Abstract
Tissue Inhibitor of Metalloproteinases-3 (TIMP3) is a tumor suppressor and a potent inhibitor of angiogenesis. TIMP3 exerts its anti-angiogenic effect via a direct interaction with vascular endothelial growth factor (VEGF) receptor-2 (KDR) and inhibition of proliferation, migration and tube formation of endothelial cells (ECs). TIMP3 has also been shown to induce apoptosis in some cancer cells and vascular smooth muscle cells via MMP inhibition and caspase-dependent mechanisms. In this study, we examined the molecular mechanisms of TIMP3-mediated apoptosis in endothelial cells. We have previously demonstrated that mice developed smaller tumors with decreased vascularity when injected with breast carcinoma cells overexpressing TIMP3, than with control breast carcinoma cells. TIMP3 overexpression resulted in increased apoptosis in human breast carcinoma (MDA-MB435) in vivo but not in vitro. However, TIMP3 could induce apoptosis in endothelial cells (ECs) in vitro. The apoptotic activity of TIMP3 in ECs appears to be independent of MMP inhibitory activity. Furthermore, the equivalent expression of functional TIMP3 promoted apoptosis and caspase activation in endothelial cells expressing KDR (PAE/KDR), but not in endothelial cells expressing PDGF beta-receptor (PAE/β-R). Surprisingly, the apoptotic activity of TIMP3 appears to be independent of caspases. TIMP3 inhibited matrix-induced focal adhesion kinase (FAK) tyrosine phosphorylation and association with paxillin and disrupted the incorporation of β3 integrin, FAK and paxillin into focal adhesion contacts on the matrix, which were not affected by caspase inhibitors. Thus, TIMP3 may induce apoptosis in ECs by triggering a caspase-independent cell death pathway and targeting a FAK-dependent survival pathway.
INTRODUCTION
Angiogenesis (the formation of new blood vessels from preexisting vasculature) plays an important role in physiological processes and in pathological conditions such as cancer and age-related macular degeneration (1-3). It is a multistep process that includes the activation of endothelial cells by growth factors, the subsequent degradation of the extracellular matrix (ECM) by proteolytic enzymes such as matrix metalloproteinases (MMPs) followed by invasion of the ECM, migration and proliferation of ECs, and finally the formation of new capillary tubes. Eventually, the newly formed capillary network is stabilized following the recruitment of pericytes (4). The initiation of angiogenesis is dependent on a dynamic balance between proangiogenic and anti-angiogenic factors. A positive balance in favor of angiogenic factors leads to new vessel formation, whereas the prevalence of anti-angiogenic factors shifts the equilibrium to vessel quiescence or under particular circumstances, even to vessel regression by inducing apoptosis in ECs (5).
VEGF is a major pro-angiogenic factor and promotes EC survival by inhibition of apoptosis (6). Interestingly, the survival effect of VEGF is dependent on the binding of VEGF to its receptor VEGFR-2, whereas VEGFR-1-specific ligands (such as PIGF) do not promote survival of ECs (7). ECM components comprise a major group of angiogenesis mediators (8). The adhesion of ECs to ECM proteins is essential for EC survival and angiogenesis. Integrins such as ανβ3 are critical for mediating the adhesion of ECs to ECM proteins and providing a potent survival signal (6, 9).
Naturally occurring inhibitors of angiogenesis i.e. anti-angiogenic factors are found in mammalian tissues, where they help maintain the quiescence of the normal vasculature. Thus, angiogenic inhibitors have been considered as potent anticancer drugs. Tissue Inhibitors of Metalloproteinase-3 (TIMP3), one of four members of a family of proteins that were originally classified according to their ability to inhibit MMPs (10, 11) is a naturally occurring inhibitor of angiogenesis that limits vessel density in the vascular bed of tumors and curtails tumor growth (12-14). Unlike the other TIMPs, which are soluble, TIMP-3 is unique in being a component of ECM (11). It is also the only TIMP that can inhibit tumor necrosis factor alpha (TNF-α) converting enzyme (TACE/ADAM17), and aggrecanase 1 and 2 (ADAMTS4 and ADAMTS5) (15). TIMP3 (but not TIMP1 or TIMP2) induces apoptosis in certain non-endothelial cells such as retinal pigment epithelial cells (16), vascular smooth muscle cells(17) melanoma (18) human colon carcinoma (19), moderately invasive HeLa cervical carcinoma cells, highly invasive HT1080 fibrosarcoma cells and non-invasive MCF-7 adenocarcinoma cells (20) but not in COS-7 cells(21). The pro-death domain of TIMP3 has been localized to the N terminus, the region associated with MMP inhibitory activity (22), and it has been proposed, at least in colon cancer cells and melanoma, that TIMP3 promotes apoptosis through stabilization of TNF-α receptors on the cell surface, leading to increased susceptibility to apoptosis (19, 23). Bond et al have reported that TIMP3 induces a Fas-associated death domain-dependent type II apoptotic pathway (24). On the other hand, deficiency of TIMP3 in homozygous knockout mice resulted in enhanced apoptosis during mammary gland involution (25), as well as a failure of liver regeneration and hepatocyte apoptosis via activation of TNF (26).
Whether TIMP3 induces endothelial apoptosis is unknown. Our previous data has established that TIMP3 exerts its anti-angiogenic effect by inhibiting proliferation, migration and tube formation of endothelial cells (ECs) via a direct interaction with VEGF receptor-2 (27). Since apoptosis of ECs in the vascular bed of tumors has been suggested to precede apoptosis of tumor cells (28), and the induction of EC apoptosis may counteract angiogenesis, we hypothesized that TIMP3 may induce EC apoptosis, and subsequently inhibit tumor angiogenesis and tumor growth.
RESULTS
TIMP3 overexpression induces apoptosis in human breast carcinoma in vivo but not in vitro
We have previously demonstrated that increased expression of TIMP-3 in a breast cancer cell line, MDA-MB 435 inhibits angiogenesis and tumor growth in mice (12). Since TIMP3 induces apoptosis in a number of cancer cell lines, we examined the effect of TIMP3 over-expression in MDA-MB 435 on apoptosis in vitro and in vivo. Western blot analysis (Fig. 1a) and reverse zymography (Fig. 1b) demonstrated that a 24-kDa functional TIMP3 was expressed in the ECM of MDA-MB 435 tumor cells transfected with TIMP3 when compared with control cells transfected with empty vector. To assess tumor cell apoptosis, tumor cells expressing TIMP3 and vector control cells were serum-starved and subsequently subjected to ApopTag kit that detects DNA fragments associated with apoptosis. As shown in Fig. 1c and d, ApopTag positive apoptotic cells were not observed in either vector control cells (Fig 1c,e) or tumor cells expressing TIMP3 (Fig. 1d,f), indicating that TIMP3 does not directly induce apoptosis in this tumor cell line (Fig. 1i). Similarly, expression of TIMP3 had no significant effect on growth rate of tumor cells as we have reported previously(12).
Figure 1. Increased levels of TIMP3 in breast cancer cells induces apoptosis in vivo but not in vitro.
a) Western blot analysis b) Reverse Zymography of ECM of MDA-MB 435 cells transfected with empty vector or vector containing TIMP3. c-f) Apoptosis in MDA-MB 435 cell cultures was evaluated with DAPI staining (c,d) and the Apoptag kit (e,f) in vector control transfected cells (c,e) and TIMP3 transfected cells (d,f). Apoptotic Index was quantitated in cultured cells following 1, 2 and 3 days of serum starvation (i). g,h, j) Apoptosis was evaluated in breast cancer tumors from mice injected with MDA-MB 435 cells transfected with empty vector (g) or TIMP3 (h) and Apoptotic Index quantitated (j).
We next investigated the effect of TIMP3 expression on tumor apoptosis in vivo. ApopTag kit analysis of tumor sections determined a significant increase in apoptotic cells in TIMP3-derived tumors (Fig. 1h) compared with vector-derived tumors (Fig. 1g, j). Taken together, the results from in vitro and in vivo experiments suggest that TIMP3 may induce MDA-MB 435 cell apoptosis in an angiogenesis inhibition-dependent manner by inducing endothelial cell apoptosis in tumors.
Adenoviral wild-type TIMP3 and TIMP3 Cys1-Ser induce apoptosis in choroidal endothelial cells
To determine if TIMP3 can induce apoptosis directly in endothelial cells, we over-expressed TIMP3 in a monkey choroidal endothelial cell line CRL-1780 using a recombinant adenovirus (Rad: WT-TIMP3). As a comparison the cells were also infected with recombinant adenovirus (Rad: TIMP3cys1-ser) that has the cys1 mutated to ser, which renders the TIMP3 to be an inactive MMP2 inhibitor. The cell matrix extracts from CRL-1780 72-h post-infection were analyzed for the expression or MMP inhibitory activity, by western blots or reverse zymography respectively. Like WT-TIMP3, TIMP3 cys1-ser was predominantly expressed in the ECM but at lower levels (Fig. 2A). As expected, reverse zymography confirmed that TIMP3cys1-ser lacked MMP-inhibitory activity relative to WT-TIMP3 (Fig.2A). RT-PCR experiments determined that infection with Rad: TIMP3 cys1-ser resulted in lower levels of TIMP3 mRNA as compared with infection with Rad: WT-TIMP3 (Fig.2B), which is consistent with the results from the western blot experiments. These results suggest that Rad: TIMP3cys1-ser had a lower infection efficiency relative to Rad: WT-TIMP3. We next analyzed apoptosis in cells transfected with the adenoviruses. Most of CRL-1780 and some of ARPE-19 cells transfected with RAD:TIMP3 cys1-ser became increasingly rounded and detached from the substratum and stained positive with ApopTag Direct kit (Fig. 2C) but to a lesser extent than. Rad:WT-TIMP3 (Fig. 2C). However, RAD:TIMP3 cys1-ser induced apoptosis in a dose-dependent manner and showed a similar extent of adjusted apoptotic index to WT-TIMP3 (ratio of apoptotic index to TIMP3 mRNA expression). Therefore, these results suggest that TIMP3 may induce endothelial cell apoptosis in an MMP inhibition-independent manner.
Figure 2. TIMP3 induces endothelial cell apoptosis in an MMP inhibition-independent manner.
A) Western blot analysis (upper panel) and reverse Zymography (lower panel) of ECM from endothelial cells infected with control adenovirus (Rad:66), adenovirus with wild type TIMP3 (rad:wtTIMP3) and mutant TIMP3 lacking MMP inhibitory activity (rad:TIMP3C1S) at the indicated doses for 72 hours. B) RT-PCR for TIMP3 expression in endothelial cells infected with control adenovirus (Rad:66), adenovirus with wild type TIMP3 (rad:wtTIMP3) and mutant TIMP3 lacking MMP inhibitory activity (rad:TIMP3C1S). C) Apoptosis in endothelial cells infected with control adenovirus (Rad:66), adenovirus with wild type TIMP3 (rad:wtTIMP3) and mutant TIMP3 lacking MMP inhibitory activity (rad:TIMP3C1S). D) Adjusted apoptotic index corrected for infection efficiency(mRNA expression).
TIMP3 expression promotes apoptosis and caspase activation in angiogenic endothelial cells expressing VEGF receptor-2 (KDR) but not in non-angiogenic endothelial cells expressing PDGF-beta receptor
We analyzed endothelial cell apoptosis using porcine aortic endothelial (PAE) cell lines expressing VEGFR-2 (PAE/KDR) or PDGF beta receptor (PAE/β-R), parental control (PAE) cells and PAE/KDR or PAE/β-R cells expressing TIMP3 (Fig. 3A). Initially, we examined the effect of TIMP3 expression on the angiogenic ability of these cell lines using an in vitro tube formation assay. Cells were sandwiched between two layers of Matrigel matrix and incubated in serum-free medium. After an 48-h incubation, we observed that PAE/KDR but not PAE/β-R nor parental PAE cells autonomously formed tube-like structures (Fig. 3B), suggesting that ectopic expression of KDR rather than β-R results in an angiogenic phenotype of endothelial cells. In contrast, PAE/KDR clones expressing TIMP3 (W1 and W3) failed to form tube-like structures but rather underwent morphological changes of death (Fig. 3B). On the other hand, PAE/β-R clones expressing TIMP3 neither formed tubes nor showed obvious morphological changes of death, suggesting that TIMP3 could specifically inhibit VEGF-dependent angiogenesis by directly inducing apoptosis. To evaluate TIMP3-induced apoptosis, PAE/KDR/TIMP3 clone (W1) and vector were incubated in serum-free medium for different time periods and analyzed by ApopTag direct kit. ApopTag positive apoptotic cells with the corresponding apoptotic index were increased in PAE/KDR/TIMP3 in a time-dependent manner relative to vector controls (Fig. 3C). Flow cytometry of propidium iodide-stained cells was employed as a second method to quantify apoptosis in PAE/KDR/TIMP3 and PAE/β-R/TIMP3 cells.. After a two-day starvation, positive apoptotic cells were increased by 14-28 % in PAE/KDR cells expressing TIMP3 (clones W1 and W3) but only by 0.7-1.2% in PAE/β-R clones expressing TIMP3 clones (W1 and W2) compared with the corresponding vector controls (V) (Fig. 3D). It has been previously reported that caspase activation plays a role in TIMP3-induced apoptosis in some non-endothelial cells. Therefore, we measured caspase activity in PAE/KDR and PAE/β-R cells expressing TIMP3 or empty vector using CasPASE™ apoptosis assays. As shown in Fig. 4A, caspase activity was significantly increased in PAE/KDR/TIMP3 cells (clones W1 and W3) but not in PAE/β-R/TIMP3 cells ((clone W1) compared with the corresponding vector controls following two-day serum starvation. Therefore, these results suggest that TIMP3 may selectively promote apoptosis via increased caspase activity in angiogenic endothelial cells.
Figure 3. TIMP3 promotes apoptosis exclusively in angiogenic endothelial cells.
A) Western Blot analysis (upper panel) and Reverse Zymography analysis (lower panel) of ECM extracts of endothelial cells expressing VEGFR2 (PAE-KDR) or PDGF-β receptor (PAE-βR) transfected with control vector (V) or wild-type TIMP3 (W1,W2). B) Tube formation assay of PAE (parental endothelial cells), PAE-KDR or PAE-βR expressing empty vector (V) or wild type TIMP3 (W1,W3). Cells were sandwiched between 2 layers of Matrigel. Tube formation was analyzed by phase contrast microscopy after a 2 day incubation. Original magnification X40. C) Representative photomicrograph images of subconfluent cells (PAE-KDR/V or PAE-KDR/W, serum starved for the indicated time periods and analyzed for apoptotic cells with the Apoptag kit (upper panel) and Apoptotic Index quantitation (lower panel). Apoptotic index is expressed as the percentage of apoptotic cells (Apoptag positive cells)/total DAPI-stained cells per HPF. Data are expressed as mean ± SD of triplicates of a typical experiment. At least 2 independent experiments gave comparable results. **P< 0.01 Vs vector controls. D) Apoptotic cells were analyzed by flow cytometric analysis following serum starvation and propidium iodide staining . Representative flow cytometry plots of PAE-KDR and PAE-βR cells transfected with control vector (V) or wild-type TIMP3 (W1, W2, W3). Apoptotic cells stain positive for both propidium iodide-PI (DNA) and FITC-BrdU (upper panel). Boxes are drawn to depict the gating of apoptotic and non-apoptotic cells. Quantitation of percent apoptotic cells is depicted in the lower panel. All data are represented as mean ± SD of three independent experiments. **P<0.01 when comparing significant differences between PAE-KDR and PAE-βR.
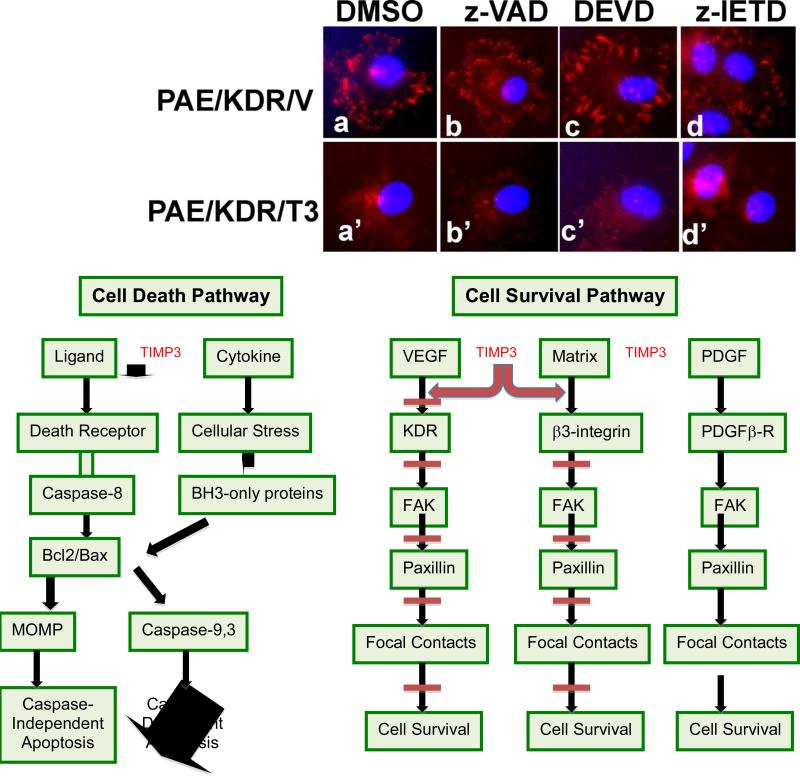
Figure 4. TIMP3 mediated apoptosis in endothelial cells is independent of Caspase activity.
A) Caspase activity was measured in serum starved PAE-KDR and PAE-βR cells expressing control vector (V) or wild-type TIMP3 (W) in the presence or absence of a pan-caspase inhibitor Z-VAD-FMK (10 μM) for 1 hour. B) PAE-KDR/V and PAE-KDR-W1 cells were serum starved and treated with 10 μM inhibitors of pan-caspase (zVAD) or caspase-3 (DEVD, 10μM) or caspase-8 (z-IETD, 30μM). Upper panel shows representative photomicrographs of endothelial cells subjected to tube formation assay and lower panel depicts apoptotic cells stained with Apoptag kit. C) Quantitation of apoptotic index. All data are mean ± SD of triplicates of a representative experiment. At least two independent experiments gave comparable results. *P<0.05 Vs DMSO
TIMP3 overexpression promotes apoptosis in angiogenic PAE/KDR cells independent of caspases
To address the contribution of caspases to apoptosis induction in response to TIMP3 over-expression, we employed a set of caspase inhibitors including a pan-capase inhibitor ZVAD-FMK, a specific caspase-8 inhibitor Z-IETD-FMK and a specific caspase-3 inhibitor DEVD. Although Z-VAD-FMK inhibited TIMP3-incuced activation of caspases (Fig.4A), it failed to prevent TIMP3-induced apoptosis in PAE/KDR cells including morphological changes of cell death in a tube formation and increased DNA fragments detected by ApopTag direct kit (Fig. 4B and 4C). Z-IETD-FMK and DEVD were also ineffective in their ability to inhibit the apoptotic activity of TIMP3 (Fig. 4B and 4C). Both Z-IETD and DEVD were effective in inhibiting caspase activity in PAE cells (Supplementary Fig. 1). Focal adhesion kinase (FAK) and paxillin have been previously shown to be cleaved in caspase-induced apoptosis (29). We wanted to determine if the increased caspase activity in endothelial cells expressing TIMP-3 resulted in FAK and paxillin cleavage. PAE/KDR or PAE/β cells expressing TIMP3 or vector were serum-starved for two days and analyzed by immunoprecipitation and/or immunoblotting with anti-FAK or anti-paxillin antibodies. We found that endothelial cells expressing VEGFR2 (PAE/KDR/V cells) had increased FAK fragments in the range of 70-100 kDa compared to endothelial cells expressing PDGF-β receptor (PAE/βR/V) (Fig. 5 left panel). However, FAK fragments were not increased in PAE/KDR/TIMP3 cells (clonesW1 or W3) or PAE/β/TIMP3 cells (clone W1) compared with the corresponding vector cells (Fig. 5 left panel). A paxillin fragment (55 kDa) was also increased in PAE/KDR/V cells compared parental PAE cells (Fig. 5 middle panel) and the expression of TIMP3 (PAE/KDR/TIMP3 cells (clone W1) had no effect on the amount of paxillin fragmentation (Fig. 5 middle panel).
Figure 5. TIMP3 does not mediate cleavage of FAK or Paxillin in endothelial cells.
Western Blot analysis of FAK (left panel), and Bcl2, Bax (right panel) and immunoprecipitation and immunoblot analysis of paxillin (middle panel) in PAE-KDR cells expressing wild-type TIMP3.
Mitochondria outer membrane permeabilization (MOMP) has been shown to promote caspase-independent death (30). Since Bax or Bcl2 protein are critical mitochondrial effectors or regulators, we investigated the effect of TIMP3 expression on the level of Bcl2 and Bax protein in PAE/KDR cells. Cells expressing TIMP3 (W1) or empty vector (V) were serum-starved for two days and analyzed by Western blots with anti-Bax or anti-Bcl2 antibody. We found that Bax protein levels were dramatically increased in PAE/KDR/TIMP3, and BCl2 levels were significantly decreased relative to vector controls (Fig. 5 right panel). Taken together, these results suggest that TIMP3 promotes apoptosis in angiogeneic cells by triggering a caspase-independent cell death pathway.
TIMP3 overexpression predominantly inhibits FAK-dependent pathway in angiogenic PAE/KDR cells but not in non-angiogeneic PAE/β cells
It has been demonstrated that FAK mediates cell survival by promoting downstream signaling and focal adhesion formation on matrix (31). To determine early signaling events leading to EC apoptosis induced by TIMP3, we initially investigated the effect of TIMP3 expression on FAK tyrosine phosphorylation and its association with paxillin, a critical downstream effector. Confluent PAE/KDR cells expressing TIMP3 or empty vector and parental PAE cells were plated on to Matrigel-coated culture dishes. After a 15-min incubation, cells were analyzed by immunoprecipitation with anti-FAK antibodies and immunoblotting with anti-FAK, phosphotyrosine and paxillin antibodies. We found that endothelial cells expressing VEGFR2 (PAE/KDR/V) had increased FAK, FAK tyrosine phosphorylation and association with paxillin relative to parental PAE cells that did not express VEGFR2 (Fig. 6A). However, FAK tyrosine phosphorylation and its association with paxillin were decreased in endothelial cells expressing VEGFR2 and TIMP3 (PAE/KDR/TIMP3) compared with vector control cells (Fig. 6A). We also investigated the effect of TIMP3 expression on focal adhesion formation in PAE/KDR and PAE/β-R cells plated on a Matrigel matrix. Cells were plated and allowed to attach and spread on matrigel for 1h, and subsequently analyzed for focal adhesion formation by immunofluorescence staining with antibodies recognizing β3 integrin, phosphotyrosine, FAK and paxillin, all which have been shown to be localized in focal adhesion contacts. As shown in Fig. 6B, anti-phosphotyrosine, anti- β3 integrin, anti-FAK or anti-paxillin antibodies revealed a punctate radial staining pattern around both PAE/KDR/vector and PAE/β-R/vector control cells which are typical for focal adhesion contacts. Focal adhesion contacts were also observed in PAE/KDR/TIMP3 cells and PAE/β-R/TIMP3 cells when stained with anti-phosphotyrosine (Fig. 6B). However, the incorporation of β3, FAK and paxillin into focal adhesion contacts was significantly reduced in PAE/KDR/TIMP3 cells (Fig. 6B). In contrast, TIMP3 expression in endothelial cells expressing PDGF β-receptor (PAE/β-R/TIMP3) resulted in decreased incorporation of β3 into focal adhesion contacts but had no any effect on FAK-and paxillin-dependent focal adhesion formation. These results suggest that TIMP3 may selectively promote apoptosis in angiogenic endothelial cells by targeting FAK- and paxillin-dependent cell survival pathway.
Figure 6. TIMP3 inhibits FAK dependent pathway in angiogenic PAE-KDR cells.
A) Analysis by immunoprecipitation and western blot analysis of FAK, phosphorylated FAK and FAK/paxillin complex in PAE-KDR/V and PAE-KDR/T3 cells B) Analysis of focal adhesion formation by immunofluorescence staining with antibodies recognizing β3 integrin, phosphotyrosine, FAK and paxillin in PAE-KDR/V, PAE-KDR/T3, PAE-βR/V and PAE-βR/T3 following exposure to matrigel for 1 hour.
TIMP3 overexpression disrupts focal adhesion formation in angiogenic PAE/KDR cells independent of caspases
We examined whether caspases contribute to the inhibition of focal adhesion contacts mediated by TIMP3 using caspase inhibitors Z-VAD-FMK, Z-IETD-FMK and DEVD. PAE/KDR/Vector or PAE/KDR/TIMP3 cells were plated and allowed to spread on matrigel in the presence or absence of inhibitors of pan-caspase (zVAD), caspase-3 (DEVD) and caspase-8 (zIETD) for 1h and subsequently analyzed for focal adhesion formation by immunofluorescence staining with anti-paxillin antibody. As shown in Fig 7, none of the caspase inhibitors inhibitors had any effect on the incorporation of paxillin into focal contacts in PAE/KDR/vector cells or on the inhibitory activity of TIMP3 on paxillin-dependent focal adhesion formation. This suggests that TIMP3 disrupts paxillin-dependent focal adhesion formation in angiogenic endothelial cells in a caspase-independent manner.
Figure 7. TIMP3 disrupts paxillin-dependent focal adhesion formation in angiogenic endothelial cells by a caspase-independent mechanism.
Analysis of paxillin in focal adhesion contacts of PAE-KDR/V (upper panel, a-d) and PAE-KDR/T3 (lower panel, a’-d’) cells exposed to matrigel in the presence of caspase inhibitors (z-VAD (b,b’), DEVD (c,c’)and z-IETD (d,d’)).
MATERIALS AND METHODS
Cells and Reagents
Monkey endothelial cells of choroid-retina (CRL-1780; ATCC™, Manassas, Virginia), porcine aortic endothelial (PAE) cell lines expressing KDR (PAE/KDR) and, PDGFβ-R (PAE/β-R) (27) and human retinal pigment epithelial cells (ARPE-19, American Type Culture Collection) were cultured in Ham's F-12/DMEM medium supplemented with 10% fetal calf serum (FCS) (Cambrex, East Rutherford, NJ). The following antibodies were used in this study; monoclonal anti TIMP3 antibody from Chemicon International, Inc (Temecula, CA), anti-phosphotyrosine mAb, clone 4G10 and anti-paxillin mAb, clone 5H11 from Upstate Biotechnology Inc (Lake Placid, NY), anti-FAK mAb, clone 77 from Transduction Laboratories (Lexington, KY), anti-Bax (P-19), anti-Bcl2 rabbit polyclonal antibodies and anti-actin (C-11) goat polyclonal antibody from Santa Cruz Biotechnology (Dallas, TX). Growth factor reduced matrigel was purchased from BD Biosciences (Bedford, MA). Z-VAD-FMK (pan-caspase inhibitor), Z-DEVD-FMK(caspase-3 inhibitor II) and Z-IETD-FMK(caspase inhibitor II) were purchased from Calbiochem (Darmstadt, Germany).
Generation of TIMP-3–expressing endothelial cell lines
A 550 bp TIMP-3 insert from a human cDNA clone (12, 27) was fused in frame with a FLAG epitope DYKDDDK at its COOH terminal end and cloned into expression vector pCEP4(Invitrogen, Life Technologies, Grand Island, NY). PAE cells expressing KDR or PDGFβ-R were transfected with TIMP-3 cDNA in pCEP4 using Lipofectamine reagent (Gibco-BRL, Life Technologies, Grand Island, NY) according to the manufacturer's protocol as described previously (12, 27). Stable clones were isolated by Hygromycin selection. Control cells were transfected using pCEP4 vector without any insert and selected with Hygromycin. Western blot analysis and reverse zymography was used to confirm expression of TIMP-3.
Adenovirus Infection
Recombinant adenovirus Rad66 containing the cytomegalovirus immediate early promoter and polyadenylation signal and transgene wtTIMP-3 were obtained from Dr. Andrew Baker (Univ. of Glasgow, United Kingdom). Cells were cultured in 6 well plates in complete medium. Sub-confluent cells (80%) were infected with adenovirus as described previously (17, 27). Cells were infected at 50 or 100 plaque-forming units (pfu)/cell in 1 ml of complete medium for 18 h. Medium was then replaced with 2 ml of fresh complete medium and left for 72 hours prior to analysis.
Gelatin Zymography and Reverse Zymography
Equal amounts of protein were dissolved in non-reducing Laemmli sample buffer and separated by electrophoresis using 7.5% SDS-polyacrylamide gels containing 1 mg/ml gelatin (gelatin zymography) or using 12% SDS-polyacrylamide gels containing 1mg/ml gelatin and RPE cell-conditioned media, as a source of MMPs, (reverse zymography). Following electrophoresis, gels were processed as described previously(32, 33). Briefly, gels were agitated in a solution of 25mg/ml Triton X-100 to remove SDS and to promote renaturation of proteases and inhibitors. The Triton was washed off with water and the gels then incubated for 16 hours in 50mM Tris-HCl (pH 7.5) containing 5mM CaCl2 and 0.2mg/ml sodium azide at 37°C. Gels were stained with 5mg/ml Coomassie Blue R-250 in acetic acid/methanol/water (1:3:6) for 2 hours and destained with acetic acid/methanol/water (1:3:6).
Immunoprecipitation and immunoblotting
Cells were lysed in lysis buffer composed of 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2.5 mM EDTA, 10% glycerol, 1% Triton X-100, and protease inhibitor cocktail tablets (Roche). Protein concentration was determined using a micro_BCA protein assay (ThermoScientific, Rockford, IL) according to the maunfacturer's instructions. The lysates were incubated with primary antibodies for 1hr on ice, followed by incubation with immobilized protein G (Santa Cruz Biotechnology, Inc) for 30 min at 4°C. Immunoprecipitated samples were separated by SDS-PAGE under reducing conditions, and transferred to nitrocellulose membranes (Hybond-C extra, Amersham, GE HealthCare, Pittsburgh, PA). Membranes were blocked in 5% non-fat milk containing 0.2% Tween-20 in PBS at room temperature for 1 hour and probed with the indicated antibodies for 1 hour. After washing with PBS/Tween, proteins were detected with HRP-conjugated secondary lgG antibodies followed by ECL. The blots were re-stripped with Western ReProbe™ solution (GBioSciences, St Louis, MO) for 30 minutes and reprobed.
In vitro tube formation assay
In vitro formation of tubular structures was studied with a modified three-dimensional matrix assay as previous described (27, 34, 35). Briefly, the cells pretreated with or without caspase inhibitors for 30 min were subjected to a first layer of growth factor-reduced Matrigel (Becton Dickinson, Bedford, MA) in 24 well plates at 2×104 cells/well. After a 4 h incubation, a second layer of Matrigel was added. After gelling, cultures were maintained in serum-free DMEM/F-12 medium in the presence or absence of caspase inhibitors for 72 h. The cellular morphology were examined using a phase-contrast microscopy.
Caspase assay
Subconfluent cells were starved in 0.25% (v/v) FBS. 24h later, caspase activity in cell lysates was measured using CasPASE™ apoptosis assay (GenoTech, St. Lous, MO.) following the manufacturer's instructions.
Apoptosis assay
For ApopTag fluorescein in situ apoptosis detection kit, briefly, cells were plated in 75-cm2 flasks and grown to 90% confluence. The cells were then subjected to serum-free treatment in the presence or absence caspase inhibitors. A 48 h, the treated cell were removed from the tissue culture by a gentle scraping, centrifuged and washed with 1 PBS. 5×107 cells/ml were fixed in 1% paraformaldehyde. 50-100 μl of cell suspension was dried on a microscope slide and stained using fluorescent labeled nucleotides and counter-stained with DIPA. ApoTagR and DAPI positive cells were visualized by with fluorescent microscopy. The percentage of ApoTagR positive cells out of 200-500 DAPI positive cells was calculated as an apoptotic idex. For flow cytometry method, confluent cells were starved in serum-free medium for 48 and were then removed from culture flasks by trypsizination. 2×106 cells were fixed in 1% paraformaldehyde. DNA fragmentation was detected by staining cells with propidium iodide and quantitated by flow cytometry. (Core service).
Immunocytochemical and immunoflurescent analyses
Falcon cultureslids (Becton Dickinson Labware Franklin Lakes, NJ), were coated with 5 μg/ml vitronectin or 50 μg/ml matrigel overnight at 4°C and blocked with 3% bovine serum albumin (BSA) in PBS for 2 h at room temperature at 37 °C. The subconfluent cells were removed from culture flasks by trypsizination, washed and resuspended in 0.2% (v/v) serum culture medium. 20000 cells were transfered into FALCON cultureslids and incubated for 1 h at 37 °C. Cells were then fixed in 1 % paraformaldehyde in PBS for 10 min at 4°C, and permeabilized in 0.2 % triton X-100 for 10 min at room temperature. After blocked using 3% BSA in PBS, cells were incubated with 4 μg/ml anti-FAK, anti-paxillin and anti-phosphotyrosine (PY-20) antibodies in 1% BSA in PBS for 1 h at room temperature, and visualized via a two-step staining procedure in combination of biotinylated anti-mouse lgG (1:1000) as the secondary antibody and Texas Red avidin D (1;1500). Cells were finally washed with PBS, and counterstained with an antifade medium containing DAPI, and mounted under a glass coverslip, prior to fluorescent microscopy.
Statistical Analysis
The p values were calculated from Student's t test using comparisons with control samples tested at the same time.
DISCUSSION
For a wide range of tumors, increased neovascularization is critical for the transition from hyperplasia to neoplasia and increased metastasis. Various studies have suggested that increased microvascular density often predicts a more aggressive cancer (36-39) and targeting newly forming tumor vasculature has been a promising approach for the treatment of cancers.
TIMP3 has been classified as a potential tumor suppressor due to its correlation with an aggressive phenotype and shortened disease free survival in breast cancer (40, 41). In addition epigenetic silencing of TIMP3 occurs in a variety of solid tumors (42-45). We have demonstrated previously that TIMP3 is an inhibitor of VEGF-induced angiogenesis and functions by inhibiting EC migration, proliferation and tube formation via a direct interaction with VEGFR-2(27).
A balance between endothelial cell proliferation and apoptosis regulates the homeostasis of the endothelial cells lining blood vessels. In this study, we have demonstrated that TIMP3 induces apoptosis in angiogenic endothelial cells likely via VEGFR-2. Apoptosis of ECs in turn leads to loss of survival of breast cancer cells due to angio-inhibition.
The pro-death domain of TIMP3 has been localized to the N terminus, the region associated with MMP inhibitory activity (22). It has also been proposed, at least in colon cancer cells and melanoma, that TIMP3 promotes apoptosis through stabilization of TNF-α receptors on the cell surface, leading to increased susceptibility to apoptosis (19, 23). On the other hand, deficiency of TIMP3 in homozygous knockout mice results in enhanced apoptosis during mammary gland involution. TIMP3 expression has been also shown to induce activation of initiator caspase-8 and -9 and promote caspase-mediated cleavage of the death substrates poly ADP-ribose polymerase and FAK via a Fas-assoicated death domain-dependent Type II apoptotic pathway in both rat VSMC and Hela cells (24). In contrast to these findings, our results indicate that TIMP3 induces endothelial apoptosis in an MMP inhibition-independent fashion. Endothelial cells infected with a recombinant adenovirus expressing mutant TIMP3cys1-ser that was devoid of MMP inhibitory activity induced apoptotic cell death as efficiently as when infected with adenovirus expressing wild-type TIMP3. Furthermore, equivalent expression of TIMP3 predominantly promoted apoptosis and caspase activation in endothelial cells expressing VEGFR-2 (PAE/KDR), but not in a non-angiogenic PAE lacking KDR but expressing PDGF-βR (PAE/β-R) cells. We also observed that even though TIMP3 induced an increase in caspase activity, caspase was unlikely to be a mediator in apoptosis as caspase inhibition by polycaspase and specific caspase-3 and -8 inhibitors failed to prevent TIMP3-induced morphological changes of cell death. Furthermore, the cleavage of FAK and paxillin, two caspase substrates, was not concurrently increased with TIMP3-induced apoptosis. These results suggest that TIMP3 induces endothelial cell apoptosis via a caspase-independent mechanism, which is different from that induced in non-endothelial cells.
It has been previously suggested that the release of mitochondrial proteins as a result of mitochondrial outer membrane permeabilization (MOMP) can promote caspase-independent death through mechanisms that are relatively poorly defined (46). TIMP3 overexpression has been shown to induce mitochondrial activation as demonstrated by loss of mitochondrial membrane potential and release of cytochrome c in both rat VSMC and Hela cells (24). In the present study, TIMP3 expression in endothelial cells resulted in an increase of Bax and decrease of Bcl2 proteins. Since Bax (a multidomain proapoptotic member) and Bcl2 (an antiapoptotic member) may promote or prevent the release of mitochondrial apoptogens (47) respectively, we hypothesize that TIMP3 may induce a caspase-independent endothelial apoptosis by increasing release of mitochondrial apoptogens though a Bax-dependent pathway.
Growth factors such as VEGF and PDGF may promote EC survival by inhibition of apoptosis (6, 48). Integrins such as ανβ3-integrin may function as EC survival factors by preventing anoikis by enhancing binding to the extracellular matrix as well as may function in concert with VEGF or PDGF to promote EC survival (6, 48-52). FAK has been demonstrated to be a key component of the signal transduction pathways triggered by VEGFR-2, PGGF beta-receptor or ανβ3-integrin and mediate cell survival by promoting downstream signaling and focal adhesion formation on matrix. We asked if TIMP3 could promote apoptosis by inhibiting FAK signaling through VEGFR-2, PDGF beta receptor and/or integrins in addition to activation of cell death pathway? In a previous study, we have demonstrated that TIMP3 inhibited VEGF-mediated angiogenesis by blocking binding of VEGF to VEGF receptor and downstream signaling via a direct interaction with VEGFR-2 but had no affect PDGF-BB binding to PDGF beta-receptor (33). In this study, we found that expression of TIMP3 inhibited the matrix-mediated tyrosine phosphorylation of FAK, association with paxillin and the incorporation of FAK and paxillin into focal contacts in PAE/KDR cells but not in PAE/β-R cells. Thus, one possible explanation for TIMP3-induced apoptosis in angiogenic PAE/KDR rather than nonangiogenic PAE/β-R is that there is a selective inhibition of VEGFR-2-FAK rather than PDGF beta-receptor-FAK survival pathway. We noted that TIMP3 completely blocked the incorporation of β3-integrin into focal adhesion contacts in PAE/KDR but to a less extent, in PAE/β-R cells. We also found that vitronectin but not laminin inhibited 125I-TIMP3 binding to endothelial cells (data not shown). These results suggest a potential interaction between TIMP3 and vitronection and/or β3-integrin. Since ligation of ανβ3-integrin is required for the survival of the angiogenic phenotype of ECs (5) and there is a cross talk between VEGFR-2/PDGF beta-receptor and ανβ3-integrin (38-41), we reason that TIMP3 may promote apoptosis in angiogenic PAE/KDR cells by targeting the FAK pathway trigged by both VEGFR-2 and ανβ3-integrin.
Putative pathways to caspase-independent cell death induced by TIMP3 and unanswered questions are presented in Fig. 8. Overall, we have identified an additional functional feature of TIMP3 in ECs: induction of apoptosis and suggest an MMP inhibition and caspase-independent mechanism underlying TIMP3-induced endothelial apoptosis.
Supplementary Material
Supplementary Figure 1. DEVD and Z-IETD inhibit Caspase 3 and Caspase 8 respectively in PAE cell lysates. Analysis of Caspase 3 and Caspase 8 activity in cell lysates from PAEKDR/V (V) and PAE-KDR/T3 (W1 and W3) cells treated with DEVD (10μM), Z-IETD (30μM) or DMSO as a vehicle control.
ACKNOWLEDGEMENTS
This work was supported in part by US National Institute of Health EY016490 (BA-A), EY020861 (BA-A), EY022768 (JHQ), Foundation Fighting Blindness Center Grant (BA-A), Research to Prevent Blindness (RPB) Challenge Grant and RPB Lew Wasserman award to BA-A. We are grateful to Mariya Ali and Alecia Cutler for providing technical assistance. We wish to extend a sincere apology to colleagues whose work was not cited because of space limitations.
REFERENCES
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000 Apr;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? [comment]. Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 6.Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:887–893. doi: 10.1161/01.atv.0000017728.55907.a9. [DOI] [PubMed] [Google Scholar]
- 7.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997;99:373–376. doi: 10.1172/JCI119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apte SS, Olsen B, Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J Biol Chem. 1995;270:14313–14318. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- 11.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 12.Anand-Apte B, Bao L, Smith R, et al. A Review of Tissue Inhibitor of Metalloproteinases-3 (TIMP-3) and Experimentsl Analysis of its Effect on Primary Tumor Growth. Biochem and Cell Biol. 1996;74:853–862. doi: 10.1139/o96-090. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Munoz W, Kim I, Khokha R. TIMP-3 deficiency in the host, but not in the tumor, enhances tumor growth and angiogenesis. Oncogene. 2006;25:650–655. doi: 10.1038/sj.onc.1209104. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Munoz W, Sanchez OH, Di Grappa M, English JL, Hill RP, Khokha R. Enhanced metastatic dissemination to multiple organs by melanoma and lymphoma cells in timp-3−/− mice. Oncogene. 2006;25:6489–6496. doi: 10.1038/sj.onc.1209663. [DOI] [PubMed] [Google Scholar]
- 15.Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- 16.Majid MA, Smith VA, Easty DL, Baker AH, Newby AC. Adenovirus mediated gene delivery of tissue inhibitor of metalloproteinases-3 induces death in retinal pigment epithelial cells. Br J Ophthalmol. 2002;86:97–101. doi: 10.1136/bjo.86.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310–2315. [PubMed] [Google Scholar]
- 19.Smith M, Kung H, Durum S, Colburn N, Sun Y. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine. 1997;9:p770–780. doi: 10.1006/cyto.1997.0233. [DOI] [PubMed] [Google Scholar]
- 20.Baker A, George S, Zaltsman A, Murphy G, Newby A. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:p1347–1355. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langton K, Barker M, McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem. 1998;273:p16778–16781. doi: 10.1074/jbc.273.27.16778. [DOI] [PubMed] [Google Scholar]
- 22.Bond M, Murphy G, Bennett MR, et al. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus. Metalloproteinase inhibition is associated with proapoptotic activity. J Biol Chem. 2000;275:41358–41363. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- 23.Ahonen M, Poukkula M, Baker AH, et al. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22:2121–2134. doi: 10.1038/sj.onc.1206292. [DOI] [PubMed] [Google Scholar]
- 24.Bond M, Murphy G, Bennett MR, Newby AC, Baker AH. Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol Chem. 2002;277:13787–13795. doi: 10.1074/jbc.M111507200. Epub 12002 Feb 13784. [DOI] [PubMed] [Google Scholar]
- 25.Fata JE, Leco KJ, Voura EB, et al. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest. 2001;108:831–841. doi: 10.1172/JCI13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 27.Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 29.Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 30.Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Anand-Apte B, Pepper MS, Voest E, et al. Inhibition of Angiogenesis by Tissue Inhibitor of Metalloproteinase-3. Invest Ophthal Vis Sci. 1997;38:817–823. [PubMed] [Google Scholar]
- 33.Qi JH, Ebrahem Q, Yeow K, Edwards DR, Fox PL, Anand-Apte B. Expression of Sorsby's fundus dystrophy mutations in human retinal pigment Epithelial cells reduces matrix metalloproteinase inhibition and may promote angiogenesis. J Biol Chem. 2002;30:30. doi: 10.1074/jbc.M110870200. [DOI] [PubMed] [Google Scholar]
- 34.Qi JH, Dai G, Luthert P, et al. S156C mutation in tissue inhibitor of metalloproteinases-3 induces increased angiogenesis. J Biol Chem. 2009;284:19927–19936. doi: 10.1074/jbc.M109.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi JH, Ebrahem Q, Ali M, et al. Tissue inhibitor of metalloproteinases-3 peptides inhibit angiogenesis and choroidal neovascularization in mice. PLoS ONE. 2013;8:e55667. doi: 10.1371/journal.pone.0055667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox SB, Gatter KC, Leek RD, et al. More about: Tumor angiogenesis as a prognostic assay for invasive ductal breast carcinoma [letter; comment]. J Natl Cancer Inst. 2000 Jan 19;92:161–162. doi: 10.1093/jnci/92.2.161. [DOI] [PubMed] [Google Scholar]
- 37.Gasparini G, Weidner N, Bevilacqua P, et al. Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma [see comments]. J Clin Oncol. 1994;12:454–466. doi: 10.1200/JCO.1994.12.3.454. [DOI] [PubMed] [Google Scholar]
- 38.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma [see comments]. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 39.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 40.Helleman J, Jansen MP, Ruigrok-Ritstier K, et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008;14:5555–5564. doi: 10.1158/1078-0432.CCR-08-0555. [DOI] [PubMed] [Google Scholar]
- 41.Mylona E, Magkou C, Giannopoulou I, et al. Expression of tissue inhibitor of matrix metalloproteinases (TIMP)-3 protein in invasive breast carcinoma: relation to tumor phenotype and clinical outcome. Breast Cancer Res. 2006;8:R57. doi: 10.1186/bcr1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu P, Xing X, Tanzer M, et al. Frequent loss of TIMP-3 expression in progression of esophageal and gastric adenocarcinomas. Neoplasia. 2008;10:563–572. doi: 10.1593/neo.08208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilska M, Roberts PJ, Collan YU, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–723. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 44.Lai K, Conway RM, Crouch R, Jager MJ, Madigan MC. Expression and distribution of MMPs and TIMPs in human uveal melanoma. Exp Eye Res. 2008;86:936–941. doi: 10.1016/j.exer.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Ninomiya I, Kawakami K, Fushida S, et al. Quantitative detection of TIMP-3 promoter hypermethylation and its prognostic significance in esophageal squamous cell carcinoma. Oncol Rep. 2008;20:1489–1495. [PubMed] [Google Scholar]
- 46.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 47.Putcha GV, Le S, Frank S, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Ahmad SA, Reinmuth N, et al. Endothelial cell survival and apoptosis in the tumor vasculature. Apoptosis. 2000;5:323–328. doi: 10.1023/a:1009679307513. [DOI] [PubMed] [Google Scholar]
- 49.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 50.Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. Embo J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. Embo J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of alphavbeta3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111(Pt 4):469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. DEVD and Z-IETD inhibit Caspase 3 and Caspase 8 respectively in PAE cell lysates. Analysis of Caspase 3 and Caspase 8 activity in cell lysates from PAEKDR/V (V) and PAE-KDR/T3 (W1 and W3) cells treated with DEVD (10μM), Z-IETD (30μM) or DMSO as a vehicle control.