Abstract
Beta2-glycoprotein I (β2GPI) is the most common antigen for autoimmune antibodies in antiphospholipid syndrome (APS). Thrombosis is a clinical feature of APS. We made a molecule (A1-A1) that consists of two identical β2GPI-binding modules from ApoE receptor 2. A1-A1 binds to β2GPI/anti-β2GPI antibody complexes preventing their association with ApoER2 and anionic phospholipids, and reducing thrombus size in the mouse model of APS. Here, we describe a mutant of A1-A1 (mA1-A1ND) with improved affinity for β2GPI. mA1-A1ND inhibits the binding of β2GPI to cardiolipin in the presence of anti-β2GPI antibodies and inhibits β2GPI/antibody complexes in plasma samples of APS patients affecting the clotting time. Inhibition of the clotting time demonstrates the presence of soluble β2GPI/anti-β2GPI antibody complexes in patients’ plasma. These complexes either already exist in patients’ plasma or form rapidly in the vicinity of phospholipids. All members of the LDL receptor family can bind β2GPI. Modelling studies of A1 in a complex with domain V of β2GPI (β2GPI-DV) revealed two possible orientations of a ligand-binding module from lipoprotein receptors on β2GPI-DV. In both orientations, the ligand-binding module interferes with the binding of β2GPI to anionic phospholipids, however it interacts with two different though overlapping sets of lysine residues in β2GPI-DV, depending on the orientation.
Keywords: Antiphospholipid syndrome, B2GPI, ApoH, ApoER2, lipoprotein receptor
Introduction
Beta2-glycoprotein I (β2GPI) has an important pathological role in antiphospholipid syndrome (APS) [1]. Patients with thrombosis and/or pregnancy loss are diagnosed with APS, if they have autoimmune antibodies to serum proteins, which bind to anionic phospholipids in laboratory tests for APS [2]. B2GPI is the major antigen for antiphospholipid antibodies [3–5]. Normally, β2GPI circulates in the blood at a high concentration and does not have a well-defined physiological function. B2GPI acquires prothrombotic properties in the presence of anti-β2GPI antibodies. Anti-β2GPI antibodies correlate with thrombosis in patients with APS and potentiate thrombosis in animal models of APS [6–9].
In vivo in mice significant β2GPI deposition was detected only on the endothelium of the uterus and the deposition pattern remained the same in mice with circulating anti-β2GPI antibodies [10]. In the presence of anti-β2GPI antibodies, β2GPI/antibody complexes enhance thrombus size in rodent models of thrombosis and activate endothelial cells, monocytes and platelets in vitro and in vivo [11–14]. Several receptors and cell-surface molecules including TLR4, Annexin II, ApoER2 and other receptors of the LDL receptor family, GPIbα and anionic phospholipids have been shown to bind β2GPI and contribute to cellular activation by β2GPI/antibody complexes [15–22]. ApoER2 and GPIbα act together in activation of platelets by β2GPI/antibody complexes [18]. Anti-β2GPI antibodies enhance the binding of β2GPI to cellular surfaces and, possibly, crosslink several receptors. In vivo studies in mice confirmed the contribution of ApoER2, TLR4 and Annexin II to prothrombotic properties of β2GPI/antibody complexes [14, 23–25]. Besides cell-surface receptors, β2GPI interacts with proteins involved in blood coagulation and fibrinolysis [26–29]. Whether these interactions are modified by antiβ2GPI antibodies and their role in antiphospholipid syndrome is not well established.
ApoER2 is a receptor for β2GPI/antibody complexes on endothelial cells and platelets [14, 30]. It belongs to a family of low-density lipoprotein receptor. Like other receptors of the same family, ApoER2 uses multiple small structurally homologous ligand-binding modules to interact with diverse ligands. The first ligand-binding module, A1, is critical for the binding of β2GPI to ApoER2 [30]. We created a molecule consisting of two A1 modules connected with a flexible linker and demonstrated that this molecule preferentially targets β2GPI/antibody complexes compared to β2GPI free of antibody [31]. A1-A1 not only interferes with the binding of β2GPI/antibody complexes to ApoER2, but also prevents their association with anionic phospholipids, therefore inhibiting two potentially harmful activities of β2GPI/antibody complexes [32]. Most importantly, we demonstrated that infusion of A1-A1 in mice abrogated the increase in thrombus size caused by the presence of β2GPI/antibody complexes [33].
In this study, we made a mutant of A1-A1 (mA1-A1ND) with improved affinity for β2GPI. The rationale for a point Asn 36 to Asp mutation in the β2GPI-binding region of mouse A1 was deduced from the modelling of the complex between A1 and β2GPI. mA1-A1ND inhibits the binding of β2GPI/antibody complexes to cardiolipin-coated surfaces and decreases anti-β2GPI-dependent prolongation of clotting time in plasma samples of APS patients. All receptors of the LDL receptor family can bind β2GPI. Modelling studies of A1 in a complex with domain V of β2GPI (β2GPI-DV) revealed two possible orientations of a ligand-binding module from lipoprotein receptors on β2GPI-DV. In both orientations, the ligand-binding module interferes with the binding of β2GPI to anionic phospholipids, however it interacts with two different though overlapping sets of lysine residues in β2GPI-DV, depending on the orientation.
Results and Discussion
Calcium binding by A1 variants
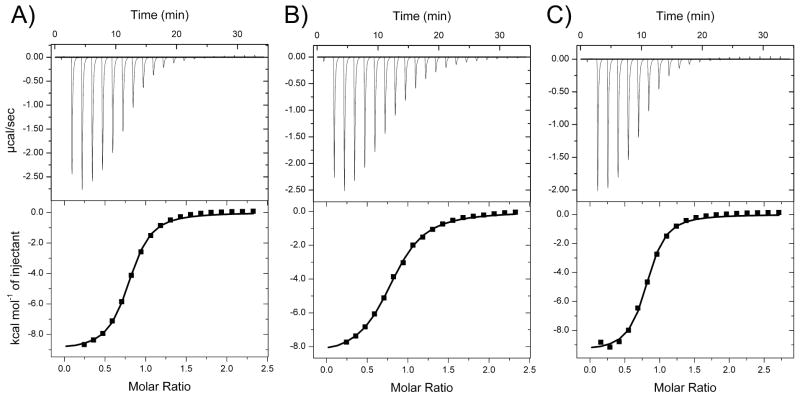
A1 is the first ligand-binding repeat from ApoE receptor 2 (ApoER2). We made A1 molecules consisting of human (hA1), mouse (mA1) and an Asn36/Asp point mutant of mouse A1 (mA1ND) sequences. Similarly to other ligand-binding repeats from lipoprotein receptors, A1 is mostly devoid of secondary structure elements, but has a well-defined three-dimensional structure [34]. The structural rigidity of a ligand-binding module is maintained by three disulfide bonds and a calcium ion coordinated by conserved aspartate and glutamate residues. In the absence of calcium, the three-dimensional structure is totally lost, affecting the ability of ligand-binding repeats to interact with their ligands [35]. Because of a strong dependence of A1 function on the bound calcium ion, we compared hA1, mA1 and mA1ND for their affinities for calcium. All three examined A1 molecules bound calcium with Kd values in the low micromolar range (Figure 1 and Table 1). The measured Kd values were 4.3 μM, 10.2 μM and 2.8 μM for hA1, mA1 and mA1ND, respectively. Of the three, mA1ND has the strongest affinity for calcium, which contributes to its conformational stability.
Figure 1.
Calcium binding by the A1 variants. ITC titrations of human A1 (A), mouse A1 (B) and Asn36/Asp mutant of the mouse A1 (C) with calcium.
Table 1.
Thermodynamic parameters of the Ca2+ binding to A1 variants measured by ITC at 298 K.
| N | Kd (μM) | ΔH (kcal/mol) | TΔS (kcal/mol) | ΔG (kcal/mol) | |
|---|---|---|---|---|---|
| hA1 | 0.81 ± 0.01 | 4.3 ± 0.4 | −9.1 ± 0.1 | −1.7 ± 0.2 | −7.4 ± 0.06 |
| mA1 | 0.80 ± 0.01 | 10.2 ± 0.6 | −8.6 ± 0.1 | −1.8 ± 0.1 | −6.8 ± 0.03 |
| mA1ND | 0.77 ± 0.01 | 2.8 ± 0.3 | −9.7 ± 0.2 | −2.1 ± 0.2 | −7.6 ± 0.06 |
Binding of A1 variants to domain V of human β2GPI
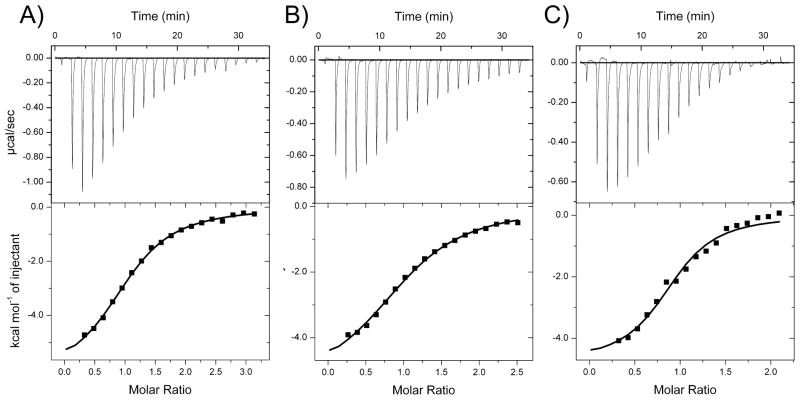
A1, which is a part of the ApoER2 receptor, is critical for the binding of β2GPI/antibody complexes to the receptor [30]. A1 binds to domain V of β2GPI (β2GPI-DV) [32, 36]. We compared the binding affinities of A1 variants for human β2GPI-DV. The Kd values measured for the binding of hA1, mA1 and mA1ND to human β2GPI-DV in a Hepes buffer containing 2 mM CaCl2 and 50 mM NaCl were 20 μM, 26 μM and 8 μM, respectively (Figure 2 and Table 2). Electrostatic interactions play a major role in the binding of all three variants of A1 to β2GPI-DV. When the concentration of NaCl in the buffer was increased to 150 mM, the Kd value of mA1ND increased from 8 μM to 14 μM (Figure 3). The binding affinity of A1 for β2GPI-DV is likely underestimated when measured in a Hepes or other sulfite containing buffer. Crystal structures of β2GPI determined in the presence of ammonium sulfate (PDB ID 3OP8 and 4JHS) have shown bound sulfate ions forming polar contacts with the lysine residues 282, 284, 308 and 317 of β2GPI-DV, which are also important for the interaction between A1 and β2GPI-DV [32].
Figure 2.
Binding of the A1 variants to domain V of β2GPI. ITC titrations of human β2GPI-DV with human A1 (A), mouse A1 (B) and Asn36/Asp mutant of the mouse A1 (C).
Table 2.
Thermodynamic parameters of A1 binding to β2GPI-DV measured by ITC at 298 K.
| ligand | N | Kd (μM) | ΔH (kcal/mol) | TΔS (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|---|---|
| hA1 | 1.06 ± 0.02 | 20 ± 1 | −6.3 ± 0.2 | 0.1 ± 0.2 | −6.39 ± 0.04 |
| mA1 | 1.06 ± 0.02 | 26 ± 2 | −5.5 ± 0.2 | 0.8 ± 0.3 | −6.25 ± 0.05 |
| mA1ND | 0.91 ± 0.03 | 8 ± 2 | −4.8 ± 0.3 | 2.2 ± 0.4 | −7.0 ± 0.1 |
Figure 3.
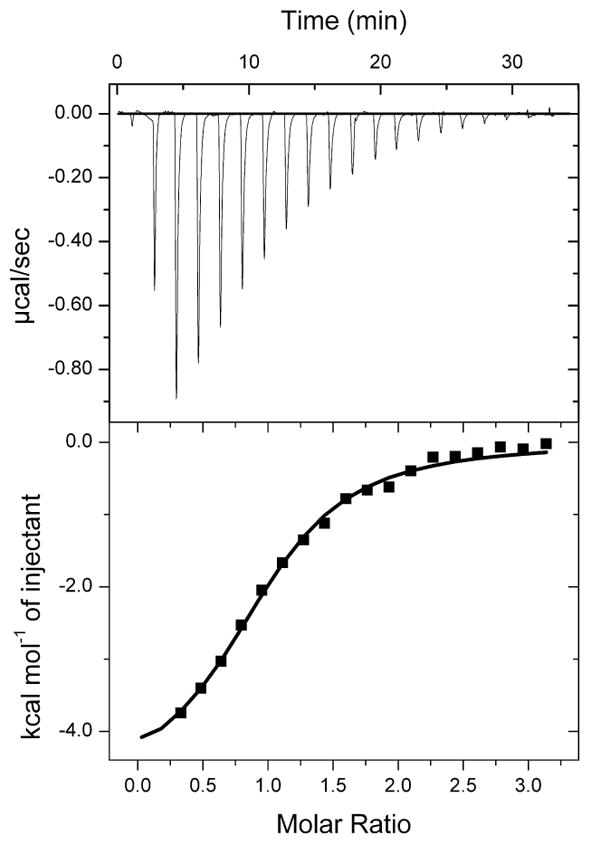
Binding of Asn36/Asp mutant of the mouse A1 to domain V of β2GPI in the presence of 150 mM NaCl. Thermodynamic parameters measured at 298 K were ΔH=−4.3 ± 0.2 (kcal/mol), TΔS=2.3 ± 0.3 (kcal/mol), ΔG=−6.6 ± 0.1 and N=1.04 ± 0.03.
Two possible orientations of the ligand-binding module from a lipoprotein receptor in the complex with β2GPI
All members of the LDL receptor family can bind β2GPI [16]. Previously, we used solution NMR spectroscopy to examine how ligand-binding modules from lipoprotein receptors interact with domain V of β2GPI (β2GPI-DV). Because the crystal structure of A1 was not available, we used LA4, a ligand-binding module from the low density lipoprotein receptor, to model the complex and confirmed the model with extensive experimental observations [32]. We also compared A1 to LA4 and determined that both molecules interact with β2GPI via residues at their calcium-coordinating site and that the lysine residues Lys 308 and 317 of β2GPI-DV are important for complex formation. Recently, the X-ray structure of human A1 was deposited to the PDB data bank (PDB ID: 3A7Q), allowing us to build a model of the complex between A1 and β2GPI-DV.
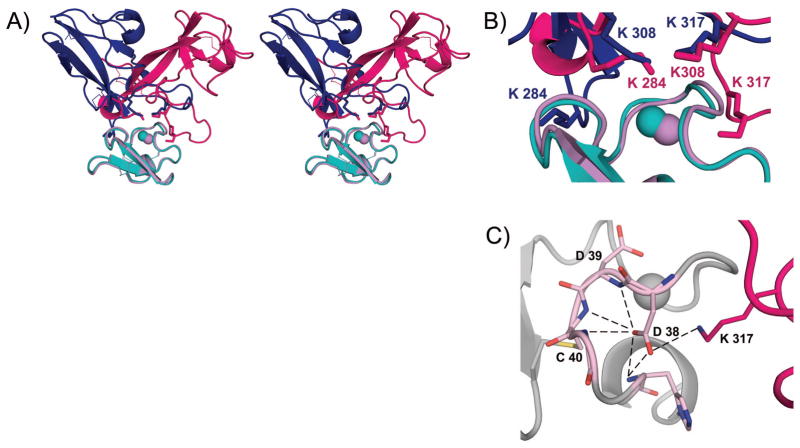
The crystal structures of human β2GPI-DV and human A1 were used for modeling the complex. The docking resulted in three clusters of structures. The top cluster was more than two times more populated than the second cluster. After superposing the representative A1/β2GPI-DV structure from the top cluster onto the LA4/β2GPI-DV complex, we observed that the two models of the complex were shifted relative to each other. To compare the two models in detail, we aligned A1 onto LA4 in the LA4/β2GPI-DV model, energy minimized, and superimposed the resultant complex, which we named A1(LA4)/β2GPI-DV, onto the model of the A1/β2GPI-DV complex (Figure 4A,B). In both models, β2GPI-DV binds to the residues around the calcium-binding site on A1 and the lysine residues of β2GPI-DV form the major contacts with A1, which is in agreement with our previous experimental observations [32]. Furthermore, in both models there are three lysine residues of β2GPI-DV, Lys 284, 308 and 317, in the vicinity of A1 and two of these lysine residues interact with the calcium-coordinating residues on A1 (Figure 4B). Interestingly, the two interacting lysine residues are Lys 308 and 317 in the A1(LA4)/β2GPI-DV complex, and Lys 284 and 308 in the A1/β2GPI-DV complex. The spatial position of ε-amino groups of Lys 284 and 308 relative to A1 in the A1/β2GPI-DV complex closely matches the spatial position of ε-amino groups of Lys 308 and 317 in the A1(LA4)/β2GPI-DV complex (Figure 4B). These two models represent two possible ways in which ligand-binding modules from lipoprotein receptors bind β2GPI. The preferential binding mode likely depends on the primary sequence of the ligand-binding module interacting with β2GPI. Both models support our previous observation that β2GPI can’t simultaneously bind to ApoER2 and anionic phospholipids [32].
Figure 4.
Two binding modes in the complex between the ligand-binding module from a lipoprotein receptor and β2GPI-DV. A) Stereo view of two superimposed complexes. Human A1 was aligned onto LA4 in the LA4/β2GPI-DV model (PDB ID: 2KRI) and energy minimized. The resultant complex, A1(LA4)/β2GPI-DV, is superimposed onto the model of the A1/β2GPI-DV complex. In the A1(LA4)/β2GPI-DV complex, A1(LA4) is cyan and β2GPI-DV is blue. In the A1/β2GPI-DV complex, A1 is pink and β2GPI-DV is magenta. B) Close-up view of the binding interface in the A1/β2GPI-DV (colored pink and magenta) complex superimposed onto the A1(LA4)/β2GPI-DV complex (colored cyan and blue). C) A network of hydrogen bonds created by Asp 38 in a complex between human A1 and human β2GPI. The backbone of A1 is gray, the residues of A1 involved in hydrogen bonds are pink and β2GPI-DV is magenta. Bound calcium ions are shown as spheres.
ITC binding studies of A1 variants support the model for the A1/β2GPI-DV complex. In this model, the sidechain of Asp 38 of human A1 forms a network of hydrogen bonds with the backbone of A1, stabilizing the calcium-coordinating residue Asp 39, and with Lys 317 of β2GPI-DV, strengthening the complex (Figure 4C). This network of hydrogen bonds is lost in mouse A1, where an asparagine residue, Asn 36 in the primary sequence of mouse A1, is located in place of Asp 38 of human A1. A point mutation, Asn36/Asp, substituting aspartate for asparagine improves both the calcium binding of mouse A1 and its affinity for β2GPI-DV.
Inhibition of the binding of human and mouse β2GPI to anionic phospholipids in cardiolipin ELISA
B2GPI/antibody complexes are prothrombotic in APS patients and in mice [7, 8, 33]. Disruption of a protective shield formed by annexin V on anionic phospholipids by β2GPI/antibody complexes is one of the thrombogenic mechanisms in antiphospholipid syndrome [21, 37]. It was also suggested that the β2GPI molecule bound to phospholipids changes its overall conformation exposing previously hidden epitopes for anti-β2GPI antibodies [38]. The inhibition of the binding of β2GPI/antibody complexes to negatively charged phospholipids, in addition to interference with cellular activation via inhibition of ApoER2, is an important property of the A1-A1 inhibitor.
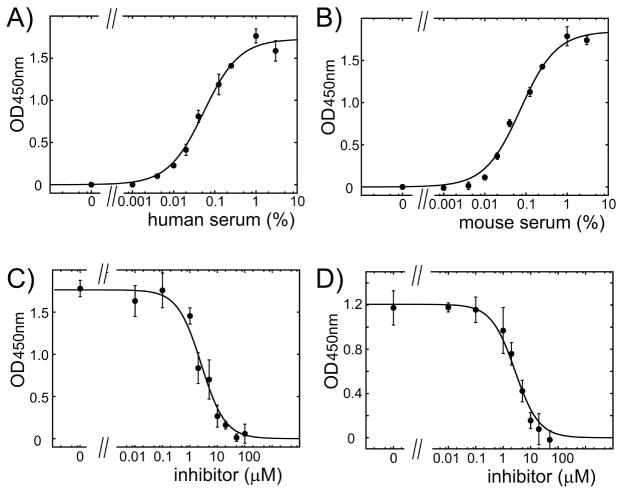
We compared how A1-A1 variants inhibit the binding of human and mouse β2GPI/antibody complexes to anionic phospholipid cardiolipin. We used normal human and mouse serum to supply β2GPI and supplemented serum with anti-β2GPI antibodies. The quantity of human and mouse serum used in the assay was selected from binding curves to yield 60% of maximal binding of β2GPI/antibody complexes to cardiolipin (Figure 5A,B). We compared the inhibitory property of A1-A1 variants composed of two mouse A1 modules (mA1-A1), two human A1 (hA1-A1) and two Asn36/Asp mutants of mouse A1 (mA1-A1ND). mA1-A1ND inhibited human and mouse β2GPI equally well in the presence of anti-β2GPI antibodies (Figure 5C,D and Table 3).
Figure 5.
Binding and inhibition of the binding of human and mouse β2GPI to cardiolipin in the presence of anti-β2GPI antibodies. The binding curves for β2GPI in human (A) and mouse (B) serum to cardiolipin-coated surface in the presence of anti-β2GPI antibodies. Representative inhibition curves for β2GPI in human (C) and mouse (D) serum titrated with mA1-A1ND in the presence of anti-β2GPI antibody. The measurement at each data point is expressed as mean ± standard deviation (n=3).
Table 3. Inhibition of the binding of β2GPI/antibody complexes to cardiolipin by A1-A1 variants.
Data is expressed as a mean calculated from at least two independent experiments. Uncertainties are calculated as mean values of errors computed for nonlinear fits of inhibition constants. Human A1-A1 (hA1-A1); mouse A1-A1 (mA1-A1); Asn36/Asp mutant of mouse A1-A1 (mA1-A1ND); human β2GPI (hβ2GPI) and mouse β2GPI (mβ2GPI).
| IC50 (μM) hβ2GPI |
IC50 (μM) mβ2GPI |
|
|---|---|---|
| hA1-A1 | 5.0 ± 0.8 | 3.1 ± 0.8 |
| mA1-A1 | 2.7 ± 0.6 | 3.0 ± 0.4 |
| mA1-A1ND | 2.5 ± 0.6 | 2.8 ± 0.5 |
Reduction of clotting time in plasma samples of APS patients
Autoimmune anti-β2GPI antibodies predispose clinically to thrombosis but cause a prolongation of clotting time in the laboratory test for APS [2]. Prolonged clotting time better correlates with thrombosis in APS patients than just a positivity in anti-β2GPI and anti-cardiolipin ELISA tests [6]. Anti-β2GPI antibodies affect clotting time by enhancing the binding of β2GPI to anionic phospholipids in test reagent, therefor competing with the assembly of the clotting factors of the coagulation cascade.
We evaluated mA1-A1ND for its ability to inhibit the clotting time in plasma samples of APS patients positive for anti-β2GPI antibodies. All patients were on long-term oral anticoagulation therapy with warfarin. The mA1-A1ND inhibitor was evaluated in a modified prothrombin time test. APS citrated plasma was incubated with mA1-A1ND for 30 min on ice in the presence of calcium. Clotting was initiated by a test reagent containing recombinant tissue factor, synthetic anionic phospholipids and calcium. Prolongation of the clotting time in patients’ plasma is a combined effect of warfarin and β2GPI/antibody complexes. mA1-A1ND reduced the clotting time dose-dependently in APS samples (Figure 6A). mA1-A1ND reduced the clotting time in normal plasma supplemented with anti-β2GPI antibodies and had no effect on clotting time in normal plasma in the absence of anti-β2GPI antibodies (Figure 6A). The lack of any effect of A1-A1 on the clotting time in normal plasma correlates with our in vivo data showing that A1-A1 is effective in interfering with thrombotic properties of anti-β2GPI antibodies and does not affect normal thrombus formation in the absence of anti-β2GPI [33]. Both mA1-A1ND and mA1-A1 inhibited the clotting time in patients’ plasma equally well (Figure 6B).
Figure 6.
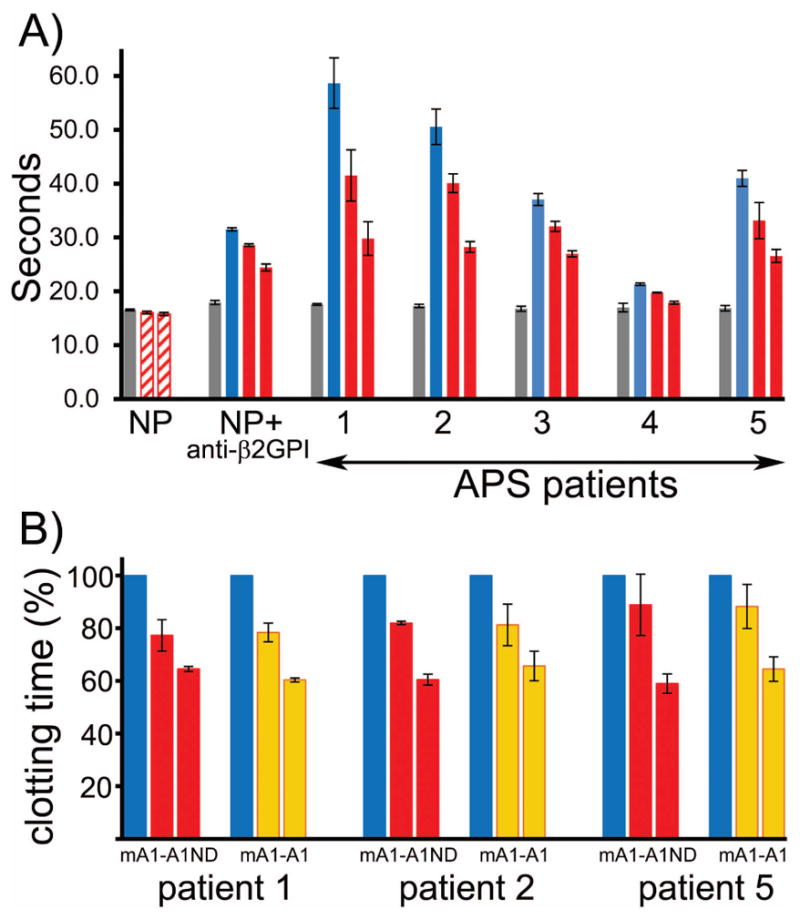
Inhibition of clotting time. A) mA1-A1ND inhibits the clotting time in plasma samples of APS patients positive for anti-β2GPI antibodies. Normal plasma (gray bars); normal plasma supplemented with anti-β2GPI antibodies and anti-β2GPI positive patients’ plasma (blue bars); normal plasma with anti-β2GPI antibodies and patients’ plasma in the presence of either 4 μM or 17 μM of mA1-A1ND (red bars) and normal plasma containing either 4 μM or 17 μM of mA1-A1ND (hatched bars). (B) mA1-A1 and mA1-A1ND inhibit equally well the prolongation of clotting time in plasma samples of APS patients positive for anti-β2GPI antibodies. APS plasma (blue bars), APS plasma in the presence of either 4 μM or 17 μM of mA1-A1ND (red bars) and mA1-A1 (orange bars). Results are expressed as mean and deviation from the mean calculated from 2 to 3 independent experiments.
The binding of A1-A1 to β2GPI free of antibody is weak. A1-A1 will only bind to β2GPI engaged by the anti-β2GPI antibody forming a stable complex containing A1-A1, two β2GPI molecules and antiβ2GPI antibody, so that the β2GPI/anti-β2GPI complex can no longer bind to phospholipids and interfere with the clotting. Observed inhibition of the clotting time demonstrates the presence of soluble β2GPI/anti-β2GPI antibody complexes in patients’ plasma. These complexes either already exist in patients’ plasma or form rapidly in the vicinity of phospholipids after addition of phospholipids with the test reagent.
Conclusions
The A1-A1 inhibitor, which contains two β2GPI-binding A1 modules from ApoER2, preferentially binds pathological β2GPI/antibody complexes compared to free β2GPI, which circulates in the blood at high concentration, and reduces thrombus size in vivo in a mouse thrombosis model [31, 33]. In this study, we made A1 and A1-A1 molecules consisting of human (hA1 and hA1-A1), mouse (mA1 and mA1-A1) and a single Asn36/Asp mutant of mouse (mA1ND and mA1-A1ND) sequences and compared their properties. 1) We determined that mA1ND has the strongest affinity for calcium, contributing to the conformational stability and improving the yield of purified A1 and A1-A1. 2) mA1ND is more than two fold more effective than either human or mouse A1 in binding to human β2GPI, suggesting that the mA1-A1ND inhibitor comprised of two mA1ND modules is the most effective in inhibiting the binding of β2GPI/antibody complexes to the ApoER2 receptor compared to human or mouse A1-A1. 3) The inhibition of the binding of β2GPI/antibody complexes to anionic phospholipids is an important feature of A1-A1. mA1-A1ND inhibited the binding of human and mouse β2GPI/antibody complexes to cardiolipin equally well. 4) mA1-A1ND inhibited β2GPI/antibody complexes in plasma samples of APS patients. It inhibited the clotting time in APS plasma, but had no effect on clotting time in normal plasma in the absence of anti-β2GPI antibodies. 5) Inhibition of the clotting time demonstrates the presence of soluble β2GPI/anti-β2GPI antibody complexes in patients’ plasma. These complexes either already exist in patients’ plasma or form rapidly in the vicinity of phospholipids. 6) All members of the LDL receptor family can bind β2GPI. The model of the A1 complex with β2GPI-DV and its detailed comparison with the LA4/β2GPI-DV complex revealed two possible ways for lipoprotein receptors to interact with β2GPI-DV. The primary sequence of the ligand-binding module determines which lysine residues of β2GPI-DV are engaged in the complex. Both complexes support our previous observation that the binding site on a ligand-binding module includes the calcium coordinating residues and that β2GPI can’t simultaneously bind lipoprotein receptor and anionic phospholipids.
Experimental Procedures
Proteins
hA1 is a fragment of human ApoER2 (residues 14–49 of mature protein), mA1 is a fragment of mouse ApoER2 (residues 12–47 of mature protein) and mA1ND is a Asn36/Asp mutant of mouse A1. hA1-A1, mA1-A1 and mA1-A1ND, which were constructed of two hA1, mA1 and mA1ND, respectively, were expressed in E.coli and purified as previously described [31]. Domain V of β2GPI, β2GPI-DV, comprising residues 244–326 of human β2GPI was subcloned into a pET15b vector (Novagen), expressed and purified as described previously [32].
Patients
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional ethics committee. Blood samples were collected from APS patients after informed consent. Patients with a history of thrombosis were diagnosed with antiphospholipid syndrome (APS) based on international guidelines [2]. All patients, except for Patient#4, had primary APS, while Patient#4 had APS secondary to lupus. At the time of blood collection, all patients were on oral anticoagulation with warfarin. The levels of anti-β2GPI antibodies in the collected blood were measured with β2GPI IgG ELISA (Inova). The INR ratio was determined in a clinical laboratory. The measured values of antiβ2GPI IgG and INR in the APS patients’ blood were as follows: in Patient#1 the level of anti-β2GPI was 98 SGU and INR was 2.3, Patient#2- 166 SGU and INR 2.3, Patient#3- 65 SGU and INR 3.4, Patient#4- 113 SGU and INR was not determined, and Patient#5- 123 SGU and INR 2.2.
Mice
BALB/c mice were obtained from Jackson Laboratories. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Isothermal Titration Calorimetry
To measure calcium binding by A1 proteins, lyophilized proteins were resuspended in a 25 mM Hepes, 150 mM NaCl, pH 7.4 buffer treated with Chelex100 (Sigma) and dialyzed overnight in the same buffer. A1 proteins in the cell were titrated with 10–15 fold molar excess of CaCl2 in an injection syringe prepared in the same buffer. The binding between A1 proteins and domain V of human β2GPI (β2GPI-DV) was measured in a 25 mM Hepes, 50 mM NaCl, 2 mM CaCl2, pH 7.4 buffer. The concentration of human β2GPI-DV in a sample cell was 100 μM and A1 proteins at a concentration between 1.0 and 1.5 mM were placed in the injection syringe. Binding isotherms were fit to a one site binding model.
Cardiolipin ELISA
ELISA 96 well plates (Costar) were coated with 50 μl per well of cardiolipin (Sigma) prepared at 200 μg/ml in ethanol. Plates were blocked for 2 hours with a 20 mM Tris, 100 mM NaCl, pH 7.4 buffer supplemented with 3% BSA. Pooled normal human serum and pooled mouse serum collected from 8–12 weeks old BALB/c mice were used as sources of human and mouse β2GPI, respectively. The concentration of serum in test samples for inhibition studies was determined from the binding curves to provide 60% of maximal binding in the presence of a given amount of anti-β2GPI antibodies.
Inhibition of human β2GPI (hβ2GPI): Samples containing 0.08% of pooled normal human serum, peroxidase-conjugated goat anti-human β2GPI antibodies (Cedarlane Laboratories, 2 mg/ml stock, 1:1000 dilution) and various concentrations of A1-A1 variants were preincubated for 1 hour at room temperature in a 50 mM Tris, pH 7.4 buffer containing 100 mM NaCl, 2mM CaCl2 and 0.5% BSA. Samples were added to wells (50 μl per well) and incubated for 1 hour at room temperature. Bound hβ2GPI/anti-β2GPI antibody complexes were detected using TMB (3,3′,5,5′-tetramethylbenzidine) substrate by measuring OD at 450 nm.
Inhibition of mouse β2GPI (mβ2GPI): Samples contained 0.1 % mouse serum, biotin-conjugated goat anti-human β2GPI antibodies (Bethyl Laboratories, 1 mg/ml stock, 1:500 dilution) and various concentrations of A1-A1 variants. Samples were preincubated for 1 hour at room temperature in a 50 mM Tris, pH 7.4 buffer containing 100 mM NaCl, 2mM CaCl2 and 0.5% BSA and applied for 1 hour at room temperature on a cardiolipin-coated plate. Wells were washed and treated with HRP-conjugated NeutrAvidin (Pierce) in 20 mM Tris, 100 mM NaCl, pH 7.4. Bound β2GPI/antibody complexes were detected with a TMB substrate.
The binding and inhibition data was fitted to a one-site model using the nonlinear least-squares Marquardt-Levenberg algorithm implemented in the GNUPLOT program. Reported inhibition constants are mean values calculated from at least two independent experiments. Uncertainties of determined inhibition constants are calculated as mean values of errors computed by GNUPLOT for nonlinear fits of inhibition constants.
Docking of A1 onto β2GPI-DV
Docking was performed using the protein-protein docking server ClusPro (http://www.cluspro.bu.edu) [39]. The coordinates of human domain V of β2GPI-DV (PDB ID: 3OP8) and human A1 (PDB ID: 3A7Q) were taken from the Protein Data Bank. All water molecules and bound sulfates were removed from the β2GPI-DV structure and only one chain was retained for docking. A calcium ion was removed from the structure of A1 prior to docking. A “Van der Waals plus Electrostatics” weighting scheme implemented in ClusPro was used for scoring. The calculated modes of the A1/β2GPI-DV complex formed three clusters with the top cluster two times more populated than the second cluster and twenty times more populated than the third cluster. Structures were visualized using PyMOL [40].
Clotting time
Blood from APS patients was collected into BD Vacutainer citrated plasma collection tubes. Citrated plasma was centrifuged twice at 2500 g for 15 min. Citrated pooled normal human plasma was from BioreclamationIVT. Platelet counts in plasma preparations were less than 10,000/μl. Clotting time was measured using Start4 (Stago) coagulometer and Dade Innovin reagent. The total sample volume was 100 μl containing 75 μl of plasma mixed with 25 μl of 25 mM HEPES, 150 mM NaCl, 40 mM CaCl2, pH 7.4 buffer. The test samples included a) pooled normal human plasma (NP), b) NP containing 10 μg of anti-β2GPI (Cedarlane), c) NP containing 10 μg of anti-β2GPI in the presence of 4 μM and 17 μM mA1-A1ND, d) anti-β2GPI positive APS plasma containing 4 μM and 17 μM mA1-A1ND or mA1-A1. The test samples were incubated on ice for 30 min. After incubation for 100 sec at 37°C, coagulation was initiated by adding 25 μl of reagent diluted 1:1 with 25 mM Hepes, 150 mM NaCl, pH 7.4 buffer.
Acknowledgments
This work was supported by a R01 grant HL096693 from the National Institute of Health.
References
- 1.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. The New England journal of medicine. 2013;368:1033–44. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, de Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.de Groot PG, Meijers JC. beta(2) -Glycoprotein I: evolution, structure and function. J Thromb Haemost. 2011;9:1275–84. doi: 10.1111/j.1538-7836.2011.04327.x. [DOI] [PubMed] [Google Scholar]
- 4.de Groot PG, Urbanus RT. The significance of auto-antibodies against beta2-Glycoprotein I. Blood. 2012;120:266–74. doi: 10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 5.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nature reviews Rheumatology. 2011;7:330–9. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 6.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104:3598–602. doi: 10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 7.Arad A, Proulle V, Furie RA, Furie BC, Furie B. beta(2)-Glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;117:3453–9. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski M, Vreys I, Wittevrongel C, Boon D, Vermylen J, Hoylaerts MF, Arnout J. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101:157–62. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 9.Pierangeli SS, Liu SW, Anderson G, Barker JH, Harris EN. Thrombogenic properties of murine anti-cardiolipin antibodies induced by beta 2 glycoprotein 1 and human immunoglobulin G antiphospholipid antibodies. Circulation. 1996;94:1746–51. doi: 10.1161/01.cir.94.7.1746. [DOI] [PubMed] [Google Scholar]
- 10.Agostinis C, Biffi S, Garrovo C, Durigutto P, Lorenzon A, Bek A, Bulla R, Grossi C, Borghi MO, Meroni P, Tedesco F. In vivo distribution of beta2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118:4231–8. doi: 10.1182/blood-2011-01-333617. [DOI] [PubMed] [Google Scholar]
- 11.Harper BE, Wills R, Pierangeli SS. Pathophysiological mechanisms in antiphospholipid syndrome. International journal of clinical rheumatology. 2011;6:157–171. doi: 10.2217/ijr.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripodi A, de Groot PG, Pengo V. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med. 2011;270:110–22. doi: 10.1111/j.1365-2796.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 13.Proulle V, Furie RA, Merrill-Skoloff G, Furie BC, Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. 2014 doi: 10.1182/blood-2014-02-554980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, Salmon JE, Shaul PW, Mineo C. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011;121:120–31. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2012;119:884–93. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennings MT, van Lummel M, Derksen RH, Urbanus RT, Romijn RA, Lenting PJ, de Groot PG. Interaction of beta2-glycoprotein I with members of the low density lipoprotein receptor family. J Thromb Haemost. 2006;4:1680–90. doi: 10.1111/j.1538-7836.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 17.Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, Rivera J, Iverson GM, Cockerill KA, Linnik MD, Krilis SA. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–67. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 18.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta(2)-glycoprotein I requires signaling via both glycoprotein Ibalpha and Apolipoprotein E Receptor 2′. J Thromb Haemost. 2008;6:1405–12. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 19.Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, VanHoorelbeke K, Urbanus RT, Lisman T, de Groot PG. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2rime; J Thromb Haemost. 2007;5:369–77. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 20.Willems GM, Janssen MP, Pelsers MM, Comfurius P, Galli M, Zwaal RF, Bevers EM. Role of divalency in the high-affinity binding of anticardiolipin antibody-beta 2-glycoprotein I complexes to lipid membranes. Biochemistry. 1996;35:13833–42. doi: 10.1021/bi960657q. [DOI] [PubMed] [Google Scholar]
- 21.Rand JH, Wu XX, Quinn AS, Taatjes DJ. Resistance to annexin A5 anticoagulant activity: a thrombogenic mechanism for the antiphospholipid syndrome. Lupus. 2008;17:922–30. doi: 10.1177/0961203308095029. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich V, Konaniah ES, Lee WR, Khadka S, Shen YM, Herz J, Salmon JE, Hui DY, Shaul PW, Mineo C. Antiphospholipid antibodies attenuate endothelial repair and promote neointima formation in mice. Journal of the American Heart Association. 2014;3:e001369. doi: 10.1161/JAHA.114.001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, Papalardo E, Vargas G, Deora AB, Wang M, Jacovina AT, Garcia-Latorre E, Reyes-Maldonado E, Hajjar KA, Pierangeli SS. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–83. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, Nguyen-Oghalai T, Meroni PL. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–33. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, Shilagard T, Vargas G, Hwang Y, de Groot PG, Pierangeli SS. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–14. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Lira F, Rosales-Leon L, Martinez VM, Ruiz Ordaz BH. The role of beta2-glycoprotein I (beta2GPI) in the activation of plasminogen. Biochimica et biophysica acta. 2006;1764:815–23. doi: 10.1016/j.bbapap.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Pozzi N, Acquasaliente L, Frasson R, Cristiani A, Moro S, Banzato A, Pengo V, Scaglione GL, Arcovito A, De Cristofaro R, De Filippis V. beta2 -Glycoprotein I binds to thrombin and selectively inhibits the enzyme procoagulant functions. J Thromb Haemost. 2013;11:1093–102. doi: 10.1111/jth.12238. [DOI] [PubMed] [Google Scholar]
- 28.Rahgozar S, Yang Q, Giannakopoulos B, Yan X, Miyakis S, Krilis SA. Beta2-glycoprotein I binds thrombin via exosite I and exosite II: anti-beta2-glycoprotein I antibodies potentiate the inhibitory effect of beta2-glycoprotein I on thrombin-mediated factor XIa generation. Arthritis Rheum. 2007;56:605–13. doi: 10.1002/art.22367. [DOI] [PubMed] [Google Scholar]
- 29.Shi T, Iverson GM, Qi JC, Cockerill KA, Linnik MD, Konecny P, Krilis SA. Beta 2-Glycoprotein I binds factor XI and inhibits its activation by thrombin and factor XIIa: loss of inhibition by clipped beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 2004;101:3939–44. doi: 10.1073/pnas.0400281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennings MT, Derksen RH, Urbanus RT, Tekelenburg WL, Hemrika W, de Groot PG. Platelets express three different splice variants of ApoER2 that are all involved in signaling. Journal Thromb Haemost. 2007;5:1538–44. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 31.Kolyada A, Lee CJ, De Biasio A, Beglova N. A novel dimeric inhibitor targeting Beta2GPI in Beta2GPI/antibody complexes implicated in antiphospholipid syndrome. PLoS One. 2010;5:e15345. doi: 10.1371/journal.pone.0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CJ, De Biasio A, Beglova N. Mode of interaction between beta2GPI and lipoprotein receptors suggests mutually exclusive binding of beta2GPI to the receptors and anionic phospholipids. Structure. 2010;18:366–76. doi: 10.1016/j.str.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Kolyada A, Porter A, Beglova N. Inhibition of thrombotic properties of persistent autoimmune anti-beta2GPI antibodies in the mouse model of antiphospholipid syndrome. Blood. 2014;123:1090–7. doi: 10.1182/blood-2013-08-520882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–3. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 35.Blacklow SC, Kim PS. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat Struct Biol. 1996;3:758–62. doi: 10.1038/nsb0996-758. [DOI] [PubMed] [Google Scholar]
- 36.van Lummel M, Pennings MT, Derksen RH, Urbanus RT, Lutters BC, Kaldenhoven N, de Groot PG. The binding site in {beta}2-glycoprotein I for ApoER2rime; on platelets is located in domain V. J Biol Chem. 2005;280:36729–36. doi: 10.1074/jbc.M504172200. [DOI] [PubMed] [Google Scholar]
- 37.Rand JH, Wu XX, Quinn AS, Taatjes DJ. The annexin A5-mediated pathogenic mechanism in the antiphospholipid syndrome: role in pregnancy losses and thrombosis. Lupus. 2010;19:460–9. doi: 10.1177/0961203310361485. [DOI] [PubMed] [Google Scholar]
- 38.Agar C, van Os GM, Morgelin M, Sprenger RR, Marquart JA, Urbanus RT, Derksen RH, Meijers JC, de Groot PG. {beta}2-Glycoprotein I can exist in two conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–43. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 39.Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics. 2004;20:45–50. doi: 10.1093/bioinformatics/btg371. [DOI] [PubMed] [Google Scholar]
- 40.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; Palo Alto, CA, USA: 2002. [Google Scholar]