.
Multivariate Analysis
| Factor | Level | n | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-value | |
|---|---|---|---|---|---|---|
| Overall Mortality^ | 897 | |||||
| Regimen | No-TBI | 515 | 1 | |||
| TBI | 382 | 1.09 | 0.91 | 1.32 | 0.35 | |
| Age | 40 – 49 | 217 | 1 | <0.001* | ||
| 50 – 59 | 406 | 1.31 | 1.03 | 1.66 | 0.03 | |
| ≥ 60 | 274 | 1.66 | 1.28 | 2.15 | <0.001 | |
| KPS | 90 – 100 | 570 | 1 | <0.001* | ||
| < 90 | 234 | 1.49 | 1.21 | 1.83 | <0.001 | |
| Unknown | 93 | 1.42 | 1.07 | 1.90 | 0.016 | |
| Donor type | HLA-identical sibling | 374 | 1 | <0.001* | ||
| 8/8 matched unrelated donors | 417 | 1.28 | 1.04 | 1.58 | 0.021 | |
| 7/8 matched unrelated donors | 106 | 1.94 | 1.46 | 2.59 | <0.001 | |
| Treated-related mortality$ | 897 | |||||
| Regimen | No-TBI | 515 | 1 | |||
| TBI | 382 | 1.10 | 0.85 | 1.41 | 0.48 | |
| Age | 40 – 49 | 217 | 1 | 0.012* | ||
| 50 – 59 | 406 | 1.11 | 0.80 | 1.55 | 0.54 | |
| ≥ 60 | 274 | 1.59 | 1.12 | 2.24 | 0.009 | |
| Donor type | HLA-identical sibling | 374 | 1 | <0.001* | ||
| 8/8 matched unrelated donors | 417 | 1.42 | 1.06 | 1.92 | 0.021 | |
| 7/8 matched unrelated donors | 106 | 2.63 | 1.79 | 3.87 | <0.001 | |
| Progression/Relapse% | 897 | |||||
| Regimen | No-TBI | 515 | 1 | |||
| TBI | 382 | 1.05 | 0.83 | 1.33 | 0.68* | |
| KPS | 90 – 100 | 570 | 1 | 0.011 | ||
| < 90 | 234 | 1.42 | 1.10 | 1.83 | 0.007 | |
| Unknown | 93 | 1.40 | 0.99 | 1.98 | 0.05 | |
| In vivo T-cell depletion | No use of in-vivo T-cell depletion | 628 | 1 | |||
| In-vivo T-cell depletion used | 269 | 1.55 | 1.22 | 1.97 | <0.001 | |
| Treatment failure& | 897 | |||||
| Regimen | No-TBI | 515 | 1 | |||
| TBI | 382 | 0.97 | 0.81 | 1.15 | 0.70 | |
| KPS | 90 – 100 | 570 | 1 | 0.005* | ||
| < 90 | 234 | 1.34 | 1.10 | 1.62 | 0.003 | |
| Missing | 93 | 1.33 | 1.01 | 1.75 | 0.04 | |
| Donor type | HLA-identical sibling | 374 | 1 | <0.001* | ||
| 8/8 matched unrelated donors | 417 | 1.21 | 1.00 | 1.47 | 0.05 | |
| 7/8 matched unrelated donors | 106 | 1.79 | 1.36 | 2.34 | <0.001 | |
| Acute GVHD Grade II–IV^^ | 896 | |||||
| Regimen | No-TBI | 515 | 1 | |||
| TBI | 382 | 1.54 | 1.19 | 2.00 | 0.001 | |
| KPS | 90 – 100 | 570 | 1 | 0.016* | ||
| < 90 | 234 | 1.45 | 1.09 | 1.92 | 0.010 | |
| Missing | 93 | 1.50 | 0.97 | 2.33 | 0.07 | |
| Sensitivity | Sensitive | 492 | 1 | <0.001* | ||
| Resistant | 129 | 2.01 | 1.35 | 2.99 | <0.001 | |
| Untreated/unknown/CLL | 276 | 1.93 | 1.47 | 2.55 | <0.001 | |
| History of prior autologous transplant | Previously received autologous transplant | 167 | 1 | |||
| No history of prior autologous transplant | 730 | 12.11 | 4.95 | 29.63 | <0.001 | |
| Chronic GVHD$$ | 896 | |||||
| Regimen | No-TBI | 514 | 1 | |||
| TBI | 382 | 1.32 | 1.10 | 1.59 | 0.003 | |
| Donor type | HLA-identical sibling | 373 | 1 | 0.003* | ||
| 8/8 matched unrelated donors | 417 | 1.39 | 1.13 | 1.71 | 0.002 | |
| 7/8 matched unrelated donors | 106 | 1.50 | 1.08 | 2.08 | 0.015 | |
| In vivo T-cell depletion | No use of in-vivo T-cell depletion | 627 | 1 | |||
| In-vivo T-cell depletion used | 269 | 0.60 | 0.47 | 0.75 | <0.001 | |
overall p-value
Model stratified by year of transplant and sensitivity. Other pair-wise contrasts: age (p-value= 0.024), donor type (p-value= 0.002)
Model stratified by year of transplant and sensitivity. Other pair-wise contrasts: age (p-value= 0.015), donor type (p-value <0.001)
Model stratified by disease group and sensitivity.
Model stratified by disease group, year of transplant, and sensitivity. Other pair-wise contrasts: donor type (p-value= 0.003)
Other pair-wise contrasts: sensitivity (p-value= 0.84)
Other pair-wise contrasts: donor type (p-value= 0.62)
Incidences of chronic GVHD at 1-year were 54% and 44% for TBI (95% CI 48–59%) and no-TBI groups (95% CI 39–48%), respectively (p=0.004, Figure 1b). Multivariate analysis showed higher rates of chronic GVHD with TBI (HR 1.32, 95% CI 1.10–1.59, p=0.003, Table 2) compared to no-TBI. Other covariates associated with this outcomes were partially matched URD graft source (HR 1.50, 95% CI, 1.08–2.08, p=0.015) and ATG or campath use (HR 0.60, 95% CI, 0.47–0.75, p<0.001, Table 2).
Transplant Related Mortality and Disease Progression
Cumulative incidence of TRM at 1 year were 18% (95% CI, 14–22%) and 16% (95% CI, 13–19%, p=0.36) for TBI and no-TBI, respectively. Multivariate analysis of TRM showed no difference between TBI and no-TBI cohorts (p=0.48, Table 2). Other covariates associated with TRM were: increasing patient age (age 60, HR 1.58 95% CI, 1.12–2.24, p=0.009) and partially matched URD source graft (HR 2.63, 95% CI, 1.79–3.87, p<0.001, Table A).
Incidences of disease progression or relapse at 5 years were 36% (95% CI, 31–41%) and 38% (95% CI, 33–42% p=0.16) for TBI and no-TBI cohorts, respectively. Multivariate analysis of progression/relapse showed no difference between two cohorts (p=0.68, Table A). Other covariates with significant association were KPS <90% (HR 1.42, 95% CI, 1.10–1.983, p=0.007) and use of ATG or campath (HR 1.55, 95% CI, 1.22–1.97, p<0.001, Table A).
Survival
Figure 1c and 1d illustrated the adjusted PFS and adjusted OS, respectively. Multivariate analysis for treatment failure preventing PFS were similar for both cohorts (HR 0.97, 95% CI, 0.81–1.51, p=0.70, Table 2). Associated covariates for treatment failure were KPS <90% (HR 1.34, 95% CI, 1.10–1.62, p=0.041) and partially matched URD source graft (HR 1.79, 95% CI 1.36–2.34, p<0.001, Table A).
Multivariate analysis for overall mortality were similar for both cohorts (HR 1.09, 95% CI, 0.91–1.32, p=0.35, Table A). Additional covariates that were associated with overall mortality in main analysis were: patient age ≥60 at transplant (HR 1.66, 95% CI, 1.28–2.15, p<0.001), KPS <90 (HR 1.49, 95% CI, 1.21–1.83, p<0.001), and partially matched URD graft source use (HR 1.94, 95% CI, 1.46–2.59, p<0.001, Table A).
Causes of Death
Primary disease was the most common cause of death in both cohorts. TBI group had more deaths due primarily to GVHD (17% vs. 8%) and organ failure (15% vs. 12%) and no-TBI group had more deaths from infection (18 vs. 11%) compared to TBI (p<0.001). Reviewing contributing causes of death, the similar trend was found. TBI cohort had 16% and no-TBI cohort had 9% deaths contributed by GVHD (p=0.007). However, deaths related to infection were similar between two cohorts (16% in TBI and 15% in no-TBI cohorts, p=0.92).
Chimerism Analysis
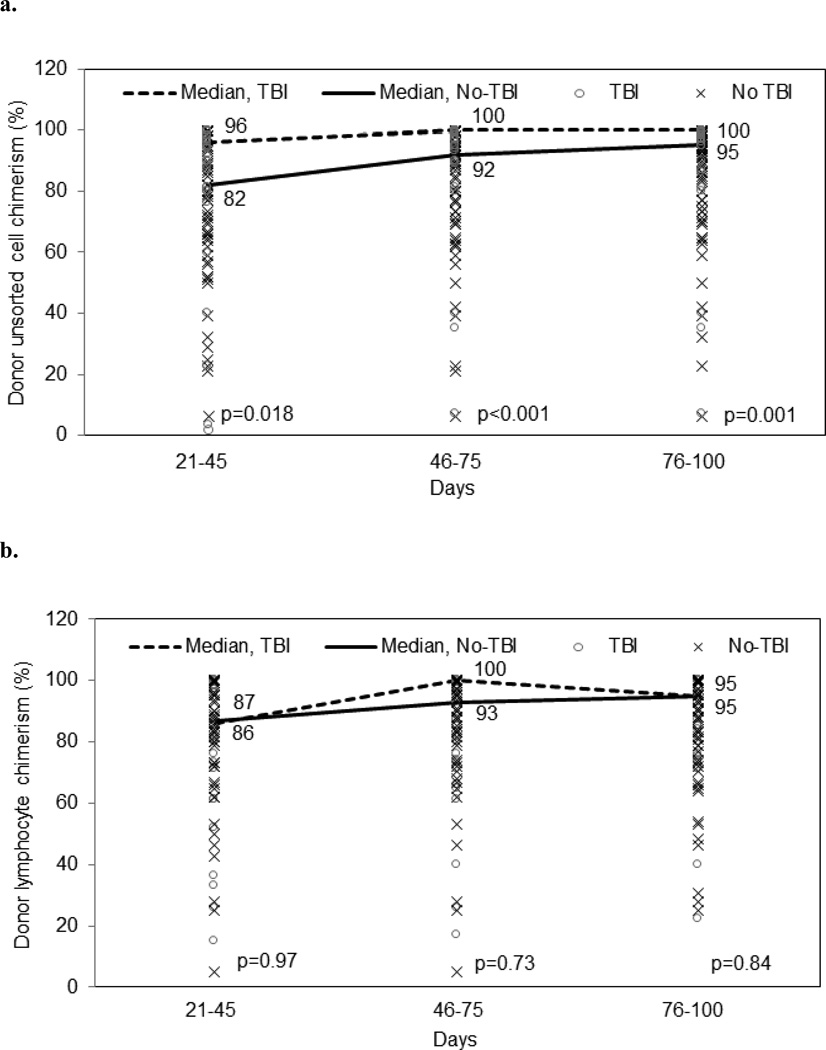
Chimerism analysis with unsorted specimen was available in 62 and 88 patients; and T-cell chimerism was available in 34 and 70 patients in the TBI and no-TBI groups, respectively. Among unsorted chimerism, achievement of full donor chimerism by day 100 was observed in 82% and 64% in the TBI and no-TBI cohorts, respectively (p=0.006). Loss of donor chimerism after initially achieving full donor chimerism was not observed in any cohort. 10% vs. 31% of TBI and no-TBI cohorts achieved only mixed chimerism. Median donor chimerism by day 100 was 100% vs. 95% for TBI and no-TBI groups, respectively (Figure 2a). The median donor chimerism among patients with mixed unsorted chimerism from both treatment groups at the last period analyzed (day 76–100) was 79% (N= 26, range 32–89%) for all patients. Among patients with lymphocyte chimerism, achievement of full donor chimerism by day 100 was observed in 53% and 59% in the TBI and no-TBI groups respectively (p=0.027). Loss of T-cell donor chimerism was not found in any cohort. 26% vs. 37% of TBI and no-TBI maintained mixed T-cell chimerism during the period. Median donor T-cell chimerism by day 100 was 94.5% and 95% for TBI and no-TBI groups (p=0.84) (Figure 2b). The median donor chimerism among patients with mixed T-cell chimerism from both treatment groups at the last period analyzed (day 76–100) was 70% (N= 37, range 19–89%) for all patients.
Figure 2.
Achievement of donor chimersim
a. Unsorted chimerism
b. Sorted lymphocyte chimerism
Subset Analysis
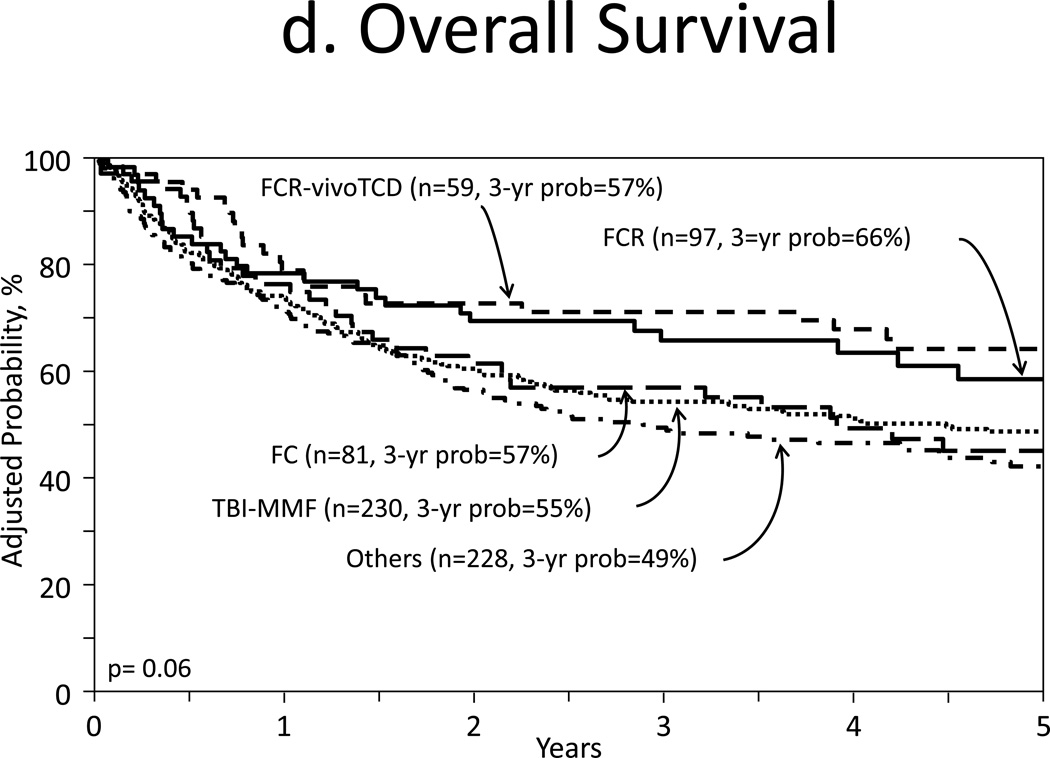
Five groups were analyzed: flu/ TBI-CNI-MMF (TBI-MMF cohort, n=230), flu/cy/rituximab-CNI- methotrexate (MTX) (FCR cohort, n=97), FCR-CNI-MTX-in vivo T-cell depletion including campath and rabbit or horse ATG (FCR-in vivo TCD cohort, n=59), flu/cy-CNI-MTX (FC cohort, n=82), and others (n=231).
When comparing the above groups, FCR contained more cases of follicular lymphoma (61% vs. 21–31%, p=0.031) in relapsed status (37% vs. 25–29%, p<0.001). TBI-MMF and FCR-in vivo TCD groups include more URD recipients (67% and 98% respectively, vs. 25–28%, p<0.001). TBI-MMF and FC groups included less number of patients with KPS 90% (31% and 26% respectively, vs. 7–11%, p<0.001).
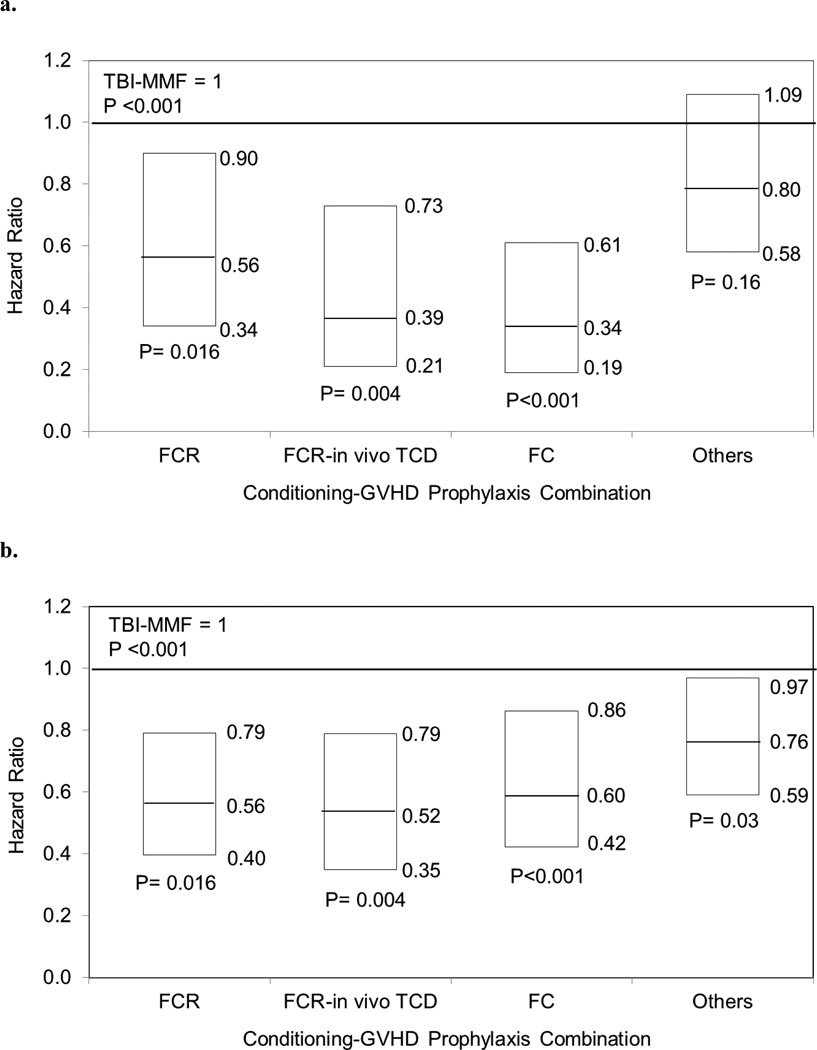
The cumulative incidences of acute GVHD, grades II to IV at 100 days were 35% of TBI-MMF (95% CI 29–42%), 20% of FCR (95% CI 12–28% for both), 12% of FC (95% CI 5–22%), 11% of FCR-in vivo TCD (95% CI 5–19%), and 29% of others (95% CI 23–35, p<0.001, Figure 3a). Chronic GVHD at 1 year in TBI-MMF cohort was 55% and in other cohorts was 41–48% (p=0.11, Figure 3b,4a). Multivariate analysis demonstrated that TBI-MMF was associated with higher incidence of chronic GVHD (Figure 4b).
Figure 3.
Outcomes of regimen-GVHD prophylaxis combinations
a. Acute GVHD, grades II–IV
b. Chronic GVHD
c. Adjusted progression free survival
d. Adjusted overall survival
Figure 4.
Multivariate analysis of regimen-GVHD prophylaxis combinations
a. Acute GVHD, grades II–IV
b. Chronic GVHD
TRM (p=0.053), progression/relapse (p=0.58), PFS (p=0.11), and OS (p=0.06) showed no statistical difference from multivariate analyses among five groups (Figures 3c, 3d).
Discussion
NMA HCTs with or without TBI have been used to treat patients with LD. This study demonstrated that use of a general immunoablative regimen with TBI results in no major differences in post-HCT outcomes compared to disease specific regimens using chemotherapy. Additionally, TBI appears to cause less ablation of hematopoiesis as a greater proportion of patients do not become neutropenic compared to chemotherapy only regimens. This translated in faster time to full donor chimerism, but no difference in proportion of donor chimerism by day 100. One important difference in outcomes noted in this study was a higher rate of GVHD with TBI regimens, which maintained after adjusting to differences in the donor type and graft source between the two cohorts. Subset analysis suggests that the choice of GVHD prophylaxis can modulate this complication post HCT as MMF based regimens associated with higher GVHD rates compared to other combinations.
Other single center experiences with NMA regimens in specific LD showed that OS and PFS for CLL (2-year OS 60% and DFS 52%) and follicular lymphoma (2-year DFS of 64% in high grade and 83% in low grade) were comparable with this study [8,10,19]. Also, median survival for patients with advanced CLL or follicular lymphoma utilizing low dose TBI (OS 64–86% at 4 years) from previous studies were comparable, when contrasted to other conditioning regimens without TBI (OS 46–84% at 4–5 years) [8,10]. Three-year rates of relapse were comparable with other reported studies for follicular lymphomas of 20–45% on average, although some studies had relapse rates 10% at 2–3 years [9,11,20].
In this study, increased acute GVHD incidence in TBI group could also be the effect of donor chimerism, suggested by a faster achievement of full donor chimerism [7,20,21]. Despite similar T-cell donor chimerism and day 100 donor chimerism between the groups, makes this argument less plausible. Alternatively, the cohorts differed in GVHD prophylaxis regimens, which could be responsible for the difference of acute GVHD incidence. For chronic GVHD, both use of ATG or campath and HLA-matching were associated with lower rates of chronic GVHD as previously observed [23]. Furthermore, the use of rituximab was more common in the no-TBI cohort which could mitigate the incidence of GVHD. Exposure to rituximab was tested in the statistical models and it was not significantly associated with any of the outcomes tested.
Moreover, a systematic review of 11 studies showed increased incidence of grades III–IV acute GVHD for patients received MMF when compared to those received MTX for GVHD prophylaxis (RR 1.61, 95% CI 1.18–2.30) [23]. Chen G et al. reported that tacrolimus (TAC)/MMF resulted in higher grades III–IV acute GVHD (49%) when compared with TAC/MTX (19%), TAC/micro-MTX/MMF (23%) following myeloablative conditioning (p<0.015) [25–26]. Also, this is comparable with other single-center NMA and MA studies which revealed that MMF-based GVHD prophylaxis increased incidences of acute GVHD (11.6–54.5%) when compared with other regimen [25–27].
In studying TBI-based NMA conditioning regimen, Kornbilt et al explained that addition of Flu-TBI (2 Gy) reduced graft rejection by hastening donor chimerism [28]. Although both groups with and without fludarabine demonstrated near-complete donor granulocyte chimerism, median number of days of absolute granulocyte counts 500 cells/L was higher in Flu/TBI.
The current study is a retrospective analysis from a large number of transplant centers. The major pitfall is based on the decision to select a particular regimen, which was based on clinical evaluation by the treating physician. The selection of the study population separated by conditioning regimens demonstrated differences between groups. Several approaches were taken to minimize the heterogeneity of the population, including restricting the age to patients older than 40, selection of common indications and excluding practices that are not commonly performed, such as T-cell depletion, cord blood and use of mismatched URD. Furthermore, the regression analysis was able to adjust for the remainder differences between the cohorts.
Nevertheless, this study was a large comparison of NMA regimens among HCT recipients with LD. This study demonstrated that TBI based and non-TBI based NMA regimens with fludarabine achieved similar disease control and survival outcomes. TBI regimens lead to faster full donor chimerism. Lastly the results of this study raises the hypotheses that differences on GVHD rates observed were possibly related to the specific GVHD prophylaxis most commonly used with TBI NMA regimens.
Acknowledgement
We thank Professor John Klein for the valuable input on the design of this study who had contributed significantly for the development of this study. We would like to dedicate this study to Dr. Klein’s memory. The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the NHLBI and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare.
References
- 1.McSweeney P, Niederwieser D, Schizuru J. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 2.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoieitc cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancie. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 3.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose TBI and fludarabine followed by HCT from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and MMF can induce durable complete chimerism and sustained remissions in patients with hematologic diseases. Blood. 2003;16(3):384–394. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP, et al. Stable Mixed Hematopoietic Chimerism in DLA-identical Littermate Dogs Given Sublethal Total Body Irradiation Before and Pharmacological Immunosuppression After Marrow Transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 5.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org. [Google Scholar]
- 6.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 7.Baron F, Baker J, Storb R. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 8.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouri IF. Allogeneic Stem Cell Transplantation in Follicular Lymphoma. Best Practice & Research Clinical Haematology. 2011;24:271–277. doi: 10.1016/j.beha.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laport G. The Role of Hematopoietic Cell Transplantation for Follicular Non-Hodgkin’s Lymphoma. Biolo Blood Marrow Transplant. 2005;12:59–65. doi: 10.1016/j.bbmt.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Besien K. The evolving role of autologous and allogeneic stem cell transplantation in follicular lymphoma. Blood Reviews. 2006;20:235–244. doi: 10.1016/j.blre.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Banerjii V, Johnston J, Seftel M. The role of hematopoietic stem cell transplantation in Chronic Lymphocytic Leukemia. Transfusion and Apheresis Science. 2007;37:57–62. doi: 10.1016/j.transci.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corazziari I, Mariotto A, Capocaccia R. Correcting the completeness bias of observed prevalence. Tumori. 1999;85:370–381. doi: 10.1177/030089169908500503. [DOI] [PubMed] [Google Scholar]
- 15.Capocaccia R, De Angelis R. Estimating the completeness of prevalence based on cancer registry data. Stat Med. 1997;16:425–440. doi: 10.1002/(sici)1097-0258(19970228)16:4<425::aid-sim414>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Bashey A, Owar K, Johnson JL, Edwards PS, Kelly M, Baxter-Lowe LA, et al. Reduced-Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation for Patients with Hematologic Malignancies Who Relapse following Autologous Transplantation: A Multi-institutional Prospective Study from the Cancer and Leukemia Group B. Biol Blood Marrow Transplant. 2011;17(4):558–565. doi: 10.1016/j.bbmt.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauter CS, Barker JN, Lechner L, Zheng J, Devline SM, Papdopoulos EB, et al. A Phase II Study of a Nonmyeloablative Allogeneic Stem Cell Transplant with Peritransplant Rituximab in Patients with B Cell Lymphoid Malignancies: Favorably Durable Event-Free Survival in Chemosensitive Patients. Biol Blood Marrow Transplant. 2014;20(3):354–360. doi: 10.1016/j.bbmt.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusumi E, Kami M, Kanda Y, et al. Reduced-intensity hematopoietic stem-cell transplantation for malignant lymphoma: a retrospective survey of 112 adult patients in Japan. Bone Marrow Transplant. 2005;36:205–213. doi: 10.1038/sj.bmt.1705027. [DOI] [PubMed] [Google Scholar]
- 20.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight Year-Experience with Allogeneic Stem Cell Transplantation for Relapsed Follicular Lymphoma after Nonmyeloablative Conditioning wiht Fludarabine, Cyclophosphamide, and Rituximab. Blood. 2008;111:5530–5336. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson J, Uzunel M, Remberger M, Ringen O. T Cell Mixed Chimerism Is Significantly Correlated to A Decreased Risk of Acute Graft-Versus-Host Disease After Allogeneic Stem Cell Transplantation. Transplantation. 2001;71(3):433–439. doi: 10.1097/00007890-200102150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Huss R, Deeg HJ, Gooley T, Bryant E, Leisenring W, Clift R, et al. Effect of mixed chimerism on graft-versus-host disease, disease recurrence and survival after HLA-identical marrow transplantation for aplastic anemia or chronic myelogenous leukemia. Bone Marrow Transplant. 1996;18(4):767–776. [PubMed] [Google Scholar]
- 23.Sykes M, Preffer F, McAfee S, Saidman SL, Weymouth D, Andrews DM, et al. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 24.Ram R, Yeschurun M, Vidal L, Shpilberg O, Gafter-Gvili A. Mycophenolate mofetil vs. methotrexate for the prevention of graft-versus-host-disease - Systematic review and meta-analysis. Leuk Res. 2013;38(3):352–360. doi: 10.1016/j.leukres.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Chen GL, Zhang T, Hahn Y, Abrams S, Ross M, Liu H, et al. Acute GVHD prophylaxis with standard-dose, micro-dose. Bone Marrow Transplant. 2014;49:248–253. doi: 10.1038/bmt.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:581–588. doi: 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabry W, Le Blanc R, Labbe AC, Sauvageau G, Couban S, Kiss T, et al. Graft-versus-Host Disease Prophylaxis with Tacrolimus and Mycophenolate Mofetil in HLA-Matched Nonmyeloablative Transplant Recipients Is Associated with Very Low Incidence of GVHD and Nonrelapse Mortality. Biol Blood Marrow Transplant. 2009;15:919–929. doi: 10.1016/j.bbmt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Kornblit B, Maloney DG, Storb R. Fludarabine and 2-Gy TBI is Superior to 2 Gy TBI as Conditioning for HLA-Matched Related Hematopoietic Cell Transplantation: A Phase III Randomized Trial. Biol Blood Marrow Transplant. 2013;19(9):1340–1347. doi: 10.1016/j.bbmt.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]